Abstract
We have previously described the antibacterial capacity of protegrin-1 (PG-1), a cysteine-rich, cationic peptide from porcine leukocytes, against Neisseria gonorrhoeae. We now report genetic and biochemical evidence that gonococcal susceptibility to the lethal action of PG-1 and other structurally unrelated antibacterial peptides, including a peptide (LL-37) that is expressed constitutively by human granulocytes and testis and inducibly by keratinocytes, is modulated by an energy-dependent efflux system termed mtr. These results indicate that such efflux systems may enable mucosal pathogens like gonococci to resist endogenous antimicrobial peptides that are thought to act during infection.
Researchers have isolated antibacterial peptides from numerous vertebrate, invertebrate, and plant sources during the past decade (1). These peptides represent part of the first line of host defense against invading pathogens and, for certain hosts, may represent the primary mechanism of host resistance against infectious agents (2). Antibacterial peptides are present constitutively in certain phagocytic cells (3). Their synthesis by epithelial cells, which line certain mucosal surfaces, can be induced by infection, inflammation, or trauma (4, 5). The broad-spectrum antimicrobial action of many peptides and the emergence of pathogens that are resistant to many currently available antibiotics have stimulated the development of antibacterial peptides as therapeutic agents (6).
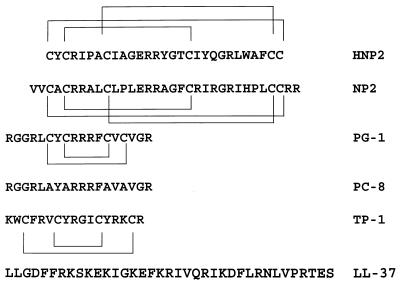
We recently described the in vitro antibacterial actions of protegrins, a family of antimicrobial peptides found in porcine leukocytes (7–9). Protegrins also display potent broad-spectrum action in vivo in experimental murine infections (10). Like the well characterized defensins, protegrins are cationic, cysteine-rich peptides that assume a β-structure in solution. However, protegrins are smaller than defensins (16–18 vs. 29–∼40 amino acids, respectively) and have two instead of three intramolecular disulfide bridges (see Fig. 1). The intramolecular disulfide bonds of protegrins appear to be crucial for their maximal bactericidal activity because a linear synthetic protegrin variant (PC-8) that lacked the four invariant cysteines found in native protegrins was at at least 100-fold less active against Neisseria gonorrhoeae (11) than the parental peptide, protegrin-1 (PG-1). The detailed, systematic structure-function analysis of PG-1 conducted by Qu et al. (11) suggested that the amphipathic central β-sheet domain of PG-1 (12) is essential for its bactericidal action against N. gonorrhoeae.
Figure 1.
The primary amino acid sequence in single letter code of the antibacterial peptides used in this study. The location and linkage of the intramolecular disulfide bonds are shown above or below each relevant sequence. HNP 2, human defensin 2; NP 2, rabbit defensin 2, PG-1, protegrin-1; PC-8, synthetic, linearized variant of PG-1; TP-1, tachyplesin-1; LL-37, antibacterial peptide derived from CAP18 (5). Note that the C-terminal arginine residue of PG-1 and PC-8 is amidated (9).
Although the structurally related human and rabbit defensins fail to exert activity against N. gonorrhoeae, the protegrins display antigonococcal action at low micromolar concentrations (9). Electron microscopic studies revealed numerous surface-associated lesions on PG-1-treated gonococci, suggesting that these peptides are membrane-acting compounds. Thus, it may be difficult for pathogens such as gonococci to develop specific mechanisms of protegrin resistance. However, nonspecific mechanisms of microbial resistance such as reduced permeability or energy-dependent efflux systems (13) that enhance bacterial resistance to multiple antimicrobial agents might decrease levels of microbial susceptibilty to antibacterial peptides.
Gonococci possess an energy-dependent efflux pump termed mtr (multiple transferrable resistance) that confers enhanced resistance to structurally diverse, antimicrobial hydrophobic agents (HAs) (14–18), including membrane-damaging compounds (e.g., bile salts and fatty acids) that bathe certain mucosal surfaces. The mtr efflux pump is encoded by a three-gene, single transcriptional unit (mtrCDE) (14). The gonococcal MtrC–MtrD–MtrE proteins are highly similar to efflux pump proteins possessed by Escherichia coli and Pseudomonas aeruginosa (19) and have been placed in the resistance/nodulation/division (RND) family of efflux pumps (20).
The known capacity of the mtr efflux system to enhance gonococcal resistance to HAs, including cationic compounds that exert their antimicrobial action at the cytoplasmic membrane, prompted us to evaluate whether this mechanism of resistance might decrease gonococcal susceptibility to antimicrobial peptides. We now report that loss of the mtr efflux pump results in increased susceptibility of gonococci to PG-1 and LL-37, a recently described (5) α-helical human antimicrobial peptide expressed constitutively by granulocytes and testis and inducibly by keratinocytes.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions.
Table 1 is a description of the genotype and source of the isogenic gonococcal strains used in this investigation. Strain FA19 is the wild-type parental strain that was used in the genetic construction of strains KH12, KH14, and RD1 (14, 15, 18). Strain BR54 is a transformant of the FA19 genetic lineage constructed previously (21) and is hypersusceptible to HAs such as erythromycin, Triton X-100, and crystal violet. Strain WV2 is a spontaneous erythromycin-resistant mutant of BR54 that is cross-resistant to additional HAs (22). Strain BR54 contains a 10-bp deletion at the 3′-end of the mtrD gene (GenBank accession no. AF037040) that causes a frame shift after codon 912, resulting in truncation of MtrD (938 vs. 1056 amino acids); this deletion has no effect on the downstream expression of the mtrE gene. Strain WV2 contains an additional 2-bp deletion in its mtrD gene that restores the correct reading frame of mtrD but leaves an internal four amino acid deletion (residues 913–916) in MtrD. All gonococcal strains were routinely cultured in their non-piliated, opacity-negative state on GCB typing agar (Difco) containing defined supplements I and II (23) under 3.8% (vol/vol) CO2 at 37°C as described previously (23). Growth from GCB agar plates was resuspended in GCB broth containing defined supplements and 0.043% (wt/vol) sodium bicarbonate and grown with shaking at 37°C to mid-logarithmic phase.
Table 1.
Susceptibility of isogenic strains to PG-1
| Strain* | Relevant genotype | PG-1 MGIC, μg/ml† |
|---|---|---|
| FA19 | Wild type | 4.37 (±0.88) |
| KH12 | mtrC::Km | 0.75 (±0.28) |
| KH14 | mtrD::Km | 0.69 (±0.22) |
| RD1 | mtrE::Km | 0.2 (±0.11) |
| BR54 | mtrD54 | 0.67 (±0.31) |
| WV2 | As BR54 but mtrD54† | 7.14 (±2.67) |
The construction of transformant strains KH12, KH14, and RD1 has been described (14–18). Strain BR54 was kindly provided by P. F. Sparling (21). Strain WV2 is a spontaneous mutant of BR54 that displays enhanced HA-resistance (22).
As determined by the growth inhibition assay using 0.2× GCB broth with 20 to 0.15 μg of PG-1 per ml. The results are mean values (±SD) from at least four independent determinations.
Antibacterial Peptides.
Fig. 1 shows the primary structures of the antibacterial peptides used in this study. PG-1 and PC-8 were synthesized as described previously (9, 11). Defensins HNP 2 and NP 2 were purified from human and rabbit granulocytes, respectively (24, 25). Tachyplesin-1 (TP-1) was purchased from Bachem (King of Prussia, PA). LL-37 was synthesized on a 0.25-mmol scale with a Perkin–Elmer Applied Biosystems model 431A synthesizer using FastMoc chemistry (26). A prederivatized polyethylene glycol polystyrene resin [Fmoc-L-Ser(tBu)-PEG-PS; PerSeptive Biosystems, Framingham, MA] was used for synthesis and all residues were single coupled. The final product was homogeneous by analytical reverse phase (RP)-HPLC and capillary zone electrophoresis and had the expected mass as determined by electrospray ionization mass-spectrometry. Peptides were purified by RP-HPLC, lyophilized, and dissolved in 0.01% (vol/vol) glacial acetic acid. Dissolved peptides were stored at −20°C and were thawed immediately before use.
Antibacterial Assays.
Mid-logarithmic phase cultures of gonococci were typically diluted 100-fold in normal, 0.2×, or 0.1× strength GCB broth, all of which lacked the glucose and iron supplements described above. Ninety microliters of the diluted cultures was added to sterile 96-well polypropylene microtiter wells that contained 10 μl of peptide solution. Different concentrations of peptide were acheived by 2-fold serial dilutions, using 0.01% (vol/vol) acetic acid, before addition of bacteria. Dilutions of bacteria from a control well that contained 10 μl of 0.01% acteic acid were plated at the beginning of the experiment and after a 45-min incubation period at 37°C under 3.8% (vol/vol) CO2. At the conclusion of the incubation period, 2.5 μl of bacteria from each well was spotted onto GCB agar plates to determine the minimal growth inhibitory concentration (MGIC) of peptides or dilutions were plated to determine the absolute reduction in colony-forming units (cfu). In the latter case, the data were calculated as log10 bactericidal units (log10 cfu/mlt=0 minus log10 cfu/mlt=45 min). All assays were performed in triplicate. The data are reported as average values ± the standard deviation. Statistical significance was determined by Student’s t test.
The cfu reduction assay was modified to test the capacity of the proton conductor carbonyl cynanide-m-chlorophenylhydrazone (CCCP) to enhance bacterial susceptibility to antibacterial peptides; CCCP was purchased from Sigma. Diluted gonococci (strain FA19) in microtiter wells were exposed to CCCP (50 μm) for 20 min at 37°C, treated with glucose (20 mM) or buffer for 10 min, and then exposed to a sublethal concentration of the test peptide. After 30 min of additional incubation at 37°C, dilutions from each sample were plated onto GCB agar.
Accumulation of Iodinated PG-1 by Gonococci.
Four hundred micrograms of PG-1 was iodinated to a specific activity of 9 × 106 dpm/μg using Iodogen-coated tubes (Pierce) and 500 μCi (1 Ci = 37 GBq) of Na [125I] (ICN), as described by the manufacturer. Unincorporated label was removed by extensive dialysis, which used tubing with a 500-Da exclusion limit (Spectrum, Houston), against 0.01% acetic acid at 4°C. (Iodination of PG-1 did not alter its antimicrobial action against the test gonococcal strains.) The accumulation of a sub-MGIC amount (0.25 μg/ml) of [125I] PG-1 with gonococci in the presence or absence of CCCP (50 μM) and/or glucose (20 mM) was assessed as described previously (16).
RESULTS AND DISCUSSION
Genetic Evidence that Antibacterial Peptide Susceptibility in Gonococci Is Linked to the mtrCDE-Encoded Efflux Pump.
Using the microtiter well growth inhibition assay described above, we found (Table 1) that isogenic transformant strains bearing insertionally inactivated mtrC (strain KH12), mtrD (strain KH14), or mtrE (strain RD1) genes were significantly (P = 0.001) more susceptible to PG-1 (MGICs range of 0.2–0.75 μg/ml) than parental strain FA19 (MGIC of 4.37 μg/ml). The extent of enhanced PG-1 susceptibility of these strains was similar to that observed previously for other antimicrobial compounds that are removed from gonococci by the mtr efflux system (14–18).
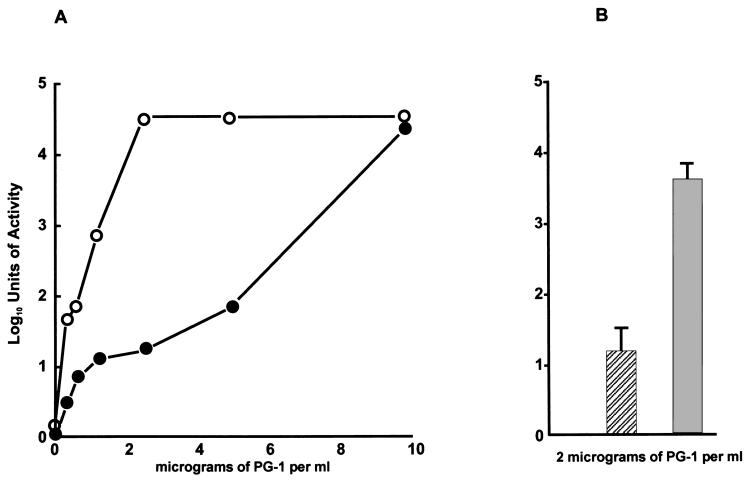
The PG-1 hypersusceptible property of strain KH14 was studied in detail and verified by a colony-reduction plating assay (Fig. 2) that compared its susceptibility to that of parental strain FA19 in the presence of increasing concentrations of PG-1 (Fig. 2A) or a fixed amount (2 μg/ml) of PG-1 (Fig. 2B). Because the kanamycin-resistance cassette within the mtrD gene, which encodes the membrane transporter protein of the mtr efflux pump (15), of strain KH14 contains a strong transcriptional terminator (15) that inhibits expression of the downstream mtrE gene, which encodes the outer membrane protein channel component (MtrE) of the mtr efflux pump (18), we also compared the PG-1 susceptibilities of strain BR54 and its spontaneous HA-resistant revertant WV2 (22). Like strain KH14, BR54 was nearly 10-fold more susceptible to PG-1 than strain FA19 (Table 1). In contrast, strain WV2 displayed increased resistance to PG-1 resembling that of strain FA19; the difference in PG-1 susceptibility between strains BR54 and WV2 was significant (P < 0.001). These results suggest that the 10-bp deletion in the mtrD gene in strain BR54 is responsible for both HA and PG-1 hypersusceptibility, supporting a role for the MtrD transporter protein in determining levels of PG-1 susceptibility in gonococci.
Figure 2.
The susceptibility of isogenic strains FA19 and KH14 to different concentrations of PG-1 (A) or a fixed concentration (B) of PG-1. The results (reported as log10 units of activity) with strain FA19 are shown in the solid circles (A) or hatched bar (B), whereas results with strain KH14 are shown in the open circles (A) or shaded bar (B). (A) The data are from a single experiment. (B) The data are from triplicate experiments.
Loss of the mtrCDE-encoded efflux pump enhanced gonococcal susceptibility to additional antibacterial peptides (Table 2), notably the linearized PG-1 variant termed PC-8, the human cathelicidin peptide LL-37 (5) and the horseshoe crab-derived peptide termed tachyplesin-1 (TP-1) (27); the amino acid sequences for these peptides are shown in Fig. 1. In both high and low salt conditions, gonococci demonstrated substantial resistance to the rabbit defensin NP 2 and the human defensin HNP 2. In contrast, when incubated with the various antibacterial peptides under high salt conditions, strain KH14 was more sensitive than strain FA19 to PG-1, TP-1, and LL-37 (Table 2). Moreover, a 10-fold reduction in salt concentration in the bactericidal assay broth enhanced the susceptibility of both strains to these three peptides, suggesting the importance of ionic interactions between antibacterial peptides and the gonococcal cell surface. The lower salt concentration also enhanced the susceptibility of strain KH14 to PC-8. These results indicated that the mtr efflux pump recognizes structurally diverse peptides such as PG-1, PC-8, LL-37, and TP-1 (Fig. 1).
Table 2.
Susceptibility of strains FA19 and KH14 to antibacterial peptides: MGICs of test strains under high and low salt
| High salt*
|
Low salt†
|
|||
|---|---|---|---|---|
| FA19 | KH14 | FA19 | KH14 | |
| PG-1 | 10 | 1.25 | 2.5 | 0.3 |
| PC-8 | >100 | 100 | 100 | 25 |
| LL-37 | >100 | 3.12 | 6.25 | 0.75 |
| HNP 2 | >200 | >200 | >200 | >200 |
| NP 2 | >200 | >200 | >200 | >200 |
| TP-1 | 5 | 1.25 | 0.62 | 0.62 |
All values are expressed in μg/ml as determined by the growth inhibition assay described in text. All values are from a single assay that are representative of two-three independent determinations. The concentrations in μg/ml of each peptide tested were: PG-1, 20-0.15; PC-8, 100-1.56; LL-37, 100-0.375; NP 2 and HNP 2, 200-6.25; TP-1, 10-0.15.
*High salt = 76.5 mM NaCl.
Low salt = 7.65 mM NaCl in the incubation media.
As an additional test of the above hypothesis, we next examined levels of PG-1 susceptibility among isogenic transformant strains bearing mutations in the mtrR gene (14, 28), which encodes a transcriptional repressor [MtrR (17)] that regulates transcription of the mtrCDE operon (28). Enhanced resistance of gonococci to HAs can be due to mutations in the mtrR gene, either as a result of a single basepair deletion in its promoter (28) or a missense mutation that causes a radical amino acid change in the helix–turn–helix motif of MtrR that abrogates binding of it to the mtrCDE promoter (29). Thus, isogenic transformants of strain FA19 or clinical isolates bearing mutations in their mtrR genes (30) were more resistant to PG-1 than strain FA19 (data not shown), displaying MGICs to PG-1 of 10–20 μg/ml. Thus, enhanced expression of the mtr efflux pump can result in decreased gonococcal susceptibility to PG-1 in a fashion reminiscent of gonococcal susceptibility to HAs (14).
Loss of the Proton Motive Force (PMF) Enhances Gonococcal Susceptibility to Antibacterial Peptides.
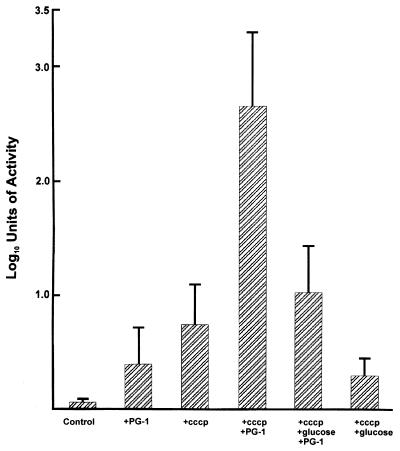
The above results suggested a role for the mtr efflux system in determining levels of gonococcal susceptibility to certain antibiotic-like peptides. Because the action of this pump requires the PMF (16), we asked whether its poisoning by the proton conductor CCCP, which depolarizes the cytoplasmic membrane (13, 31), would enhance gonococcal susceptibility to PG-1. CCCP has been used extensively by others (13, 19, 31) in their studies dealing with the PMF-dependent efflux of antibiotics and other antibacterial substances by bacteria. Typically, addition of CCCP to strains possessing PMF-dependent efflux pumps enhance accumulation of antibacterial compounds that are normally removed by the respective efflux system, by 2- to 4-fold. The loss of efflux activity due to the depolarizing effect of CCCP can be reversed by an appropriate energy source, such as glucose. In this respect, we previously showed (16) that CCCP-mediated loss of efflux of [3H]-Triton X-100 due to the gonococcal mtr system could be reversed by the addition of glucose. Accordingly, we determined the susceptibility of strain FA19 to PG-1 in the presence or absence of CCCP and/or glucose. Using a sub-MGIC amount of PG-1 (1 μg/ml), we found that gonococci pre-exposed to CCCP for 20 min were significantly (P = 0.014) more susceptible to subsequent exposure to PG-1 (Fig. 3) than gonococci not pretreated with CCCP. However, when gonococci were treated with CCCP and then with glucose, susceptiblity to PG-1 was significantly reduced compared with those cultures treated with CCCP, but not glucose, before addition of PG-1 (P = 0.013). In fact, the glucose-reengerized cultures displayed a susceptibility to PG-1 not significantly different (P = 0.15) from those that were exposed only to PG-1 (Fig. 3).
Figure 3.
The susceptibility of strain FA19 to a sublethal amount of PG-1 (1 μg/ml) in the presence or absence of CCCP (50 μM) and/or glucose (20 mM). The conditions used are described in the text and under each bar. In control experiments, it was determined that incubation of strain FA19 with PG-1 (1 μg/ml) and 20 mM of glucose did not increase or decrease the bactericidal capacity of PG-1 (data not shown). All assays were performed in triplicate and the results are from four independent experiments with the data presented as log10 units of bactericidal activity ± standard deviation.
We next examined the capacity of CCCP to enhance gonococcal susceptibility to LL-37, defensins NP 2 and HNP 2, and PC-8. Treatment of strain FA19 with CCCP did not enhance gonococcal susceptibility to 100 μg/ml of either defensin (data not shown). In contrast, CCCP treatment did enhance gonococcal susceptibility to 50 μg/ml of PC-8 by more than one order of magnitude. The results from two independent experiments showed (data not shown) that treatment of gonococci with CCCP before PC-8 addition resulted in 2.05 log10 reduction in viability as opposed to a 0.55 log10 reduction in the absence of CCCP. Addition of glucose to the CCCP-treated culture restored gonococcal resistance to PC-8 (0.95 log10 reduction in viability). Similar experiments with the antigonococcal LL-37 peptide showed that strain FA19 could be rendered more susceptible to LL-37 after treatment with CCCP. Thus, gonococci exposed to CCCP and then a normally sub-MGIC amount of LL-37 (0.5 μg/ml) were significantly (P = 0.002) more susceptible to this human peptide [log10 reduction in viability of 2.14 (±0.0085)] than in the absence of CCCP [log10 reduction = 0.243 (±0.016)]. The effect of CCCP on enhancing gonococcal susceptibility to LL-37 could be reversed by glucose addition [log10 reduction = 1.26 (±0.16)]; the difference between these samples was statistically significant (P = 0.0017).
Accumulation of PG-1 by Gonococci.
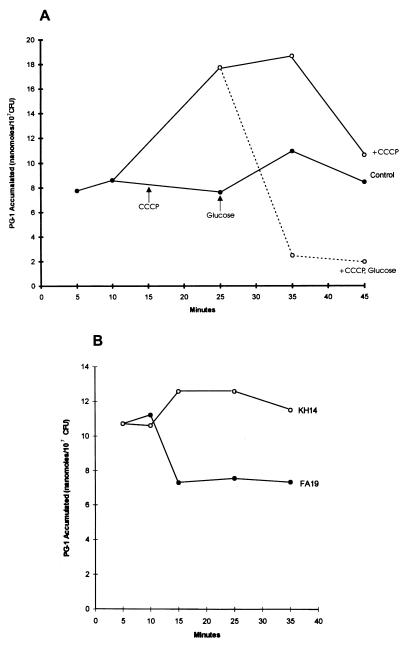
The genetic and physiologic results described above implicated the energy-dependent mtr efflux pump in determining levels of gonococcal susceptibility to structurally diverse antibacterial peptides. Biochemical evidence in support of this hypothesis was obtained by determining the accumulation of a sub-MGIC amount (0.25 μg/ml) of [125I]PG-1 by gonococci. As is shown in Fig. 4A, treatment of strain FA19 with CCCP after 15 min of incubation with PG-1 enhanced gonococcal accumulation of this peptide by 2-fold during a subsequent 20-min incubation period. This enhanced accumulation of PG-1 by strain FA19 could, however, be completely reversed by the addition of glucose 10 min after CCCP addition. Thus, loss of the gonococcal PMF due to the action of CCCP (16, 31) presumably inactivated the efflux action of the MtrC–MtrD–MtrE efflux pump, resulting in enhanced accumulation of PG-1. Once the cytoplasmic membrane was reenergized by glucose (16, 19, 31), the capacity of this efflux pump to export PG-1 was likely restored. It was verified that the MtrC–MtrD–MtrE efflux pump was indeed responsible for PG-1 export by comparing the accumulation of radiolabeled PG-1 (0.25 μg/ml) by isogenic strains FA19 and KH14, which revealed that strain KH14 accumulated at least 50% more PG-1 than parental strain FA19 over a 35-min incubation period (Fig. 4B). In triplicate, independent experiments (data not shown) that measured PG-1 accumulation by strains FA19 and KH14 after a 30-min exposure to 0.25 μg/ml of [125I]PG-1, we found that the difference between these isogenic strains [6.65 (±1.86) vs. 12.62 (±1.15) nmol, respectively] was indeed significant (P = 0.043).
Figure 4.
(A) The accumulation of 0.25 μg/ml of [125I]PG-1 by strain FA19 in the absence of CCCP (•—•), after CCCP addition (○—○) or after glucose addition to the CCCP-treated culture (○- - -○); the time points of these additions are shown. (B) The accumulation of [125I]PG-1 (0.25 μg/ml) by isogenic strains FA19 (•—•) and KH14 (○—○). All values were calculated as nanomoles of PG-1 accumulated per 107 cfu.
The MtrC–MtrD–MtrE efflux pump can recognize a broad range of substrates that includes structurally diverse antibiotics, dyes, and detergents (14–18). Based on genetic and biochemical results obtained in this study, this list now includes certain antibacterial peptides that can assume β-sheet (PG-1) or α-helical (PC-8 and LL-37) structures. While there seems to be little in common among the diverse compounds recognized by this and other RND-type efflux pumps, they typically are hydrophobic compounds and some have a charged domain. Nikaido (19) virtually predicted that RND efflux pumps would modulate bacterial resistance to antibacterial peptides by indicating that these pumps are of importance in the bacterium’s attempt to remove toxic, foreign compounds that interact with the cytoplasmic membrane. Thus, because the protegrin class of antibacterial peptides (and probably the human LL-37 as well) are thought to reach the cytoplasmic membrane, a hypothesis supported by the results presented herein, it is not surprising then that the MtrC–MtrD–MtrE pump can enhance gonococcal resistance to these membrane-active peptides.
Although most of the present work dealt with PG-1, an antibacterial peptide from porcine leukocytes, the results have much broader implications for other antibacterial peptides, including those derived from humans. In this respect, the antigonococcal action of the human LL-37 peptide is noteworthy. LL-37 synthesis can be induced in human keratinocytes (5) and the gene is expressed in granulocytes and testis (32). Unlike PG-1, LL-37 is a cysteine-free peptide that adopts an amphipathic α-helical conformation. Given its tissue and fluid distribution, LL-37 is likely to be present at the genital mucosal surface during gonococcal infection. We propose that the action of the mtr efflux pump could impede the efficacy of LL-37 (and perhaps other urethral antimicrobial peptides), thereby promoting gonococcal survival.
N. gonorrhoeae is a strict human pathogen with a propensity to circumvent several host defensive systems (33). The earlier hypothesis (17, 23, 30) that the mtr efflux system evolved to allow it to resist the antibacterial effects of fatty acids and bile salts that bathe certain mucosal surfaces (e.g., the rectum) should be expanded to encompass endogenous antibacterial peptides, expecially those—like LL-37—that gonococci might encounter on genitourinary mucosae.
Acknowledgments
We thank Jacqueline Balthazar and Nhu-Nguyen Dinh for excellent technical assistance, David Stephens for manuscript review, Lane Pucko for manuscript preparation, and P. F. Sparling for strain BR54. This work was supported by National Institutes of Health Grants AI-21150 (W.M.S.) and AI-22839 and AI-37945 (R.I.L.) and by funds from the Veterans Affairs Medical Research Service to W.M.S. W.M.S. was supported by an Associate Career Scientist Award from the Veterans Affairs Medical Research Service. X.-D.Q. is a Special Fellow of the Cystic Fibrosis Foundation.
ABBREVIATIONS
- CCCP
carbonyl cynanide-m-chlorophenylhydrazone
- HA
hydrophobic agents
- mtr
multiple transferrable resistance
- MGIC
minimal growth inhibitory concentration
- PG-1
protegrin-1
- RND
resistance/nodulation/division
- cfu
colony-forming unit
- PMF
proton motive force
References
- 1.Hancock R E W. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 2.Boman H G. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T, Lehrer R I. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 4.Schonwetter B S, Stoltzenberg E D, Zasloff M A. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 5.Frohm M, Agerberth B, Ahangari G, Ståhle-Backdähl M, Lidén S, Wigzell H, Gudmundsson G H. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 6.Kelley K J. Nat Biotechnol. 1996;14:587–590. doi: 10.1038/nbt0596-587. [DOI] [PubMed] [Google Scholar]
- 7.Kokryakov V N, Harwig S S L, Panyutich E A, Shevchenko A A, Aleshina G M, Shamova O V, Korneva H A, Lehrer R I. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 8.Yasin B, Harwig S S L, Lehrer R I, Wagar E A. Infect Immun. 1996;64:709–713. doi: 10.1128/iai.64.3.709-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu X-D, Harwig S S L, Oren A, Shafer W M, Lehrer R I. Infect Immun. 1996;64:1240–1245. doi: 10.1128/iai.64.4.1240-1245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg D A, Hurst M A, Fujii C A, Kung A H C, Ho J F, Cheng F-C, Loury D J, Fiddes J C. Antimicrob Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu X-D, Harwig S S L, Shafer W M, Lehrer R I. Infect Immun. 1997;65:636–639. doi: 10.1128/iai.65.2.636-639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahrner R L, Dieckmann T, Harwig S S L, Lehrer R I, Eisenberg D, Feigon J. Chem Biol. 1996;3:543–550. doi: 10.1016/s1074-5521(96)90145-3. [DOI] [PubMed] [Google Scholar]
- 13.Nikaido H. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 14.Hagman K E, Pan W, Spratt B G, Judd R C, Shafer W M. Microbiology. 1995;141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 15.Hagman K E, Lucas C E, Balthazar J T, Snyder L, Nilles M, Judd R C, Shafer W M. Microbiology. 1997;143:2117–2125. doi: 10.1099/00221287-143-7-2117. [DOI] [PubMed] [Google Scholar]
- 16.Lucas C E, Hagman K E, Levin J C, Stein D C, Shafer W M. Mol Microbiol. 1995;16:1001–1009. doi: 10.1111/j.1365-2958.1995.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 17.Pan W, Spratt B G. Mol Microbiol. 1994;11:769–775. doi: 10.1111/j.1365-2958.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 18.Delahay R, Robertson B D, Balthazar J T, Shafer W M, Ison C A. Microbiology. 1997;143:2127–2133. doi: 10.1099/00221287-143-7-2127. [DOI] [PubMed] [Google Scholar]
- 19.Nikaido H. J Bacteriol. 1996;178:382–388. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saier M H, Jr, Tam R, Reizer A, Reizer J. Mol Microbiol. 1994;11:841–847. doi: 10.1111/j.1365-2958.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 21.Sarubbi F A, Sparling P F, Blackman E, Lewis E. J Bacteriol. 1975;124:750–756. doi: 10.1128/jb.124.2.750-756.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veal, W. L., Yellen, A., Balthazar, J. T., Pan, W., Spratt, B. G. & Shafer, W. M. (1998) Microbiology, in press. [DOI] [PubMed]
- 23.Shafer W M, Guymon L F, Lind I, Sparling P F. Antimicrob Agents Chemother. 1984;25:767–769. doi: 10.1128/aac.25.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selsted M E, Szkllarek D, Lehrer R I. Infect Immun. 1984;25:150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harwig S S L, Ganz T, Lehrer R I. Methods Enzymol. 1994;236:163–172. doi: 10.1016/0076-6879(94)36015-4. [DOI] [PubMed] [Google Scholar]
- 26.Fields C G, Lloyd D H, Macdonald R C, Ottenson K M, Noble R L. Peptide Res. 1991;4:95–101. [PubMed] [Google Scholar]
- 27.Nakamura T, Furunaka H, Miyata T, Tokunuaga F, Muta T, Iwanaga S, Niwa M, Takao T, Shimonishi Y. J Biol Chem. 1988;263:16709–16713. [PubMed] [Google Scholar]
- 28.Hagman K E, Shafer W M. J Bacteriol. 1995;177:4162–4165. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas C E, Balthazar J T, Hagman K E, Shafer W M. J Bacteriol. 1997;179:4123–4128. doi: 10.1128/jb.179.13.4123-4128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafer W M, Balthazar J T, Hagman K E, Morse S A. Microbiology. 1995;141:907–911. doi: 10.1099/13500872-141-4-907. [DOI] [PubMed] [Google Scholar]
- 31.McMurray L M, George A M, Levy S B. Antimicrob Agents Chemother. 1994;38:542–546. doi: 10.1128/aac.38.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H G, Gudmundsson G H. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparling P F, Tsai J, Cornelissen C N. Scand J Infect Dis Suppl. 1990;69:125–136. [PubMed] [Google Scholar]