Abstract
Ras is mutated to remain in the active oncogenic state in many cancers. As Ras has proven difficult to target therapeutically, we searched for secreted, druggable proteins induced by Ras that are required for tumorigenesis. We found that Ras induces the secretion of cytokine IL6 in different cell types, and that knockdown of IL6, genetic ablation of the IL6 gene, or treatment with a neutralizing IL6 antibody retard Ras-driven tumorigenesis. IL6 appears to act in a paracrine fashion to promote angiogenesis and tumor growth. Inhibiting IL6 may therefore have therapeutic utility for treatment of cancers characterized by oncogenic Ras mutations.
Keywords: Ras, oncogene, IL6, cytokine, cancer
The small GTPase family of Ras proteins function as GDP/GTP-regulated binary switches that normally relay signals from extracellular stimulus-activated cell surface receptors to diverse cytoplasmic signaling networks in a regulated fashion. Ras is mutated to remain in a stimulus-independent, constitutively active GTP-bound state in one-third of tumors, or if Ras itself is not mutated, the Ras pathway is often inappropriately activated through mutations to upstream receptor tyrosine kinases or downstream components. Such inappropriate activation of Ras provides self-sufficiency in growth signals, leading to increased cell proliferation and survival among other phenotypes characteristic of cancer cells. As such, Ras is a desirable target for cancer therapy, although attempts to inhibit Ras have not yet borne out clinically (Downward 2003). On the other hand, secreted proteins are druggable, typically with neutralizing antibodies (Adams and Weiner 2005). We therefore speculated that inhibiting secreted proteins induced by Ras might block Ras oncogenesis.
The cancer most associated with oncogenic Ras mutations is pancreatic (Downward 2003). Proteins elevated in the serum of pancreatic cancer patients may correspondingly be the product of oncogenic Ras activity. One such protein elevated in pancreatic cancer patients is IL6 (Barber et al. 1999; Wigmore et al. 2002; Ebrahimi et al. 2004). IL6 is a pleiotropic cytokine functioning in inflammation, immunity, bone metabolism, neural development, reproduction, and hematopoiesis (Keller et al. 1996), but it has also been implicated in the etiology of some cancers (Trikha et al. 2003). Given the alluring link of elevated IL6 levels in patients with a cancer characterized by Ras mutations and the observation that Ras-induced secretion of another cytokine, IL8, is important for tumor growth of HeLa cells overexpressing oncogenic Ras (Sparmann and Bar-Sagi 2004), we examined whether IL6 plays any role in Ras-mediated cancers.
Results and Discussion
Oncogenic RasG12V induces IL6 expression
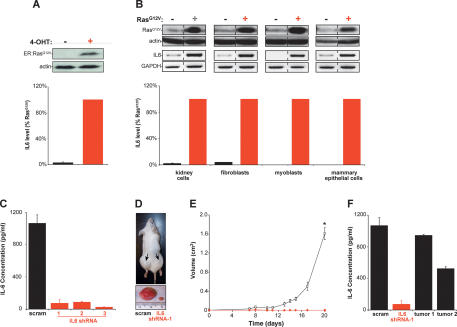
Normal human kidney cells genetically transformed with SV40 T/t-Ags, the telomerase catalytic subunit hTERT, and a 4-hydroxytamoxifen (4-OHT) inducible ER:RasG12V fusion protein were assayed for secreted IL6 levels in the absence of 4-OHT, and hence, no ER:RasG12V protein, and presence of 4-OHT, and hence, the presence of ER:RasG12V, which converts these cells to a tumorigenic state (Lim and Counter 2005). Upon induction of ER:RasG12V by 4-OHT (Fig. 1A, top panel), the level of secreted IL6 was elevated almost 40-fold (Fig. 1A, bottom panel). Thus, induction of a tumorigenic state by oncogenic Ras is associated with an increase in IL6 secretion.
Figure 1.
Oncogenic Ras-induced secretion of IL6 is required for Ras-mediated tumorigenesis. (A) IL6-secreted protein levels increase upon activation of Ras. Human kidney cells expressing T/t-Ag, hTERT, and ER:RasG12V (and p110-CAAX) were confirmed by immunoblot to up-regulate ER:RasG12V in the presence of 4-OHT (top), which led to elevated levels of IL6 (percent relative to RasG12V-expressing cells) present in cell medium as detected by ELISA (bottom). (Actin) Loading control. (B) Oncogenic Ras increases secreted IL6 independent of cell type. The indicated four primary human cell types expressing T/t-Ag and hTERT and, where indicated, also RasG12V, as assessed by immunoblot (top), were shown to increase the levels of IL6 mRNA, as assessed by RT–PCR (middle), and secreted IL6 protein (percent relative to RasG12V-expressing cells), as assessed by ELISA (bottom) upon expression of RasG12V. (Actin) Loading control; (GAPDH) RT–PCR control. (C) Primary human kidney cells expressing T/t-Ags, hTERT, RasG12V, and shRNA-1, shRNA-2, and shRNA-3, but not a scramble (scram) control sequence, exhibited a decrease in secreted IL6 as assessed by ELISA. (D) Knockdown of IL6 (IL6 shRNA-1) in the aforementioned human kidney cells induced decreased tumor growth, as visualized in a representative mouse (arrow, tumor) or ressected tumors, when compared with scramble control-treated cells (scram). (E) IL6 is required for Ras-driven tumor growth. A plot of tumor volume (cubic centimeters) ± standard error versus time in days for the aforementioned human kidney cells expressing the scramble control sequence (open box) or the three different IL6 shRNAs (as growth kinetics were identical, tumor growth in mice injected with cells expressing any of the three IL6 shRNAs is shown with one symbol [red diamond]) when injected in mice. (*) P < 0.001 (F) Two tumors (tumor 1, tumor 2), eventually arising when the aforementioned human kidney cells expressing IL6 shRNA-1 were isolated, were cultured briefly in hygromycin to enrich for tumor cells and assayed for secreted IL6 by ELISA, with the finding that these tumors exhibited increased IL6 compared with the original IL6 shRNA-1 donor cells, almost to the level of scramble (scram) control cells.
Ras can elicit very different effects in different cell backgrounds (Shields et al. 2000). To address whether induction of IL6 secretion occurred in other cell types, IL6 secretion was compared with the Ras status and tumorigenic phenotype of four different cell types that retained a constant genetic background (O’Hayer and Counter 2006). Both mRNA and protein levels of IL6 were greatly increased in normal primary human kidney cells, fibroblasts, myoblasts, and mammary epithelial cells expressing T/t-Ag and hTERT that were driven to be tumorigenic by ectopic expression of RasG12V compared with the nontumorigenic cells lacking RasG12V (Fig. 1B). Thus, an oncogenic Ras-mediated tumorigenic state is associated with elevated levels of IL6 in multiple cell types.
Ras-induced secretion of IL6 is required for human tumor cell growth in vivo
To test whether the Ras-induced secretion of IL6 is required for Ras oncogenic function, IL6 levels were stably reduced >90% by IL6 short hairpin RNA (shRNA) in the tumorigenic human kidney cells expressing T/t-Ag, hTERT, and RasG12V (Fig. 1C). The resultant cells or the scramble control counterpart were then tested for tumor growth in immunocompromised mice. While scramble control cells rapidly formed tumors, reaching maximum tumor volume within ∼20 d, IL6 shRNA-treated cells barely generated palpable masses in this time span (P < 0.001) (Fig. 1D,E). Even though tumors eventually did arise (data not shown), IL6 was re-expressed in these tumors (Fig. 1F), strongly suggesting that a loss of IL6 must be overcome for tumor growth.
We validated these results with two more independent IL6 shRNA sequences. Identical to the first IL6 shRNA, knockdown of IL6 by these two other sequences (Fig. 1C) blocked tumor growth of the aforementioned RasG12V-transformed human kidney cells (Fig. 1E). To address whether IL6 is required for Ras-driven growth independent of cell type, IL6 was knocked down by shRNA (Supplementary Fig. 1A,D) in the aforementioned human fibroblasts and myoblasts engineered to be tumorigenic by the expression of T/t-Ags, hTERT, and RasG12V (O’Hayer and Counter 2006) and again found to reduce tumor size by at least 97% compared with scramble control cells (Supplementary Fig. 1B,C,E,F). Knockdown of IL6 thus presents a formidable barrier to Ras-induced human tumor growth of cells derived from different lineages.
IL6−/− mice are highly resistant to spontaneous induced Ras-driven tumors
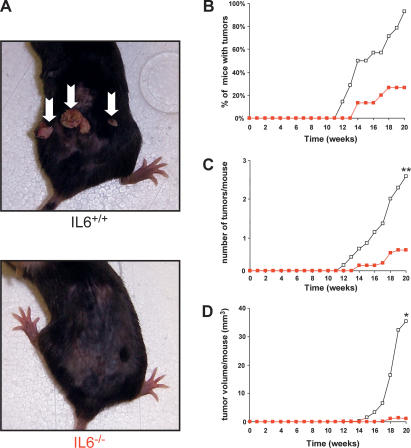
To address whether IL6 is also required for Ras-mediated tumor growth in a system that recapitulates the spontaneous process of tumorigenesis, we tested whether IL6−/− knockout mice are resistant to carcinogen-induced tumors. Premalignant papillomas with a high level of Ras mutations are induced by topical application of the carcinogen 7,12-Dimethylbenzanthracene (DMBA), followed by repetitive application of 12-O-tetradecanoylphorbol-13-acetate (TPA) (Quintanilla et al. 1986). Thus, IL6+/+ and IL6−/− mice were treated topically with DMBA and then TPA for 20 wk, and tumor growth monitored. Tumors appeared within 12 wk of initiation in IL6+/+ mice, and by termination of the experiment, 93% of the mice had tumors, with an average of 2.4 tumors per mouse (Fig. 2A–C). In sharp contrast, tumors appeared 2 wk later in the IL6−/− mice (Fig. 2A,B), and by the termination of the experiment, only 27% of the mice had tumors (Fig. 2B) and the average number of tumors dropped to 0.5 per mouse (P < 0.01) (Fig. 2C). Perhaps most telling, the total tumor volume per mouse at week 20, a measurement of both the size and number of tumors dropped almost 30-fold in IL6−/− mice (P < 0.05) (Fig. 2D). In agreement, a 1-wk delay in tumor onset and a 20% reduction in mice with tumors had been described for DMBA/TPA-treated IL6−/− mice, although in this case, a more aggressive carcinogen protocol was used (Suganuma et al. 2002). Thus, loss of IL6 inhibits spontaneous formation of Ras-driven tumors.
Figure 2.
IL6−/− mice are resistant to carcinogen-induced skin tumors. Fourteen IL6+/+ and 15 IL6−/− mice were treated with a single topical application of DMBA, followed 1 wk later by twice-weekly topical applications of TPA for 20 wk to induce skin tumors. (A) Reduction of spontaneous tumors in IL6−/− mice. Representative mice of the indicated genotype at 20 wk. (Arrows) Tumors. (B) Reduction in the number of IL6−/− mice having tumors. Percentage of IL6+/+ (open box) and IL6−/− (red box) mice with tumors versus time after initial application of DMBA (weeks). (C) Reduction in the number of tumors in IL6−/− mice. Mean number of tumors per IL6+/+ (open box) and IL6−/− (red box) mouse versus time after initial application of DMBA (weeks). (**) P < 0.01 (D) Reduction in tumor volume in IL6−/− mice. Mean tumor volume per IL6+/+ (open box) and IL6−/− (red box) mouse versus time after initial application of DMBA (weeks). (*) P < 0.05.
Ras-induced IL6 secretion acts in a paracrine fashion to promote tumor growth
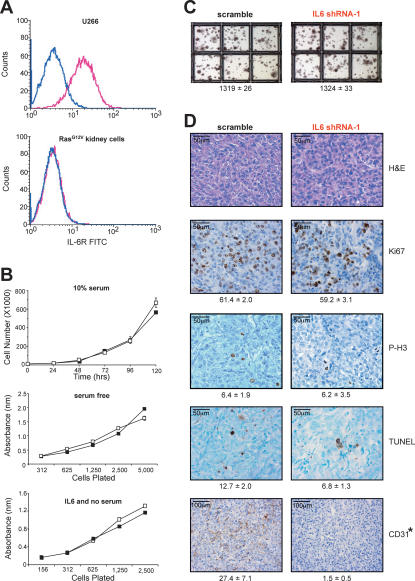
To address how Ras-induced secretion of IL6 promotes tumor growth in vivo, we tested whether the tumor cells were themselves the target of IL6. However, we did not find any evidence for autocrine signaling. RasG12V-transformed human kidney cells, which require IL6 for tumor growth, do not express detectable IL6 receptor (IL6R) (Fig. 3A), and knockdown of IL6 in these cells did not, when compared with vector control cells, have any effect on cell proliferation under normal serum (Fig. 3B, top panel) or in the stress condition of low serum (Fig. 3B, middle panel) or growth in soft agar (Fig. 3C), nor did addition of exogenous IL6 foster the growth of the same cells in the absence of Ras (Fig. 3B, bottom panel). We thus explored the possibility that Ras-induced secretion of IL6 acts in a paracrine fashion to promote Ras tumorigenesis. Immunohistological staining revealed that tumors eventually arising from IL6 shRNA-treated RasG12V-transformed human kidney cells did not exhibit any gross histological differences, or changes in cell proliferation, as assessed by Ki67 and phospho-histone H3 staining, or apoptosis, as detected by the TUNEL assay, compared with scramble control tumors (P > 0.05) (Fig. 3D). This could mean that either loss of IL6 has no effect on apoptosis or proliferation, or that by the time we are able to study these tumors, the observed reactivation of IL6 (Fig. 1F) masked these effects. Nevertheless, we did find that CD31-positive (endothelial) cells were reduced almost 18-fold in IL6 shRNA-treated tumors (P < 0.01) (Fig. 3D). In agreement, IL6 has been shown to promote angiogenesis (Cohen et al. 1996; Wei et al. 2003; Huang et al. 2004; Loeffler et al. 2005; Nilsson et al. 2005). However, endothelial cells typically do not express detectable levels of IL6R (Supplementary Fig. 2) (Romano et al. 1997) or respond to IL6 (Podor et al. 1989; Sironi et al. 1989), arguing that the reduction of CD31-positive cells by knocking down IL6 in tumorigenic cells is indirect. This paracine effect must also be local, as tumors arising from scramble control cells on one flank of the mice failed to promote tumorigenic growth of IL6 shRNA-treated cells on the opposite flank (Fig. 1D).
Figure 3.
IL6 acts in a paracrine manner to promote angiogenesis. (A) Detection of IL6R (pink profile) by flow-cytometric analysis in positive control U266 cells (top graph), but not RasG12V-transformed human kidney cells (bottom graph). No antibody (blue profile) serves as a negative control. (B) IL6 does not alter the growth of RasG12V-transformed human kidney cells. Absorbance ± standard error versus time (days) as measured by the MTT assay of RasG12V-transformed human kidney cells stably expressing IL6 shRNA-1 (black box) or the appropriate scramble sequence (open box) cultured in 10% serum (top) or serum-free (middle) medium, or the same kidney cells lacking RasG12V plated at the indicated densities in serum-free medium (black box) or serum-free medium containing 100 pg/mL IL6 (open box). (C) Similar anchorage-independent growth of RasG12V-transformed human kidney cells stably expressing IL6 shRNA-1 versus the appropriate scramble sequence. Average number of colonies ± standard error calculated from three independent experiments conducted in triplicate. (D) Loss of IL6 inhibits angiogenesis. Tumors from RasG12V-transformed human kidney cells stably expressing IL6 shRNA-1 or the appropriate scramble sequence were excised, formalin-fixed, and stained for H&E or assayed for Ki67, phospho-histone H3 (P-H3), TUNEL, or CD31-positive (dark brown) cells. (Bottom) Average number of marker-positive cells ± standard deviation from five independent fields of two to four different tumors. (*) P < 0.01)
IL6 as a target for cancer therapy
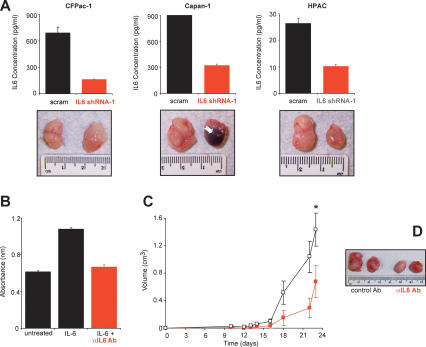
To expand our studies to more clinically relevant settings, we tested whether stable knockdown of IL6 (Fig. 4A, top panels) inhibited the tumorigenic growth in the human pancreatic cancer cell lines CFPac-1, Capan-1, and HPAC that contain an oncogenic K-Ras allele (Kita et al. 1999; Moore et al. 2001; Lim et al. 2006), as such cell lines better model the genetic chaos of human cancers that is thought to underlie the variability in the effectiveness of cancer therapies. While knockdown of IL6 had no obvious effect on CFPac-1 cells, it did cause necrosis in the tumors arising from Capan-1 cells and retarded the tumor growth of HPAC cells compared with scramble control-treated cells (Fig. 4A, bottom panels). Thus, IL6 is important for tumorigenic growth in two of the three tested human mutant K-Ras-positive cancer cell lines, suggesting that an anti-IL6 therapy could be effective in the heterogeneous situation of human cancers.
Figure 4.
IL6 as a therapeutic target. (A) Knockdown of IL6 inhibits tumor growth. (Top panel) Reduction of IL6, as detected by ELISA from the conditioned medium of the indicated three human pancreatic cancer cell lines expressing IL6 shRNA-1, but not the scramble (scram) control counterpart. (Bottom panel) Representative tumors from the indicated three human pancreatic cancer cell lines stably expressing an IL6 shRNA-1 or scramble sequence after injection subcutaneously into the flanks of mice. (Arrow) Region of necrosis. (B) Neutralization of IL6 activity by an anti-IL6 antibody (αIL6 Ab). Cell numbers as measured by average absorbance ± standard error using the MTT assay 48 h after B9 cells were plated in serum-free medium (untreated), in the presence of IL6 to stimulate cell proliferation (IL6) or in the presence of IL6 and αIL6 Ab (IL6 + αIL6 Ab). (C) An IL6-neutralizing antibody inhibits Ras-driven tumor growth. Tumor volume (cubic centimeters) ± standard error versus time (days) of RasG12V-transformed human kidney cells growing in mice injected at the tumor site every 3 d with 100 μg of either the IgG1 control antibody (black box) or the IL6-neutralizing antibody (red box). (*) P < 0.01. (D) Representative tumors of RasG12V-transformed human kidney cells growing in mice injected every 3 d with 100 μg of either the IgG1 control antibody (control Ab) or the IL6-neutralizing antibody (αIL6 Ab).
We next tested whether a more clinically relevant mode of inhibiting IL6 function could impede Ras-driven tumor growth. As IL6 is a secreted protein, it is amendable to targeting by neutralizing antibodies. Moreover, such an antibody is tolerated in humans (Trikha et al. 2003). Thus, we tested whether a neutralizing IL6 antibody could inhibit the tumor growth of the RasG12V-transformed human kidney cells. We first confirmed that such an antibody effectively neutralized IL6 in a biological assay. Addition of the neutralizing IL6 antibody to culture medium reduced the IL6-dependant growth of B9 cells to the same level as cells that were not induced by IL6 (Fig. 4B). Next, mice pretreated with either the IL6-neutralizing antibody or a control IgG antibody were injected with RasG12V-transformed human kidney cells, and then treated again every 3 d by intratumor injections of either the anti-IL6 or control antibody. Tumors in mice treated with the control IgG antibody first appeared at week 2 and reached maximum tumor volume by day 23. This growth was retarded in the IL6-neutralizing antibody treatment group, and tumors were approximately half the size (P < 0.01) of control tumors (Fig. 4C,D). Consistent with these data, anti-IL6 antibodies have been reported to reduce the tumor growth of prostate cancer cells (Smith and Keller 2001; Wallner et al. 2006). These experiments suggest the intriguing possibility that IL6-neutralizing antibodies may have therapeutic value in the treatment of Ras-driven cancers.
Summary
We show that oncogenic Ras induces the secretion of the cytokine IL6 from a variety of cell types, and that knockdown or genetic ablation of IL6 thwarts Ras-mediated tumor growth of human tumor cell lines and chemical carcinogen-induced tumors. IL6 is thus a bona fide downstream effector of oncogenic Ras that promotes the tumorigenic effects of Ras. IL6 can also act upstream of Ras (Rowley and Van Ness 2002) and, correspondingly, the tumor-promoting effects of IL6 can be context dependant. Nevertheless, in the case of the tested oncogenic Ras-driven tumor cells, IL6 acts downstream from Ras in a paracrine fashion to promote angiogenesis. Overexpression of oncogenic Ras in the tumorigenic HeLa cancer cell line also induced the secretion of a different cytokine IL8, and inhibiting IL8 reduced tumor growth of these cells and the number of CD31-positive cells in a tumor (Sparmann and Bar-Sagi 2004). Like IL6, IL8 was also secreted upon expression of Ras in different human cell types (Supplementary Fig. 3). Thus, Ras-induced secretion of multiple cytokines may underlie the ability of this oncogene to potently induce angiogenesis. Secreted proteins that promote angiogenesis have been successfully inhibited with neutralizing antibodies with desirable clinical outcomes in the treatment of many different human cancers (Adams and Weiner 2005). Consistent with this promising therapeutic strategy to target angiogenesis, and the dependence of oncogenic Ras-driven tumorigenesis on IL6 for angiogenesis, we demonstrate that an IL6-neutralizing antibody inhibits Ras-driven tumor growth. IL6 is thus a druggable protein that holds promise as a target for the treatment of Ras-driven cancers.
Materials and methods
Retroviral vectors
pBabepuro, pBabepuro-H-RasG12V, and pBabepuro-ER:RasG12V were previously described (Lim and Counter 2005). IL6 shRNA-1, shRNA-2, and shRNA-3, and scramble control sequences (5′-AGATGGATGCTTC CAATCTGG-3′, 5′-AAGGCAAAGAATCTAGATGCA-3′, 5′-AGACAT GTAACAAGAGTAA-3′, and 5′-AGACGGAGGCTTACAGTCTGG-3′, respectively) were cloned into pSUPER-RETRO-PURO.
Cell lines
Human (embryonic) kidney cells, BJ fibroblasts, mammary epithelial cells, and skeletal muscle myoblasts stably expressing the early region of SV40 (which produce the proteins T-Ag and t-Ag); hTERT; and either H-RasG12V or no transgene (O’Hayer and Counter 2006) were stably infected with retroviruses generated from the indicated vectors to generate polyclonal populations as previously described (O’Hayer and Counter 2006). The ER:RasG12V-expressing tumor kidney cells also expressed p110-CAAX for unrelated reasons (Lim and Counter 2005). B9 cells (Aarden et al. 1987) were a kind gift of Peter Lansdorp (University of British Columbia, Vancouver, BC, Canada). U266 cells were obtained from American Type Culture Collection. HMVECs were a kind gift of Xiao-Fan Wang (Duke University Medical Center, Durham, NC).
RT–PCR
Total RNA was isolated, reverse-transcribed with an oligodT primer, and PCR-amplified with the primers 5′-ATGTAGCCGCCCCACACAGA-3′ and 5′-CATCCATCTTTTTCAGCCAT-3′ to detect IL6, and 5′-GAAG GTGAAGGTCGGAGACAA-3′ and 5′-GCAGAGGGGGCAGAGATGA T-3′ to detect GAPDH, using a previously described protocol (Hamad et al. 2002). Cycle number varied between 25 and 40 cycles, depending on cell type and transcript.
Immunoblot
Lysates from the described cell lines were immunoblotted with the primary antibody α-pan-Ras (Oncogene) or α-actin C-2 (Santa Cruz Biotechnology) using standard methods.
IL6R detection
Cells (2 × 106) were incubated with 20 μL of IL6R FITC-conjugated antibody (Abcam) in 3% BSA-PBS for 1 h at 4°C. Cells were then washed three times in PBS and IL6R-positive cells were detected by flow cytometry.
ELISA
Cells were plated at ∼80% confluency. Twenty-four hours later, cells were washed three times with PBS and cultured in serum-free medium, and 48 h later, cells were collected and analyzed in duplicate with a human IL6 or IL8 ELISA Kit (R&D Systems). Results are reported as means ± standard deviation.
Cell proliferation
Cells (1 × 104 per 6-cm dish) were seeded in triplicate, and viable trypan blue-negative cells were counted daily for 5 d. To measure cell proliferation rate under stress conditions, cells were plated at varying densities in a 96-well plate. Twenty-four hours later, medium was replaced with serum-free medium. Four days later, 50 μL of 5 mg/mL 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma) was added to each well, and 4 h later, medium was aspirated and cells were resuspended in 200 μL of DMSO. Absorbencies were recorded at 540 nm. IL6 antibody neutralization was assayed by seeding 3 × 104 B9 cells per well in a 96-well plate in medium supplemented with 10 pg/mL human IL6 and 0.04 ug/mL monoclonal anti-human IL6 antibody (R&D Systems), which was preincubated to allow binding of the antibody to the cytokine. Forty-eight hours later, 10 μL of 5 mg/mL MTT was added per well. Four hours later, 50 μL of 20% SDS/50% DMF was added per well, and 24 h later, the absorbencies at 570–650 nm were measured.
Soft agar
Of the indicated cells, 5 × 104 cells were suspended in soft agar in triplicate three times independently, and colonies >30 cells were scored after 3 wk, as previously described (Cifone and Fidler 1980; Hamad et al. 2002).
Tumor growth
Cells (1 × 107) mixed with Matrigel were injected subcutaneously into one or both flanks of SCID/beige mice for a total of four injections per cell line, after which tumor volumes were determined at regular intervals as described previously (Hamad et al. 2002). In cases in which tumor-derived cells were retested for IL6 levels, tumors were established in culture under hygromycin selection to enrich for tumor cells as previously described (Lim and Counter 2005). For antibody neutralization of IL6, four animals were pretreated with an injection of 100 μg per mouse IL6-neutralizing antibody (MAB206; R&D Systems) or isotype control antibody (R&D Systems) 2 d prior to tumor cell injection. Starting 1 d after tumor cell injection, mice were treated with neutralizing IL6 antibody or control antibody every 3 d at the tumor site. For chemical carcinogenesis, the backs of 15 control (IL6+/+) C57BL/6J mice and 15 experimental (IL6−/−) C57BL/6J mice, in which both alleles of IL6 were disrupted (B6.129S2-IL6tm1Kopf/J) (Kopf et al. 1994) (Jackson Laboratory) were shaved, and the following day, 150 μL of 125 μg/mL DMBA (Sigma) in DMSO were applied topically, followed 1 wk later by twice-weekly topical applications of 150 μL of 10−4 M TPA (Sigma) in DMSO for 20 wk. One IL6+/+ mouse died at week 11 for unrelated reasons and was excluded from analysis. Tumor number and size were recorded weekly. Student’s t-test was used to compare tumor growth in the various models. The differences between means were considered significant if P < 0.05. All procedures with mice were done under an Institutional Animal Care and Use Committee-approved protocol.
Immunohistochemistry
Excised tumors were fixed in formalin and sectioned. H&E staining, Ki67 (αKi-67 Ab, Zymed), phospho-histone H3 (α phospho-Histone H3 [Ser10] Ab; Upstate Biotechnology), and CD31 (α-PECAM-1 Ab; Santa Cruz Biotechnology) immunohistochemistry, and TUNEL assay (Apoptag Plus Peroxidase Apoptosis Detection Kit; Chemicon) were preformed using standard protocols.
Acknowledgments
We thank the Counter laboratory for thoughtful discussions. This work was supported by NIH grant CA94184. C.M.C. is a Leukemia and Lymphoma Scholar, K.-H.L. is a Department of Defence Breast Cancer Research Predoctoral Scholar.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1549407
References
- Aarden L.A., De Groot E.R., Schaap O.L., Lansdorp P.M., De Groot E.R., Schaap O.L., Lansdorp P.M., Schaap O.L., Lansdorp P.M., Lansdorp P.M. Production of hybridoma growth factor by human monocytes. Eur. J. Immunol. 1987;17:1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Adams G.P., Weiner L.M., Weiner L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- Barber M.D., Fearon K.C., Ross J.A., Fearon K.C., Ross J.A., Ross J.A. Relationship of serum levels of interleukin-6, soluble interleukin-6 receptor and tumour necrosis factor receptors to the acute-phase protein response in advanced pancreatic cancer. Clin. Sci. 1999;96:83–87. [PubMed] [Google Scholar]
- Cifone M.A., Fidler I.J., Fidler I.J. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc. Natl. Acad. Sci. 1980;77:1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T., Nahari D., Cerem L.W., Neufeld G., Levi B.Z., Nahari D., Cerem L.W., Neufeld G., Levi B.Z., Cerem L.W., Neufeld G., Levi B.Z., Neufeld G., Levi B.Z., Levi B.Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Ebrahimi B., Tucker S.L., Li D., Abbruzzese J.L., Kurzrock R., Tucker S.L., Li D., Abbruzzese J.L., Kurzrock R., Li D., Abbruzzese J.L., Kurzrock R., Abbruzzese J.L., Kurzrock R., Kurzrock R. Cytokines in pancreatic carcinoma: Correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- Hamad N.M., Elconin J.H., Karnoub A.E., Bai W., Rich J.N., Abraham R.T., Der C.J., Counter C.M., Elconin J.H., Karnoub A.E., Bai W., Rich J.N., Abraham R.T., Der C.J., Counter C.M., Karnoub A.E., Bai W., Rich J.N., Abraham R.T., Der C.J., Counter C.M., Bai W., Rich J.N., Abraham R.T., Der C.J., Counter C.M., Rich J.N., Abraham R.T., Der C.J., Counter C.M., Abraham R.T., Der C.J., Counter C.M., Der C.J., Counter C.M., Counter C.M. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes & Dev. 2002;16:2045–2057. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.P., Wu M.S., Shun C.T., Wang H.P., Lin M.T., Kuo M.L., Lin J.T., Wu M.S., Shun C.T., Wang H.P., Lin M.T., Kuo M.L., Lin J.T., Shun C.T., Wang H.P., Lin M.T., Kuo M.L., Lin J.T., Wang H.P., Lin M.T., Kuo M.L., Lin J.T., Lin M.T., Kuo M.L., Lin J.T., Kuo M.L., Lin J.T., Lin J.T. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J. Biomed. Sci. 2004;11:517–527. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- Keller E.T., Wanagat J., Ershler W.B., Wanagat J., Ershler W.B., Ershler W.B. Molecular and cellular biology of interleukin-6 and its receptor. Front. Biosci. 1996;1:d340–d357. doi: 10.2741/a136. [DOI] [PubMed] [Google Scholar]
- Kita K., Saito S., Morioka C.Y., Watanabe A., Saito S., Morioka C.Y., Watanabe A., Morioka C.Y., Watanabe A., Watanabe A. Growth inhibition of human pancreatic cancer cell lines by anti-sense oligonucleotides specific to mutated K-ras genes. Int. J. Cancer. 1999;80:553–558. doi: 10.1002/(sici)1097-0215(19990209)80:4<553::aid-ijc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kopf M., Baumann H., Freer G., Freudenberg M., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Kohler G., Baumann H., Freer G., Freudenberg M., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Kohler G., Freer G., Freudenberg M., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Kohler G., Freudenberg M., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Kohler G., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Kohler G., Kishimoto T., Zinkernagel R., Bluethmann H., Kohler G., Zinkernagel R., Bluethmann H., Kohler G., Bluethmann H., Kohler G., Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Lim K.H., Counter C.M., Counter C.M. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Lim K.H., O’Hayer K., Adam S.J., Kendall S.D., Campbell P.M., Der C.J., Counter C.M., O’Hayer K., Adam S.J., Kendall S.D., Campbell P.M., Der C.J., Counter C.M., Adam S.J., Kendall S.D., Campbell P.M., Der C.J., Counter C.M., Kendall S.D., Campbell P.M., Der C.J., Counter C.M., Campbell P.M., Der C.J., Counter C.M., Der C.J., Counter C.M., Counter C.M. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr. Biol. 2006;16:2385–2394. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Loeffler S., Fayard B., Weis J., Weissenberger J., Fayard B., Weis J., Weissenberger J., Weis J., Weissenberger J., Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int. J. Cancer. 2005;115:202–213. doi: 10.1002/ijc.20871. [DOI] [PubMed] [Google Scholar]
- Moore P.S., Sipos B., Orlandini S., Sorio C., Real F.X., Lemoine N.R., Gress T., Bassi C., Kloppel G., Kalthoff H., Sipos B., Orlandini S., Sorio C., Real F.X., Lemoine N.R., Gress T., Bassi C., Kloppel G., Kalthoff H., Orlandini S., Sorio C., Real F.X., Lemoine N.R., Gress T., Bassi C., Kloppel G., Kalthoff H., Sorio C., Real F.X., Lemoine N.R., Gress T., Bassi C., Kloppel G., Kalthoff H., Real F.X., Lemoine N.R., Gress T., Bassi C., Kloppel G., Kalthoff H., Lemoine N.R., Gress T., Bassi C., Kloppel G., Kalthoff H., Gress T., Bassi C., Kloppel G., Kalthoff H., Bassi C., Kloppel G., Kalthoff H., Kloppel G., Kalthoff H., Kalthoff H., et al. Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439:798–802. doi: 10.1007/s004280100474. [DOI] [PubMed] [Google Scholar]
- Nilsson M.B., Langley R.R., Fidler I.J., Langley R.R., Fidler I.J., Fidler I.J. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hayer K.M., Counter C.M., Counter C.M. A genetically defined normal somatic human cell system to study ras oncogenesis in vitro and in vivo. Methods Enzymol. 2006;407:637–647. doi: 10.1016/S0076-6879(05)07050-3. [DOI] [PubMed] [Google Scholar]
- Podor T.J., Jirik F.R., Loskutoff D.J., Carson D.A., Lotz M., Jirik F.R., Loskutoff D.J., Carson D.A., Lotz M., Loskutoff D.J., Carson D.A., Lotz M., Carson D.A., Lotz M., Lotz M. Human endothelial cells produce IL-6. Lack of responses to exogenous IL-6. Ann. N. Y. Acad. Sci. 1989;557:374–385. [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A., Brown K., Ramsden M., Balmain A., Ramsden M., Balmain A., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Romano M., Sironi M., Toniatti C., Polentarutti N., Fruscella P., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., Sironi M., Toniatti C., Polentarutti N., Fruscella P., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., Toniatti C., Polentarutti N., Fruscella P., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., Polentarutti N., Fruscella P., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., Fruscella P., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., Luini W., van Hinsbergh V., Sozzani S., van Hinsbergh V., Sozzani S., Sozzani S. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- Rowley M., Van Ness B., Van Ness B. Activation of N-ras and K-ras induced by interleukin-6 in a myeloma cell line: Implications for disease progression and therapeutic response. Oncogene. 2002;21:8769–8775. doi: 10.1038/sj.onc.1205387. [DOI] [PubMed] [Google Scholar]
- Shields J.M., Pruitt K., McFall A., Shaub A., Der C.J., Pruitt K., McFall A., Shaub A., Der C.J., McFall A., Shaub A., Der C.J., Shaub A., Der C.J., Der C.J. Understanding Ras: ‘It ain’t over ’til it’s over.’. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A., Vecchi A., Van Damme J., Dejana E., Mantovani A., Van Damme J., Dejana E., Mantovani A., Dejana E., Mantovani A., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J. Immunol. 1989;142:549–553. [PubMed] [Google Scholar]
- Smith P.C., Keller E.T., Keller E.T. Anti-interleukin-6 monoclonal antibody induces regression of human prostate cancer xenografts in nude mice. Prostate. 2001;48:47–53. doi: 10.1002/pros.1080. [DOI] [PubMed] [Google Scholar]
- Sparmann A., Bar-Sagi D., Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Okabe S., Kurusu M., Iida N., Ohshima S., Saeki Y., Kishimoto T., Fujiki H., Okabe S., Kurusu M., Iida N., Ohshima S., Saeki Y., Kishimoto T., Fujiki H., Kurusu M., Iida N., Ohshima S., Saeki Y., Kishimoto T., Fujiki H., Iida N., Ohshima S., Saeki Y., Kishimoto T., Fujiki H., Ohshima S., Saeki Y., Kishimoto T., Fujiki H., Saeki Y., Kishimoto T., Fujiki H., Kishimoto T., Fujiki H., Fujiki H. Discrete roles of cytokines, TNF-α, IL-1, IL-6 in tumor promotion and cell transformation. Int. J. Oncol. 2002;20:131–136. [PubMed] [Google Scholar]
- Trikha M., Corringham R., Klein B., Rossi J.F., Corringham R., Klein B., Rossi J.F., Klein B., Rossi J.F., Rossi J.F. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: A review of the rationale and clinical evidence. Clin. Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- Wallner L., Dai J., Escara-Wilke J., Zhang J., Yao Z., Lu Y., Trikha M., Nemeth J.A., Zaki M.H., Keller E.T., Dai J., Escara-Wilke J., Zhang J., Yao Z., Lu Y., Trikha M., Nemeth J.A., Zaki M.H., Keller E.T., Escara-Wilke J., Zhang J., Yao Z., Lu Y., Trikha M., Nemeth J.A., Zaki M.H., Keller E.T., Zhang J., Yao Z., Lu Y., Trikha M., Nemeth J.A., Zaki M.H., Keller E.T., Yao Z., Lu Y., Trikha M., Nemeth J.A., Zaki M.H., Keller E.T., Lu Y., Trikha M., Nemeth J.A., Zaki M.H., Keller E.T., Trikha M., Nemeth J.A., Zaki M.H., Keller E.T., Nemeth J.A., Zaki M.H., Keller E.T., Zaki M.H., Keller E.T., Keller E.T. Inhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized mice. Cancer Res. 2006;66:3087–3095. doi: 10.1158/0008-5472.CAN-05-3447. [DOI] [PubMed] [Google Scholar]
- Wei L.H., Kuo M.L., Chen C.A., Chou C.H., Lai K.B., Lee C.N., Hsieh C.Y., Kuo M.L., Chen C.A., Chou C.H., Lai K.B., Lee C.N., Hsieh C.Y., Chen C.A., Chou C.H., Lai K.B., Lee C.N., Hsieh C.Y., Chou C.H., Lai K.B., Lee C.N., Hsieh C.Y., Lai K.B., Lee C.N., Hsieh C.Y., Lee C.N., Hsieh C.Y., Hsieh C.Y. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- Wigmore S.J., Fearon K.C., Sangster K., Maingay J.P., Garden O.J., Ross J.A., Fearon K.C., Sangster K., Maingay J.P., Garden O.J., Ross J.A., Sangster K., Maingay J.P., Garden O.J., Ross J.A., Maingay J.P., Garden O.J., Ross J.A., Garden O.J., Ross J.A., Ross J.A. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int. J. Oncol. 2002;21:881–886. doi: 10.3892/ijo.21.4.881. [DOI] [PubMed] [Google Scholar]