Abstract
Background
Although obesity is known to increase the risk of hypertension, few studies have prospectively evaluated body mass index (BMI) across the range of normal weight and overweight as a primary risk factor.
Methods
In this prospective cohort, we evaluated the association between BMI and risk of incident hypertension. We studied 13,563 initially healthy, non-hypertensive men who participated in the Physicians’ Health Study. We calculated BMI from self-reported weight and height and defined hypertension as self-reported systolic blood pressure (BP) ≥140 mmHg, diastolic BP ≥90 mmHg, or new antihypertensive medication use.
Results
After a median 14.5 years, 4920 participants developed hypertension. Higher baseline BMI, even within the “normal” range, was consistently associated with increased risk of hypertension. Compared to participants in the lowest BMI quintile (<22.4 kg/m2), the relative risks (95% confidence interval) of developing hypertension for men with a BMI of 22.4–23.6, 23.7–24.7, 24.8–26.4, and >26.4 kg/m2 were 1.20 (1.09–1.32), 1.31 (1.19–1.44), 1.56 (1.42–1.72), and 1.85 (1.69–2.03), respectively (P for trend, <0.0001). Further adjustment for diabetes, high cholesterol, and baseline BP did not substantially alter these results. We found similar associations using other BMI categories and after excluding men with smoking history, those who developed hypertension in the first 2 years, and those with diabetes, obesity, or high cholesterol at baseline.
Conclusion
In this large cohort, we found a strong gradient between higher BMI and increased risk of hypertension, even among men within the “normal” and mildly “overweight” BMI range. Approaches to reduce the risk of developing hypertension may include prevention of overweight and obesity.
Keywords: hypertension, obesity, body mass index
Introduction
Hypertension represents one of the most prevalent chronic conditions in the US, with normotensive middle-aged adults estimated to have a 90% residual lifetime risk of developing hypertension.1 Lifestyle interventions recommended in national guidelines for the prevention and management of hypertension include weight reduction, physical activity, and dietary modification.2 Although clinical studies indicate that weight loss among the overweight and obese can reduce the risk of hypertension,3-6 few epidemiologic studies have evaluated the risk associated with excess body weight across the range of normal weight and overweight.
Cross-sectional studies have identified direct associations between BMI and hypertension, across various levels of BMI.7 Early prospective data from Framingham suggested that adiposity and 8-year changes in body fat may be associated with increased risk of hypertension.8 Small prospective studies have also reported links between abdominal accumulation of body fat with the risk of hypertension.9 In addition, body weight in childhood10 and adolescence11 has been associated with the long-term risk of hypertension. Other follow-up studies have demonstrated associations between body mass index (BMI) and changes in blood pressure (BP) over 8 years.12 In a Finnish cohort, being overweight or obese was associated with an increased risk of initiating antihypertensive drug treatment.13 A large cohort study of US women further demonstrated that higher BMI was directly associated with an increased risk of developing hypertension, and long-term weight changes were inversely correlated with risk of hypertension.14
We therefore aimed to clarify the association between excess body weight and incident hypertension in a large prospective cohort of over 13,500 initially healthy US male physicians with 14.5 years of follow-up.
Methods
Study Population
Study subjects were 22,071 participants in the Physicians’ Health Study (PHS), a completed randomized trial of aspirin and beta-carotene in the primary prevention of cardiovascular disease and cancer.15, 16 Subjects had no baseline history of cardiovascular disease, cancer (except nonmelanoma skin cancer), current liver disease or decreased kidney function (defined as renal failure or insufficiency), or other major illnesses.
The baseline population was restricted to 13,563 men without hypertension, defined as having self-reported systolic BP of less than 140 mmHg, diastolic BP of less than 90 mmHg, and no self-reported past or current history of antihypertensive medication use at study entry. Individuals with missing information for BP, antihypertensive medication use, or BMI at baseline were also excluded from analyses.
Assessment of Body Mass Index and Other Covariates
Baseline and follow-up data were self-reported and collected through mailed questionnaires starting in 1982 and continuing every 6 months for the first year and annually thereafter. Information was collected on a wide variety of participant demographics, personal characteristics, medical history, and health behaviors.
BMI was calculated from self-reported weight (converted from pounds to kilograms) divided by height (converted from inches to meters) squared. Covariates at baseline included age (continuous), cigarette smoking (never, past, current), alcohol consumption (rarely/never, 1 drink/month to 1 drink/week, 2 to 6 drinks/week, and ≥1 drink/day), exercise (none, 1 time/month to 1 time/week, ≥2 times/week), parental history of myocardial infarction before the age of 60 years (no, yes), history of diabetes (no, yes; defined as any history of diabetes), and history of elevated cholesterol (no, yes; defined as level ≥6.21 mmol/L [240 mg/dL] or history of past or current use of cholesterol-lowering medication).
Ascertainment of Hypertension
Men were defined as incident cases of hypertension by meeting one of the following criteria during follow-up: (i) self-reported systolic BP of at least 140 mmHg or diastolic BP of at least 90 mmHg on follow-up questionnaires at years 2 or 7; or (ii) self-reported new antihypertensive medication use on follow-up questionnaires at years 2, 7, and annually thereafter. A single measurement of self-reported BP in health professionals is highly correlated with measured systolic BP (r=0.72) and diastolic BP (r=0.60).17
Men with a missing date for the start of antihypertensive medication use were assigned a date of incident hypertension by randomly selecting a date between the current and previous annual questionnaires where antihypertensive status was assessed. Individuals developing major concomitant diseases, the management of which may influence BP at or after baseline but before the development of hypertension, including myocardial infarction and stroke, were censored at that date of diagnosis.
Data Analyses
We compared baseline participant characteristics according to BMI categories by chi-square tests for categorical variables and analysis of variance for continuous variables. We used Cox proportional hazards models to compute the relative risks (RRs) and 95% confidence intervals (CIs) of incident hypertension for various classifications of BMI, with the lowest level as the referent.
We considered two multivariable-adjusted models. The first included the potential confounders of the association between baseline BMI and risk of hypertension: age at baseline, cigarette smoking, alcohol consumption, exercise, and parental history of myocardial infarction before the age of 60 years. The second multivariable model additionally adjusted for possible mediators of the association between BMI and risk of hypertension: history of diabetes and elevated cholesterol. An indicator variable accounted for the 1521 individuals (11.2%) with missing information on elevated cholesterol. In addition to quintiles of BMI, we considered BMI as a continuous variable in models, reflecting the RR of hypertension for 1 kg/m2 increases in BMI. We also examined deciles of BMI to illustrate better the association between body weight and the risk of developing hypertension. We further evaluated the risk of developing hypertension associated with changes in BMI between baseline and 8-year follow-up for men with available BMI at both time points. For these analyses, we excluded men who developed hypertension prior to 8 years.
Several important subanalyses were performed to clarify the association between BMI and the risk of developing hypertension. We limited analyses to men who reported never having smoked cigarettes at baseline (n=7016), due to potential unmeasured confounding among individuals reporting a history of past or current smoking at baseline. We further excluded men who were censored or who developed hypertension during the first 2 years of follow-up (n=1397), since death or the development of hypertension early after study enrollment may be related to unmeasured, preexisting disease and illness-related weight loss not reflected in baseline BMI.
We also examined whether the association between BMI and the risk of developing hypertension was modified by baseline BP or smoking status. We considered both stratified and joint models, incorporating baseline BP as dichotomous variables (<120 vs. 120–139 mmHg for systolic BP; <80 vs. 80–89 mmHg for diastolic BP) and cigarette smoking in 3 categories (never, past, or current smoking at baseline). To assess for effect modification, we used the likelihood ratio test (LRT) contrasting age-adjusted models with and without interaction terms for baseline BMI in quintiles and blood pressure (dichotomous) or smoking status (never, past, or current at baseline). Finally, we excluded participants who were obese (BMI ≥30.0 kg/m2), those who reported a history of diabetes or high cholesterol, and those missing information on self-reported cholesterol levels at baseline. All data analyses were performed using SAS Software Version 8.2 (SAS Institute, Cary, NC).
Results
The baseline characteristics of the 13,563 normotensive participants are shown according to BMI quintiles in Table 1. Men with higher BMI reported higher systolic and diastolic BP at baseline. As expected, men with higher BMI were more likely to have diabetes and high cholesterol. They were also more likely to smoke, consumed less alcohol, and were less physically active.
Table 1.
Baseline Characteristics of 13,563 Participants According to Baseline Body Mass Index (BMI, in kg/m2)
| Characteristics | Baseline BMI quintile (kg/m2) | P* | ||||
|---|---|---|---|---|---|---|
| <22.4 | 22.4–23.6 | 23.7–24.7 | 24.8–26.4 | >26.4 | ||
| Age at baseline (y) | 51.1 ± 9.4 | 51.8 ± 9.2 | 52.1 ± 8.9 | 52.1 ± 8.6 | 52.1 ± 8.4 | <0.001 |
| Systolic BP 120–139, mmHg (%) | 62.9 | 69.3 | 72.1 | 75.4 | 80.1 | <0.001 |
| Diastolic BP 80–89, mmHg (%) | 45.0 | 47.7 | 52.7 | 56.3 | 61.8 | <0.001 |
| Diabetes mellitus (%)† | 1.7 | 1.5 | 1.3 | 1.4 | 2.4 | 0.01 |
| Cigarette smoking (%) | ||||||
| Never | 56.4 | 53.1 | 51.7 | 50.7 | 47.2 | <0.001 |
| Past | 34.0 | 36.7 | 38.7 | 38.1 | 40.3 | |
| Current | 9.6 | 10.2 | 9.6 | 11.2 | 12.5 | |
| Alcohol intake ≥1 drink/day (%) | 24.6 | 23.3 | 22.4 | 22.8 | 16.5 | <0.001 |
| Physical activity ≥2 times/wk | 62.0 | 60.3 | 57.8 | 54.3 | 47.1 | <0.001 |
| High cholesterol or treatment† | 7.8 | 9.8 | 10.3 | 11.2 | 11.0 | <0.001 |
| Parental history of myocardial infarction at <60 years of age | 9.7 | 8.9 | 9.1 | 10.0 | 9.7 | 0.63 |
P-values from chi-square tests for categorical variables and analysis of variance for continuous variables.
Diabetes defined as any self-reported history of diabetes. High cholesterol or treatment defined as self-reported level ≥6.21 mmol/L (240 mg/dL) or past or current cholesterol-lowering medication use.
Among 13,563 men, 4,920 cases of incident hypertension developed during a median follow-up of 14.5 years (maximum follow-up, 20.5 years). In age- and multivariable-adjusted analyses, higher baseline BMI was significantly associated with increased risk of incident hypertension (Table 2). The age-adjusted estimated risk of hypertension generally increased across BMI quintiles relative to the referent group (BMI <22.4 kg/m2), even within the range of “normal” BMI. Higher BMI remained associated with risk of hypertension after adjustment for the potential confounders (age, cigarette smoking status, alcohol consumption, exercise, and parental history of premature myocardial infarction). The multivariable-adjusted RR of hypertension for men in the highest BMI quintile (>26.4 kg/m2) was 1.85 (95% CI, 1.69–2.03; P for trend, <0.001), as compared to those in the lowest BMI quintile (<22.4 kg/m2).
Table 2.
Relative Risks of Developing Hypertension, According to Baseline Body Mass Index
| Baseline BMI quintile (kg/m2) | P for trend | |||||
|---|---|---|---|---|---|---|
| <22.4 | 22.4–23.6 | 23.7–24.7 | 24.8–26.4 | >26.4 | ||
| No. of men | 2727 | 2752 | 2666 | 2690 | 2728 | |
| Mean weight – lb (kg) | 150 (68) | 162 (73) | 172 (77) | 180 (81) | 199 (90) | |
| All men (n=13,563) | ||||||
| No. of hypertension cases | 756 | 909 | 945 | 1091 | 1219 | |
| Rate (No. per 1000 person-yrs) | 22.5 | 27.7 | 30.3 | 36.0 | 42.2 | |
| Age-Adjusted RR (95% CI) | 1.00 | 1.19 (1.08–1.32) | 1.30 (1.18–1.43) | 1.56 (1.42–1.71) | 1.83 (1.67–2.00) | <0.001 |
| Model 1* RR (95% CI) | 1.00 | 1.20 (1.09–1.32) | 1.31 (1.19–1.44) | 1.56 (1.42–1.72) | 1.85 (1.69–2.03) | <0.001 |
| Model 2† RR (95% CI) | 1.00 | 1.20 (1.09–1.32) | 1.31 (1.19–1.44) | 1.56 (1.42–1.72) | 1.84 (1.68–2.02) | <0.001 |
| Men who never smoked (n=7016) | ||||||
| No. of hypertension cases | 408 | 451 | 457 | 532 | 545 | |
| Rate (No. per 1000 person-yrs) | 21.1 | 25.2 | 27.5 | 34.3 | 38.6 | |
| Age-Adjusted RR (95% CI) | 1.00 | 1.17 (1.02–1.34) | 1.24 (1.09–1.42) | 1.61 (1.42–1.83) | 1.75 (1.54–1.99) | <0.001 |
| Model 1* RR (95% CI) | 1.00 | 1.17 (1.02–1.34) | 1.25 (1.09–1.43) | 1.63 (1.43–1.85) | 1.77 (1.55–2.01) | <0.001 |
| Model 2† RR (95% CI) | 1.00 | 1.16 (1.01–1.33) | 1.24 (1.08–1.42) | 1.62 (1.42–1.85) | 1.75 (1.53–1.99) | <0.001 |
| Men who never smoked with ≥ 2 yrs of follow-up (n=6376) | ||||||
| No. of hypertension cases | 333 | 359 | 365 | 414 | 405 | |
| Rate (No. per 1000 person-yrs) | 17.3 | 20.2 | 22.1 | 26.9 | 29.0 | |
| Age-Adjusted RR (95% CI) | 1.00 | 1.15 (0.99–1.33) | 1.22 (1.05–1.42) | 1.57 (1.36–1.82) | 1.64 (1.42–1.90) | <0.001 |
| Model 1* RR (95% CI) | 1.00 | 1.15 (0.99–1.34) | 1.21 (1.04–1.41) | 1.58 (1.37–1.84) | 1.66 (1.43–1.92) | <0.001 |
| Model 2† RR (95% CI) | 1.00 | 1.14 (0.98–1.33) | 1.21 (1.04–1.41) | 1.58 (1.37–1.84) | 1.65 (1.42–1.91) | <0.001 |
Model 1 – adjusted for baseline age, cigarette smoking, alcohol consumption, exercise, and parental history of myocardial infarction before the age of 60 years.
Model 2 – adjusted for all variables in Model 1 plus history of diabetes and elevated cholesterol.
Further adjustment for the potential mediators (diabetes and high cholesterol) did not materially alter the results (Model 2, Table 2). Additionally adjusting for baseline systolic and diastolic BP only partly attenuated the effect estimates of the association between BMI and risk of hypertension. Compared to men in the lowest BMI quintile, the RR of hypertension for men in the highest quintile was 1.47 (95% CI, 1.34–1.62; P for trend, <0.001).
Examining baseline BMI as a continuous term, each 1-unit increase in BMI was associated in multivariable models with an 8% (95% CI, 7–9%) increase in the risk of incident hypertension (Model 2). We found similar associations evaluating BMI according to WHO categories and in deciles. The multivariable-adjusted RRs of hypertension were 1.42 (95% CI, 1.33–1.50) for overweight men and 1.95 (95% CI, 1.68–2.25) for obese men (P for trend, <0.001), as compared to men of normal BMIs. Men in the highest decile of baseline BMI (>27.8 kg/m2) had a multivariable-adjusted RR of hypertension of 2.33 (95% CI, 2.04–2.66; P for trend, <0.001), as compared to men with BMI <21.6 kg/m2 (Figure 1).
Figure 1. Multivariable-adjusted relative risk of developing hypertension, according to baseline body mass index decile (BMI kg/m2).
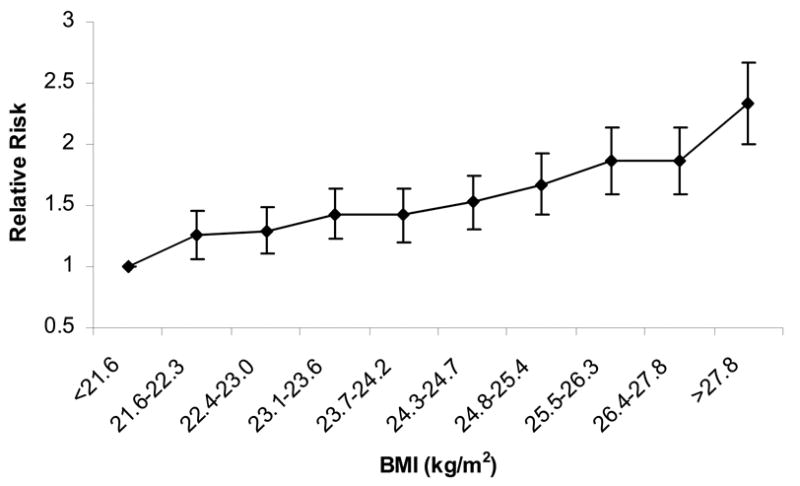
Adjusted for baseline age, cigarette smoking, alcohol consumption, exercise, parental history of myocardial infarction before the age of 60 years, history of diabetes, and elevated cholesterol. Referent group (RR=1.0): BMI <21.6 kg/m2 at baseline. P for trend <0.001.
We found similar associations between baseline BMI and risk of hypertension when limiting analyses to the 7,016 men without a history of cigarette smoking at baseline. Further excluding men who developed hypertension or who were censored during the first 2 years of follow-up only slightly attenuated the results (Table 2).
In additional analyses, we found similar associations between baseline BMI and risk of hypertension after excluding those who were obese, had high cholesterol or missing cholesterol information, or who reported diabetes at baseline (n=3,361). Adjusting for potential confounders, men in the highest BMI quintile (>26.4 kg/m2) had a RR of incident hypertension of 1.75 (95% CI, 1.57–1.95; P for trend, <0.001), as compared to men in the lowest quintile (<22.4 kg/m2).
Regardless of smoking status, the risk of developing hypertension significantly increased across BMI quintiles relative to the lowest quintile in both age- and multivariable-adjusted models (Table 3; P for trend, <0.001). Although the magnitude of the association between BMI and risk of hypertension was stronger for past smokers, we did not detect effect modification by baseline smoking status (LRT, P interaction = 0.60).
Table 3.
Relative Risks of Developing Hypertension, According to Baseline Body Mass Index and Cigarette Smoking Status
| Baseline BMI quintile (kg/m2) | P for trend | |||||
|---|---|---|---|---|---|---|
| <22.4 | 22.4–23.6 | 23.7–24.7 | 24.8–26.4 | >26.4 | ||
| Current smokers at baseline (n=1440) | ||||||
| No. (%) of men | 261 (18.1) | 281 (19.5) | 257 (17.8) | 301 (20.9) | 340 (23.6) | |
| No. of hypertension cases | 78 | 96 | 101 | 122 | 146 | |
| Rate (No. per 1000 person-yrs) | 26.8 | 30.5 | 36.1 | 36.0 | 42.6 | |
| Age-Adjusted RR (95% CI) | 1.00 | 1.07 (0.80–1.45) | 1.33 (0.99–1.79) | 1.35 (1.01–1.79) | 1.67 (1.27–2.20) | <0.001 |
| Model 1* RR (95% CI) | 1.00 | 1.09 (0.81–1.48) | 1.35 (1.00–1.83) | 1.37 (1.03–1.83) | 1.69 (1.27–2.24) | <0.001 |
| Model 2† RR (95% CI) | 1.00 | 1.08 (0.80–1.47) | 1.33 (0.98–1.80) | 1.35 (1.01–1.80) | 1.68 (1.27–2.23) | <0.001 |
| Never smokers at baseline (n=7016) | ||||||
| No. (%) of men | 1535 (21.9) | 1458 (20.8) | 1376 (19.6) | 1362 (19.4) | 1285 (18.3) | |
| No. of hypertension cases | 408 | 451 | 457 | 532 | 545 | |
| Rate (No. per 1000 person-yrs) | 21.1 | 25.2 | 27.5 | 34.3 | 38.6 | |
| Age-Adjusted RR (95% CI) | 1.00 | 1.17 (1.02–1.34) | 1.24 (1.09–1.42) | 1.61 (1.42–1.83) | 1.75 (1.54–1.99) | <0.001 |
| Model 1* RR (95% CI) | 1.00 | 1.17 (1.02–1.34) | 1.25 (1.09–1.43) | 1.63 (1.43–1.85) | 1.77 (1.55–2.01) | <0.001 |
| Model 2† RR (95% CI) | 1.00 | 1.16 (1.01–1.33) | 1.24 (1.08–1.42) | 1.62 (1.42–1.85) | 1.75 (1.53–1.99) | <0.001 |
| Past smokers at baseline (n=5083) | ||||||
| No.(%) of men | 925 (18.2) | 1007 (19.8) | 1030 (20.3) | 1023 (20.1) | 1098 (21.6) | |
| No. of hypertension cases | 267 | 360 | 385 | 434 | 525 | |
| Rate (No. per 1000 person-yrs) | 23.8 | 30.9 | 32.7 | 38.1 | 46.6 | |
| Age-Adjusted RR (95% CI) | 1.00 | 1.27 (1.08–1.48) | 1.35 (1.16–1.58) | 1.54 (1.32–1.80) | 1.95 (1.68–2.26) | <0.001 |
| Model 1* RR (95% CI) | 1.00 | 1.27 (1.08–1.49) | 1.36 (1.16–1.60) | 1.56 (1.33–1.82) | 2.00 (1.72–2.33) | <0.001 |
| Model 2† RR (95% CI) | 1.00 | 1.28 (1.09–1.50) | 1.38 (1.17–1.61) | 1.57 (1.34–1.83) | 2.01 (1.73–2.34) | <0.001 |
Model 1 – adjusted for baseline age, cigarette smoking, alcohol consumption, exercise, and parental history of myocardial infarction before the age of 60 years.
Model 2 – adjusted for all variables in Model 1 plus history of diabetes and elevated cholesterol.
We also performed analyses on BMI and hypertension stratified by either baseline systolic or diastolic BP. Again, for each baseline BP category, the risk of developing hypertension significantly increased across BMI quintiles in both age- and multivariable-adjusted models (P for trend, <0.001). For systolic BP, the multivariable-adjusted RR (95% CI) comparing the highest and lowest BMI quintiles was 1.82 (1.45–2.28) among those with systolic BP <120 mmHg, versus 1.64 (1.48–1.82) among those with systolic BP 120–139 mmHg (LRT, P interaction = 0.19). By contrast, the association between BMI and risk of hypertension was stronger for men with diastolic BP <80 mmHg (RR [95% CI], 1.84 [1.56–2.16]), as compared to men with diastolic BP 80–89 mmHg at baseline (RR [95% CI], 1.58 [1.40–1.77]); LRT, P interaction = 0.02.
The risk of developing hypertension was similar between men <60 years of age at baseline and men >60 years in models considering the joint effects of age and BMI (Figure 2). In models including joint effects of baseline BMI quintiles and BP, the RR of developing hypertension was consistently highest among men with higher BP at baseline, across all BMI quintiles (Figure 2).
Figure 2. Multivariable-adjusted relative risk for developing hypertension, according to BMI quintile (kg/m2), age, and blood pressure at baseline.
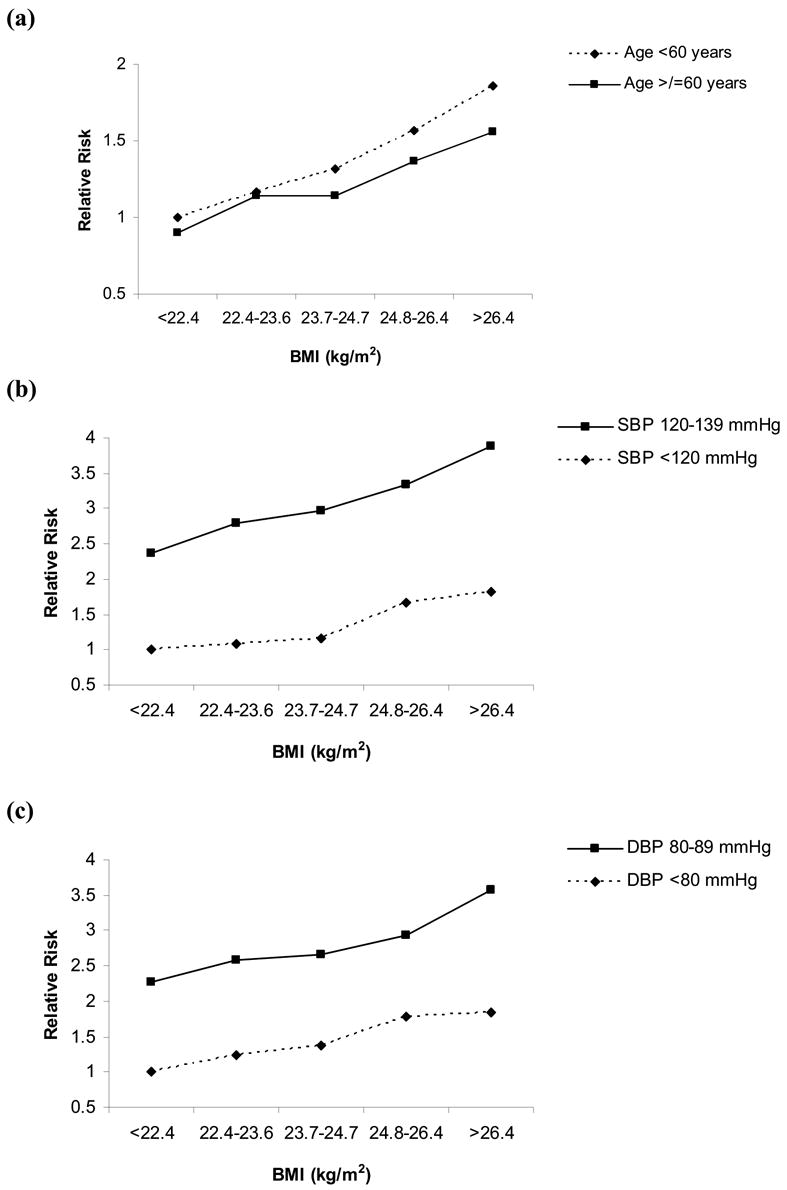
Adjusted for baseline age, cigarette smoking, alcohol consumption, exercise, parental history of myocardial infarction before the age of 60 years, history of diabetes, and elevated cholesterol. SBP = systolic blood pressure at baseline. DBP = diastolic blood pressure at baseline. (a) Referent group (RR=1.0): age <60 years with BMI <22.4 kg/m2 at baseline. P for trend <0.001 for both age categories. Likelihood ratio test for effect modification by baseline age, P=0.15. (b) Referent group (RR=1.0): SBP <120 mmHg with BMI <22.4 kg/m2 at baseline. P for trend <0.001 for both BP categories. Likelihood ratio test for effect modification by baseline SBP, P=0.19. (c) Referent group (RR=1.0): DBP <80 mmHg with BMI <22.4 kg/m2 at baseline. P for trend <0.001 for both DBP categories. Likelihood ratio test for effect modification by baseline DBP, P=0.02.
In secondary analyses, we examined the associations between weight change over 8 years and subsequent risk of developing hypertension. Overall, 8-year mean change in BMI (SD) was +2.0 (5.9)%, varying from +0.35 (8.4)% among those who were obese at baseline to +2.2 (5.6)% among those with BMI <25.0 kg/m2 at baseline (P<0.001). Adjusting for the potential confounders, men whose BMI increased by more than 5% had a significantly increased risk of developing hypertension after 8 years. Men whose BMI increased by >5 – 10% had a RR of 1.21 (95% CI, 1.09–1.35), and men whose BMI increased by >10% had a RR of 1.72 (95% CI, 1.48–1.99), as compared to men whose weight remained stable (BMI within 5% of baseline). Men whose BMI decreased by more than 5% were at similar risk for developing hypertension as compared to those with stable BMI (RR, 0.91; 95% CI, 0.77–1.08). Again, further adjustment for the potential mediators did not alter the associations. Across all baseline BMI levels, 8-year weight gains of >5% were associated with a significantly increased risk of developing subsequent hypertension. In stratified analyses adjusting for both potential confounders and mediators, weight gain was most strongly associated with increased hypertension risk among men who were obese at baseline (RR, 2.49; 95% CI, 1.05–5.91, for BMI increases >10% vs. stable weight), as compared to those who were overweight (RR, 1.77; 95% CI, 1.41–2.22) or normal weight (RR, 1.58; 95% CI, 1.30–1.93) at baseline.
Discussion
In this large, prospective study, progressively higher BMI was positively and significantly associated with an increased risk of incident hypertension. This association existed across the full spectrum of BMI, even within the “normal” BMI range, and persisted after adjusting for multiple potential confounders and intermediates. The increased risk incurred by higher BMI was not entirely explained by confounding due to smoking and was consistently demonstrated among men with normal blood pressures at baseline. Furthermore, 8-year weight gain of >5% was associated with increased risk of developing subsequent hypertension, even among those whose BMI was in the normal range (<25.0 kg/m2) at baseline.
Prior studies have demonstrated benefits of weight reduction in both the primary prevention3-5 and treatment18-21 of hypertension. Recent observational studies also confirm the benefits of weight loss in the primary prevention of hypertension among the overweight and obese.6 Furthermore, national recommendations support weight loss, both in the treatment of hypertension among overweight and obese adults2 and in the primary prevention of hypertension.22 These data confirm the adverse effects of long-term elevations in BMI on an increased risk of hypertension in middle-aged and older men.
Our findings are consistent with a cohort of Finnish men and women followed for a mean of 11 years. In that study, baseline BMI was associated with increased risk of hypertension defined as new antihypertensive medication use. Obese men had a RR of 1.66 (95% CI, 1.35–2.04) compared to men who had a normal BMI 13. Among US female nurses followed from 1976 to 1992, higher BMI at 18 years of age and midlife was monotonically associated with an increased risk of hypertension, with an increase in BMI of 1 kg/m2 associated with a 12% increase in risk of hypertension (95% CI, 11–12%).14 However, these results were not adjusted for physical activity, alcohol consumption, or high cholesterol.
The specific biologic mechanisms by which higher BMI increases the risk of developing hypertension remain unclear. Several metabolic and neurohormonal pathways likely have complex interactions underlying the development of hypertension among the overweight and obese, including alterations in insulin resistance, the renin-angiotensin-aldosterone system, and sympathetic tone.23-27 Weight loss among obese individuals has been associated with improvements in insulin resistance, as well as decreases in norepinephrine, plasma renin activity, and aldosterone levels.25, 26
Strengths of our study include the long duration of follow-up and resulting large number of hypertension cases accrued, increasing the statistical power of our analyses. In addition, the relative homogeneity of these predominantly Caucasian male physicians reduced potential confounding by race/ethnicity, socioeconomic status, and access to health care.
Limitations should be considered in the interpretation of our findings. First, information on height, weight, and blood pressure were self-reported, so random misclassification of BMI, hypertension, and blood pressure is possible. However, validation studies of other health professional cohorts have demonstrated extremely high reliability and validity of self-reported information on height, weight, and cardiovascular risk factors (r=0.97 for self-reported and measured weight).28, 29 Furthermore, any potential misclassification of BMI would most likely result in underestimation of the association between BMI and risk of hypertension. Second, lack of information on diet, an important component of hypertension prevention,3–5 may lead to residual confounding. However, we have otherwise comprehensively controlled for major lifestyle and clinical risk factors for hypertension. Finally, our results may not extrapolate to other populations. Although the association between BMI and risk of hypertension has been previously demonstrated among women,14 further study is warranted in more ethnically diverse populations.
We found a positive, significant association between BMI and risk of developing hypertension in middle-aged and older men. Small downward shifts in the BP distribution in the general population can result in large reductions in hypertension and related diseases.22, 30, 31 The identification of BMI and weight gain as potentially independent risk factors in the development of hypertension, and prior studies demonstrating BP reductions with weight loss,3-6, 18-21 suggest that population-based approaches to weight reduction may have considerable benefits in reducing the burden of hypertension and subsequent cardiovascular disease.
Acknowledgments
We are indebted to the participants in the Physician’s Health Study for their outstanding commitment and cooperation; to the entire Physicians’ Health Study staff for their expert and unfailing assistance.
Footnotes
This work was supported by grants CA 34944 and CA 40360 from the National Cancer Institute, and grants HL 26490 and HL 34595 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Stamler R, Stamler J, Gosch FC, Civinelli J, Fishman J, McKeever P, McDonald A, Dyer AR. Primary prevention of hypertension by nutritional-hygienic means. Final report of a randomized, controlled trial. JAMA. 1989;262:1801–1807. [PubMed] [Google Scholar]
- 4.He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35:544–549. doi: 10.1161/01.hyp.35.2.544. [DOI] [PubMed] [Google Scholar]
- 5.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 6.Moore LL, Visioni AJ, Qureshi MM, Bradlee ML, Ellison RC, D’Agostino R. Weight loss in overweight adults and the long-term risk of hypertension: the Framingham study. Arch Intern Med. 2005;165:1298–1303. doi: 10.1001/archinte.165.11.1298. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Wang B, Chen C, Jin Y, Yang J, Stampfer MJ, Xu X. Body mass index and cardiovascular risk factors in a rural Chinese population. Am J Epidemiol. 2000;151:88–97. doi: 10.1093/oxfordjournals.aje.a010127. [DOI] [PubMed] [Google Scholar]
- 8.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 9.Cassano PA, Segal MR, Vokonas PS, Weiss ST. Body fat distribution, blood pressure, and hypertension.A prospective cohort study of men in the normative aging study. Ann Epidemiol. 1990;1:33–48. doi: 10.1016/1047-2797(90)90017-m. [DOI] [PubMed] [Google Scholar]
- 10.Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res. 2005;13:163–169. doi: 10.1038/oby.2005.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong LC, Kuller LH, Rutan G, Bunker C. Longitudinal study of blood pressure: changes and determinants from adolescence to middle age. The Dormont High School follow-up study, 1957–1963 to 1989–1990. Am J Epidemiol. 1993;138:973–983. doi: 10.1093/oxfordjournals.aje.a116817. [DOI] [PubMed] [Google Scholar]
- 12.Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986–1995. Arch Intern Med. 2000;160:2847–2853. doi: 10.1001/archinte.160.18.2847. [DOI] [PubMed] [Google Scholar]
- 13.Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 16.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 17.Klag MJ, He J, Mead LA, Ford DE, Pearson TA, Levine DM. Validity of physicians’ self-reports of cardiovascular disease risk factors. Ann Epidemiol. 1993;3:442–447. doi: 10.1016/1047-2797(93)90074-e. [DOI] [PubMed] [Google Scholar]
- 18.The treatment of mild hypertension study. A randomized, placebo-controlled trial of a nutritional-hygienic regimen along with various drug monotherapies. The Treatment of Mild Hypertension Research Group Arch Intern Med. 1991;151:1413–1423. doi: 10.1001/archinte.151.7.1413. [DOI] [PubMed] [Google Scholar]
- 19.Davis BR, Blaufox MD, Oberman A, Wassertheil-Smoller S, Zimbaldi N, Cutler JA, Kirchner K, Langford HG. Reduction in long-term antihypertensive medication requirements. Effects of weight reduction by dietary intervention in overweight persons with mild hypertension. Arch Intern Med. 1993;153:1773–1782. doi: 10.1001/archinte.153.15.1773. [DOI] [PubMed] [Google Scholar]
- 20.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 21.Reisin E, Abel R, Modan M, Silverberg DS, Eliahou HE, Modan B. Effect of weight loss without salt restriction on the reduction of blood pressure in overweight hypertensive patients. N Engl J Med. 1978;298:1–6. doi: 10.1056/NEJM197801052980101. [DOI] [PubMed] [Google Scholar]
- 22.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 23.Kolanowski J. Obesity and hypertension: from pathophysiology to treatment. Int J Obes Relat Metab Disord. 1999;23 (Suppl 1):42–46. doi: 10.1038/sj.ijo.0800794. [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med. 1997;50:191–197. doi: 10.1016/s0300-2977(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda T, Gomi T, Hirawa N, Sakurai J, Yoshikawa N. Improvement of insulin sensitivity contributes to blood pressure reduction after weight loss in hypertensive subjects with obesity. Hypertension. 1996;27:1180–1186. doi: 10.1161/01.hyp.27.5.1180. [DOI] [PubMed] [Google Scholar]
- 26.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 27.Hsueh WA, Buchanan TA. Obesity and hypertension. Endocrinol Metab Clin North Am. 1994;23:405–427. [PubMed] [Google Scholar]
- 28.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 30.Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17:I16–20. doi: 10.1161/01.hyp.17.1_suppl.i16. [DOI] [PubMed] [Google Scholar]
- 31.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. [PubMed] [Google Scholar]


