Abstract
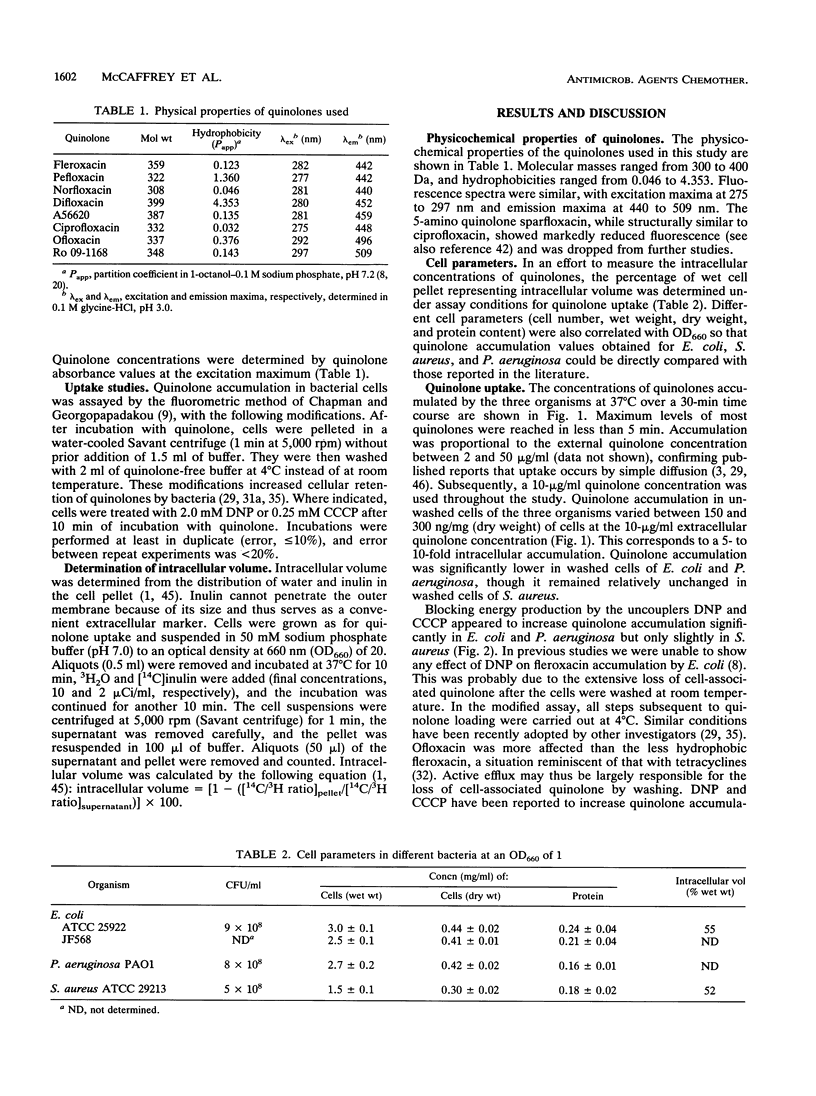
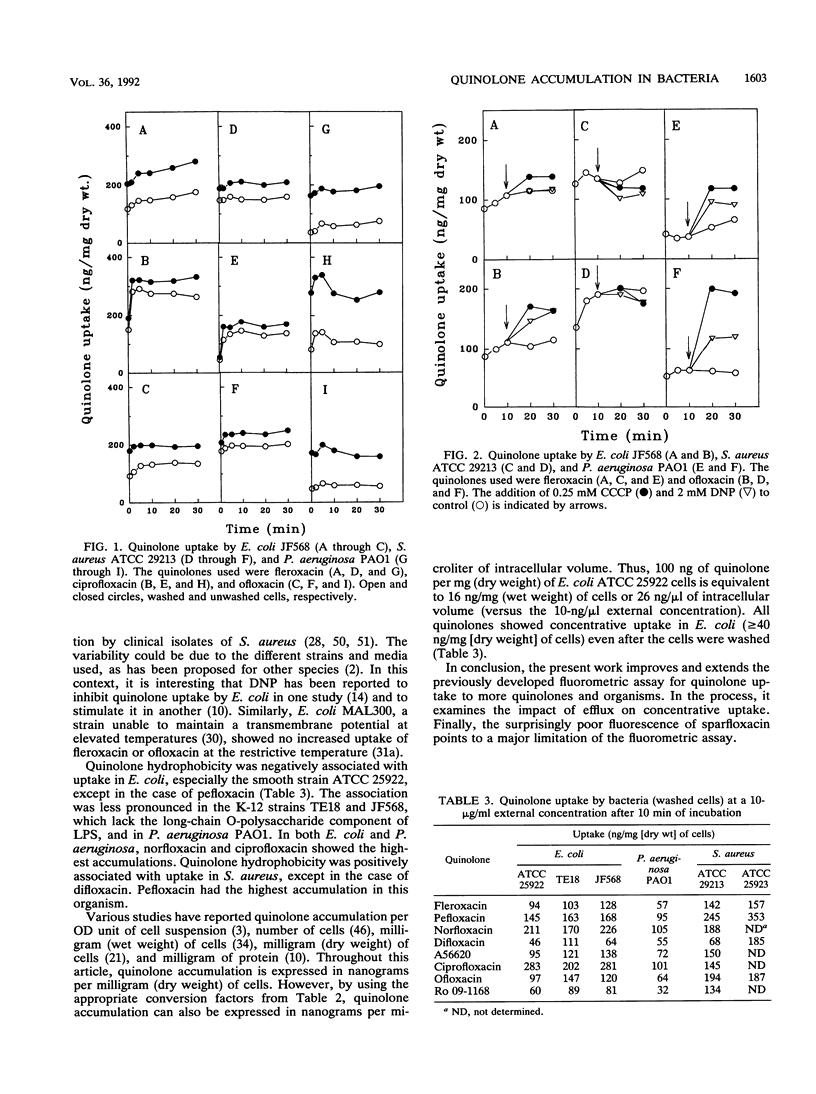
The accumulation of quinolones by Escherichia coli JF568, Pseudomonas aeruginosa PAO1, and Staphylococcus aureus ATCC 29213 was measured by a modified fluorometric assay (J. S. Chapman and N. H. Georgopapadakou, Antimicrob. Agents Chemother. 33:27-29, 1989). The quinolones examined were fleroxacin, pefloxacin, norfloxacin, difloxacin, A56620, ciprofloxacin, ofloxacin, and Ro 09-1168. In all three organisms, uptake was complete in less than 5 min and was proportional to extracellular quinolone concentrations between 2 and 50 micrograms/ml, which is consistent with simple diffusion. Washing cells with quinolone-free buffer decreased accumulation by up to 70% in E. coli and P. aeruginosa but not in S. aureus. Similarly, incubation with the uncouplers 2,4-dinitrophenol and carbonyl cyanide m-chlorophenylhydrazone increased accumulation up to fourfold in E. coli and P. aeruginosa, though not in S. aureus, suggesting endogenous, energy-dependent efflux. High quinolone hydrophobicity was generally associated with decreased accumulation in E. coli and P. aeruginosa (except in the case of pefloxacin) but was associated with increased accumulation in S. aureus (except in the case of difloxacin). Ciprofloxacin had the highest accumulation in E. coli and P. aeruginosa, while pefloxacin had the highest accumulation in S. aureus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Rottenberg H., Caplan S. R. An estimation of the light-induced electrochemical potential difference of protons across the membrane of Halobacterium halobium. Biochim Biophys Acta. 1976 Sep 13;440(3):557–572. doi: 10.1016/0005-2728(76)90042-6. [DOI] [PubMed] [Google Scholar]
- Bedard J., Chamberland S., Wong S., Schollaardt T., Bryan L. E. Contribution of permeability and sensitivity to inhibition of DNA synthesis in determining susceptibilities of Escherichia coli, Pseudomonas aeruginosa, and Alcaligenes faecalis to ciprofloxacin. Antimicrob Agents Chemother. 1989 Sep;33(9):1457–1464. doi: 10.1128/aac.33.9.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Bedard J. Impermeability to quinolones in gram-positive and gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1991 Apr;10(4):232–239. doi: 10.1007/BF01966995. [DOI] [PubMed] [Google Scholar]
- Celesk R. A., Robillard N. J. Factors influencing the accumulation of ciprofloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Nov;33(11):1921–1926. doi: 10.1128/aac.33.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Bertasso A., Georgopapadakou N. H. Fleroxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1989 Feb;33(2):239–241. doi: 10.1128/aac.33.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Fluorometric assay for fleroxacin uptake by bacterial cells. Antimicrob Agents Chemother. 1989 Jan;33(1):27–29. doi: 10.1128/aac.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Hooper D. C., Wolfson J. S., Levy S. B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989 Aug;33(8):1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. Alterations in outer membrane proteins of Pseudomonas aeruginosa associated with selective resistance to quinolones. Antimicrob Agents Chemother. 1988 May;32(5):785–787. doi: 10.1128/aac.32.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechène M., Leying H., Cullmann W. Role of the outer membrane for quinolone resistance in enterobacteria. Chemotherapy. 1990;36(1):13–23. doi: 10.1159/000238743. [DOI] [PubMed] [Google Scholar]
- Diver J. M., Piddock L. J., Wise R. The accumulation of five quinolone antibacterial agents by Escherichia coli. J Antimicrob Chemother. 1990 Mar;25(3):319–333. doi: 10.1093/jac/25.3.319. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H., Hosaka M., Hirai K., Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990 Sep;34(9):1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Menzel R., Mizuuchi K., O'Dea M. H., Friedman D. I. Regulation of DNA supercoiling in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):763–767. doi: 10.1101/sqb.1983.047.01.087. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Dix B. A., Angehrn P., Wick A., Olson G. L. Monocyclic and tricyclic analogs of quinolones: mechanism of action. Antimicrob Agents Chemother. 1987 Apr;31(4):614–616. doi: 10.1128/aac.31.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991 Feb 7;324(6):384–394. doi: 10.1056/NEJM199102073240606. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Mode of action of the new quinolones: new data. Eur J Clin Microbiol Infect Dis. 1991 Apr;10(4):223–231. doi: 10.1007/BF01966994. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Ruble C. A. Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J Infect Dis. 1991 May;163(5):1080–1086. doi: 10.1093/infdis/163.5.1080. [DOI] [PubMed] [Google Scholar]
- Lei Y., Sato K., Nakae T. Ofloxacin-resistant Pseudomonas aeruginosa mutants with elevated drug extrusion across the inner membrane. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1043–1048. doi: 10.1016/0006-291x(91)90997-l. [DOI] [PubMed] [Google Scholar]
- Lieberman M. A., Simon M., Hong J. S. Characterization of Escherichia coli mutant incapable of maintaining a transmembrane potential. MetC ecfts mutations. J Biol Chem. 1977 Jun 25;252(12):4056–4067. [PubMed] [Google Scholar]
- Masecar B. L., Celesk R. A., Robillard N. J. Analysis of acquired ciprofloxacin resistance in a clinical strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Feb;34(2):281–286. doi: 10.1128/aac.34.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Aronson D. A., Levy S. B. Susceptible Escherichia coli cells can actively excrete tetracyclines. Antimicrob Agents Chemother. 1983 Oct;24(4):544–551. doi: 10.1128/aac.24.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Furet Y. X., Pechère J. C. Role of protein D2 and lipopolysaccharide in diffusion of quinolones through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Oct;35(10):2091–2097. doi: 10.1128/aac.35.10.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniot-Ville N., Guibert J., Moreau N., Acar J. F., Collatz E., Gutmann L. Mechanisms of quinolone resistance in a clinical isolate of Escherichia coli highly resistant to fluoroquinolones but susceptible to nalidixic acid. Antimicrob Agents Chemother. 1991 Mar;35(3):519–523. doi: 10.1128/aac.35.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer P. G., Piddock L. J. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother. 1991 Nov;28(5):639–653. doi: 10.1093/jac/28.5.639. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Clinical utility of DNA gyrase inhibitors. Pharmacol Ther. 1989;41(1-2):207–221. doi: 10.1016/0163-7258(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J. Mechanism of quinolone uptake into bacterial cells. J Antimicrob Chemother. 1991 Apr;27(4):399–403. doi: 10.1093/jac/27.4.399. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Zhu M. Mechanism of action of sparfloxacin against and mechanism of resistance in gram-negative and gram-positive bacteria. Antimicrob Agents Chemother. 1991 Nov;35(11):2423–2427. doi: 10.1128/aac.35.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siporin C. The evolution of fluorinated quinolones: pharmacology, microbiological activity, clinical uses, and toxicities. Annu Rev Microbiol. 1989;43:601–627. doi: 10.1146/annurev.mi.43.100189.003125. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Valisena S., Palumbo M., Parolin C., Palú G., Meloni G. A. Relevance of ionic effects on norfloxacin uptake by Escherichia coli. Biochem Pharmacol. 1990 Aug 1;40(3):431–436. doi: 10.1016/0006-2952(90)90540-2. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano Y., Nishikawa T., Komatsu Y. Outer membrane proteins responsible for the penetration of beta-lactams and quinolones in Pseudomonas aeruginosa. J Antimicrob Chemother. 1990 Aug;26(2):175–184. doi: 10.1093/jac/26.2.175. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura S., Ubukata K., Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990 Dec;172(12):6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kojima T., Inoue M., Mitsuhashi S. Uptake of sparfloxacin and norfloxacin by clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Feb;35(2):368–370. doi: 10.1128/aac.35.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]



