Abstract
The cell cycle inhibitor p27Kip1 also has cyclin–cyclin-dependent kinase (CDK)-independent functions. To investigate the significance of these functions in vivo, we generated a knock-in mouse in which four amino acid substitutions in the cdkn1b gene product prevent its interaction with cyclins and CDKs (p27CK−). In striking contrast to complete deletion of the cdkn1b gene, which causes spontaneous tumorigenesis only in the pituitary, the p27CK− protein dominantly caused hyperplastic lesions and tumors in multiple organs, including the lung, retina, pituitary, ovary, adrenals, spleen, and lymphomas. Moreover, the high incidence of spontaneous tumors in the lung and retina was associated with amplification of stem/progenitor cell populations. Therefore, independently of its role as a CDK inhibitor, p27Kip1 promoted stem cell expansion and functioned as a dominant oncogene in vivo. Thus, the p27CK− mouse unveils a dual role for p27 during tumorigenesis: It is a tumor suppressor by virtue of its cyclin–CDK regulatory function, and also an oncogene through a cyclin–CDK-independent function. This may explain why the cdkn1b gene is rarely inactivated in human tumors, and the p27CK− mouse in which the tumor suppressor function is lost but the cyclin–CDK-independent—oncogenic—function is maintained may represent a more faithful model for the widespread role of p27 misregulation in human cancers than the p27 null.
Keywords: p27Kip1, lung tumor, oncogene, retina, bronchioalveolar stem cell, desquamative interstitial pneumonitis
p27Kip1 (p27) is an inhibitor of the cyclin/cyclin-dependent kinase (CDK) complexes that are necessary for progression through the cell cycle, and can stop proliferation in the G1 phase of the cell cycle in response to anti-mitogenic signals (Polyak et al. 1994a, b; Coats et al. 1996; Sherr and Roberts 1999). p27 exerts its anti-proliferative activity both by occluding a substrate interaction domain located on the surface of the cyclin, and by inserting itself in the catalytic cleft of the CDK, thereby preventing the binding of ATP (Russo et al. 1996). Each of these inhibitory functions is mediated by a specific cyclin or CDK-binding site, which are both located in the N-terminal portion of the p27 protein (Russo et al. 1996; Vlach et al. 1997). The important role of p27 as a cell cycle regulator in vivo was revealed by the generation of the p27−/− mice, which display an ∼30% increase in body size, multiple organ hyperplasia, and disorganization of sensory epithelia in the retina and inner ear, all of which are attributed to an overall increased cellular proliferation; other phenotypes of the p27-null mice include female sterility due to defective ovarian and uterine function, but these have not been entirely linked to abnormal cell proliferation (Fero et al. 1996; Kiyokawa et al. 1996; Nakayama et al. 1996; Tong et al. 1998; Chen and Segil 1999; Levine et al. 2000; Dyer and Cepko 2001).
p27 is a tumor suppressor. This was first demonstrated in p27−/− mice, which spontaneously develop adenomas of the intermediate lobe of the pituitary gland and are more susceptible to tumorigenesis induced by chemical carcinogens or irradiation (Fero et al. 1996, 1998; Kiyokawa et al. 1996; Nakayama et al. 1996). Moreover, the tumor-prone phenotypes caused by the loss of other tumor suppressor genes are usually enhanced by concomitant loss of p27 (Franklin et al. 2000; Malumbres et al. 2000; Di Cristofano et al. 2001). In rats, an inherited syndrome of multiple endocrine neoplasia (MEN) is caused by a germline nonsense mutation in the p27 gene, and germline mutations in the human p27 gene are also linked to MEN (Pellegata et al. 2006; Georgitsi et al. 2007).
p27 deficiency is also associated with sporadic tumorigenesis in humans. It was first reported that breast and colon cancers frequently express abnormally low amounts of nuclear p27, and this is associated with increased tumor aggressiveness and a relatively poor clinical outcome (Catzavelos et al. 1997; Loda et al. 1997; Porter et al. 1997; Tan et al. 1997). Similar observations have subsequently been made in a broad array of human cancers, including carcinomas of the prostate, ovary, lung, brain, stomach, and others (Slingerland and Pagano 2000; Philipp-Staheli et al. 2001). Nevertheless, p27 is an unusual tumor suppressor because, unlike the canonical tumor suppressor genes Rb or p53, homozygous inactivation of the p27 gene in sporadic tumors is extremely rare (Ponce-Castaneda et al. 1995; Fero et al. 1998; Slingerland and Pagano 2000; Philipp-Staheli et al. 2001).
More recent clinical studies suggest that the role of p27 misregulation in tumorigenesis may extend beyond cyclin–CDK inhibition and modulation of cell proliferation. Indeed, some studies have indicated that p27 levels in tumors do not always correlate with proliferative index, and increasing evidence points to the importance of the subcellular localization of p27 in the control of its function, with cytoplasmic localization being a negative prognostic factor in certain instances (Singh et al. 1998; Nakasu et al. 1999; Saez et al. 1999; Sanchez-Beato et al. 1999; Slingerland and Pagano 2000; Philipp-Staheli et al. 2001; Kouvaraki et al. 2002; Liang et al. 2002; Besson et al. 2004a; Rosen et al. 2005; Qi et al. 2006). This possibility is supported by in vivo studies: p27+/− mice are more susceptible to tumor development than p27−/− animals in mammary and prostate tumor models, suggesting an active contribution of the remaining p27 allele (Muraoka et al. 2002; Gao et al. 2004). In contrast, mice carrying a p27S10A allele, which is mostly nuclear, are partially resistant to urethane-induced tumorigenesis despite reduced overall abundance of the p27 protein (Besson et al. 2006). One interpretation of these studies is that cytoplasmic localization of p27 may be critical for its contribution to tumorigenesis.
Several studies have begun to characterize the cyclin–CDK-independent functions of p27. These novel functions include the regulation of the actin cytoskeleton and cell migration through the modulation of RhoA activity, an activity that lies in the C-terminal half of p27 (McAllister et al. 2003; Besson et al. 2004b; Wu et al. 2006). This function is critical for the migration of cortical neurons during mouse embryonic development (Kawauchi et al. 2006; Nguyen et al. 2006). In addition, p27 modulates the differentiation of neuronal progenitors in vivo via the stabilization of Neurogenin-2, an activity that lies in the N-terminal half of p27 but does not involve the cyclin–CDK-binding activity of the protein (Nguyen et al. 2006).
These studies show that the function of p27 in vivo is not entirely explained by its inhibitory effect on CDK activity, and raise the important question as to whether its role in tumorigenesis similarly extends beyond its ability to regulate CDKs. Here, using a mouse knock-in model in which the cdkn1b gene has been replaced by an allele that cannot bind cyclins and CDKs, we addressed this question by comparing the tumor-prone phenotype caused by deletion of the p27 gene to that caused by expression of the p27CK− protein (Besson et al. 2006). We found that the p27CK− allele caused a dominant increase in spontaneous tumorigenesis in many tissues, compared with either wild-type or p27−/− animals. Focusing on lung tumorigenesis, we found that tumors arising in p27CK− mice likely originated from the aberrant expansion of a bronchioalveolar stem cell (BASC) pool (Kim et al. 2005).
In conclusion we show that p27 has genetically separable tumor suppressing and oncogenic functions. The tumor suppressor function is mediated by its inhibitory interactions with cyclins and CDKs, whereas its dominant oncogenic function is cyclin–CDK-independent.
Results
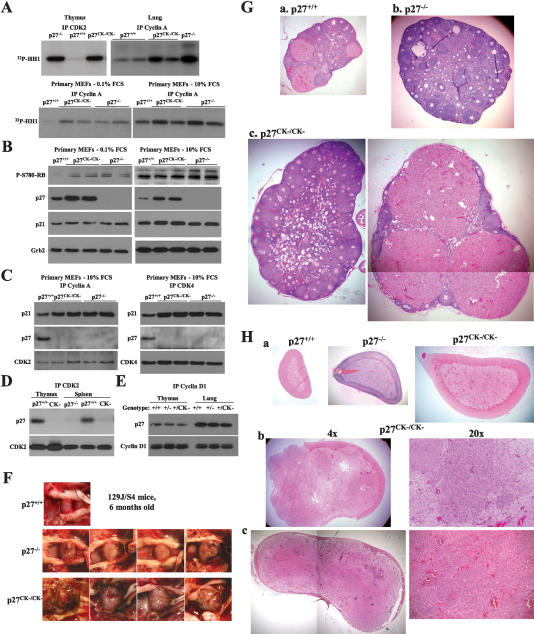
To determine the importance of the cyclin–CDK-independent functions of p27 in vivo we compared the phenotypes of p27CK− and p27-null mice. The p27CK− protein carries four point mutations, Arg30 to Ala and Leu32 to Ala in the cyclin-binding domain, and Phe62 to Ala and Phe64 to Ala in the CDK-binding domain of p27, and as a consequence can no longer bind to cyclins and CDKs (Vlach et al. 1997; Besson et al. 2006). We used several methods to ascertain that p27CK− was incapable of regulating cyclin/CDK complexes, and did not interfere with the interaction between CDKs and other casein kinase Is (CKIs). Thus, cyclin A and CDK2-associated kinase activities were similarly elevated in p27CK− and p27-null animals compared with wild-type in the thymus, lung, spleen, and primary mouse embryonic fibroblasts (MEFs) (Fig. 1A,B; data not shown). Moreover, in vivo phosphorylation of Rb on the CDK-dependent site Ser 780 was similarly elevated in both p27CK− and p27−/− proliferating and serum-starved MEFs cells compared with wild-type cells (Fig. 1B). Lastly, similarly increased amounts of p21Waf1/Cip1 were coprecipitated with cyclin A or CDK4 in p27CK− and p27−/− primary MEFs compared with wild-type cells (Fig. 1C). Notably, in these experiments, no p27CK− was detected in association with cyclin/CDK complexes (Fig. 1C,D; Besson et al. 2006). In addition, similar amounts of wild-type p27 protein were coprecipitated with cyclin D1 in lungs and thymus from p27+/CK− and p27+/− mice (Fig. 1E). Thus, in each assay p27CK− acted as a null mutation with respect to its interaction with and regulation of cyclin–CDK complexes.
Figure 1.
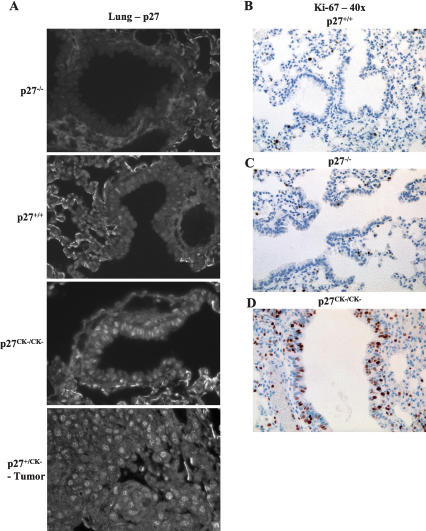
The p27CK− allele does not regulate cyclin–CDK complexes and causes multiple organs hyperplasia. (A) Comparison of cyclin A and CDK2-associated kinase activities in lung, thymus, and primary MEFs (either serum-starved for 48 h [0.1% FCS] or exponentially growing [10% FCS]) derived from wild-type, p27−/−, and p27CK−/CK− mice. (B) Increased phospho-Rb levels in p27CK−/CK− and p27−/− primary fibroblasts. Serum-starved or exponentially growing MEFs were probed sequentially for Phospho-Ser780-Rb (a CDK phosphorylation site), p27, p21, and Grb2 for loading control. (C) p27CK− does not interact with cyclins or CDKs and does not prevent p21 from binding to them. Exponentially growing primary MEF extracts were immunoprecipitated for cyclin A and CDK4 and were probed for p21, p27, and CDK2 (in cyclin A immunoprecipitates) and CDK4 (in CDK4 immunoprecipitates). (D) p27CK− does not bind to CDK2 in thymus and spleen. CDK2 immunoprecipitates from thymus and spleen extracts from wild-type, p27-null, and p27CK− mice were probed for p27 and CDK2. (E) p27CK− in p27+/CK− mice does not prevent the remaining wild-type p27 from interacting with cyclin D1. Cyclin D1 immunoprecipitates from thymus and lung of wild-type, p27+/−, and p27+/CK− mice were probed for p27 and cyclin D1. (F) Accelerated pituitary tumorigenesis in p27CK−/CK− mice compared with wild-type and p27−/− animals at 6 mo of age. (G) H&E staining of ovaries from 5- to 6-mo-old mice (magnification, 4×). (Panel a) Wild-type ovary showing normal follicle maturation. (Panel b) Ovaries from p27−/− mice are enlarged and lack corpora lutea. (Panel c) Twenty-five percent of p27CK− ovaries form corpora lutea, which are often grossly enlarged (as shown in right panel). (H) Spontaneous pheochromocytoma in p27CK−/CK− mice. (Panel a) H&E-stained sections of adrenal glands from 6-mo-old wild-type, p27−/−, and p27CK−/CK− mice (magnification, 4×). (Panels b,c) Pheochromocytomas developing in 12-mo-old (panel b) and 14-mo-old (panel c) p27CK−/CK− mice.
In accord with these molecular observations, some phenotypes of the p27CK− mice, such as a generalized organomegaly and overall increase in body size, were similar to that observed in p27−/− animals. This confirmed the importance of the cell cycle regulatory function of p27 in limiting organ growth in the adult (Besson et al. 2006). However, other phenotypes differed from that of p27−/− mice, thus demonstrating the existence of a cyclin–CDK-independent function(s) for p27 in vivo. Here, we focused on tumorigenesis.
The phenotypic differences between the p27CK− and p27−/− mice were observed in both a pure 129S4 genetic background and in a mixed C57BL6/129S4 background. In addition, two independently derived p27CK− mouse strains were compared with each other and found to be indistinguishable (data not shown). The most striking phenotype of p27CK− animals was the development of spontaneous tumors in multiple tissues (Table 1), suggesting that independently of its function as a cell cycle inhibitor, p27 may act as an oncogene. Moreover, this widespread tumorigenesis was also observed in p27+/CK− heterozygous mice, albeit with some delay relative to p27CK−/CK− homozygotes, indicating that the oncogenic effect of the p27CK− mutation was dominant. The p27CK−/CK− mice had a median survival of only 26 wk in the 126S4 background (Supplementary Table 1), with none surviving beyond 34 wk of age; this compared with a median survival of 35 wk for p27−/− mice in the same genetic background (M. Fero, pers. comm.). In all genotypes, lethality was primarily caused by the development of pituitary tumors. Most prevalent were adenomas of the intermediate lobe, similar to that described in p27−/− mice (Fero et al. 1996; Kiyokawa et al. 1996; Nakayama et al. 1996). However, comparison of 6-mo-old wild-type, p27−/−, and p27CK−/CK− mice in the 129S4 background revealed that tumors arising in the p27CK−/CK− mice were consistently larger, hypervascular, and caused more damage to surrounding tissue than those developing in the p27-null mice (Fig. 1F).
Table 1.
Incidence of spontaneous tumors
aGross evidence of DIP or diffuse adenocarcinoma, which can only be differentiated histologically; see Figure 4.
Note that some mice had both nodular tumor(s) and lesion on the lung.
T-cell lymphomas (in the thymus or lymph nodes) occurred at a 15%–20% frequency in p27CK−/CK− and p27+/CK− mice, but only in the mixed C57BL6/129S4 genetic background. There was also a low incidence of histiocytic tumors in the spleens of p27CK−/CK− mice (Table 1; data not shown).
Hyperplastic lesions also occurred in the ovaries, adrenal glands, and retinae of p27CK− mice. p27−/− females are infertile, and although ovulation can occur, corpora lutea are absent, demonstrating a defect in the luteal phase of follicular maturation (Fero et al. 1996; Kiyokawa et al. 1996; Nakayama et al. 1996). There is a uterine defect as well in p27−/− mice as fertilized oocytes can be detected after exogenous administration of supraphysiological amounts of gonadotrophins, but these fail to implant in the uterine wall at day 4.5 (Tong et al. 1998). In 75% of p27CK−/CK− ovaries (15 of 20), we did not detect any corpora lutea, and therefore these resembled the ovaries of p27-null mice (Fig. 1G, panels a–c). However, in 25% of the p27CK−/CK− mice (five of 20), corpora lutea were present. Remarkably, these were often greatly enlarged, hypercellular, and cystic (Fig. 1G, panel c, right panel). Luteal function was confirmed by the occurrence of infrequent pregnancies in p27CK−/CK− females. Embryonic development was noted as late as embryonic day 18–19 (E18–E19), but no live pups have been recovered.
Hyperplasia of chromaffin cells in the medulla of the adrenal gland was frequently observed in p27CK−/CK− mice and to a lesser extent in p27+/CK−, with a progressive thinning of the adrenal cortex over time and in some cases histological features of pheochromocytoma after 12 mo of age (Fig. 1H, panels b,c). Although some limited hyperplasia of the medulla was observed in p27−/− mice, as reported previously (Nakayama et al. 1996), it was not as extensive as that observed in p27CK− mice (Fig. 1H, panel a).
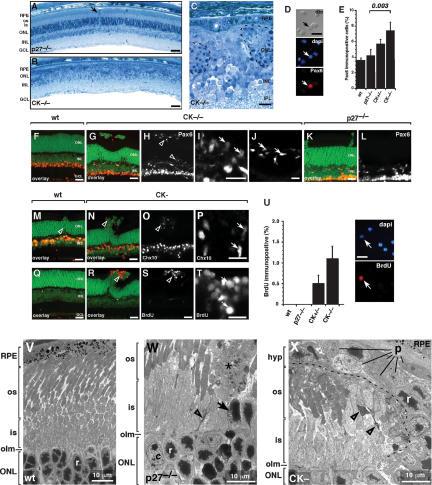
The p27CK− mutation had a striking effect on retinal organization. Unlike the retinae in p27-null or wild-type littermates, the outer nuclear layer (ONL) was hypocellular in adult p27CK− retinae and the laminar organization was severely disrupted, with immature cells from the inner nuclear layer (INL) protruding through the outer plexiform layer (OPL) (Fig. 2A–C; Supplementary Fig. 1A–C). In 50% (four of eight) of the p27CK− adult mice, the immature cells extended to the apical surface of the retina, disrupted the interaction between photoreceptor outer segments and the retinal pigment epithelium (RPE), and led to photoreceptor cell death (Fig. 2C). In the remaining p27CK− retinae, immature cells disrupted the OPL contributing to retinal dysplasia, but they did not extend to the apical surface.
Figure 2.
Hyperplasia in p27CK− retinae. (A–C) Toluidine blue-stained 1-μm plastic sections of adult p27−/− (A) and p27CK− (B) retinae. The ONL is severely disrupted and hypocellular, and the inner and outer segments are absent in the p27CK−. (C) Higher-magnification view of the p27CK− retina, showing hyperplasia of cells with an immature morphology invading the ONL (dashed line). (D,E) Dissociated cell scoring of adult retinae for Pax6 immunoreactivity. (D) Example of a Pax6 immunopositive cell from the p27CK− retina. (E) Five-hundred cells were scored in duplicate from three independent animals, and the mean and standard deviation are presented. There was a statistically significant increase in Pax6 immunopositive cells in the p27CK− retina as compared with the p27-null retina (P = 0.003). (F–L) Immunostained vibratome sections from wild-type, p27-null, and p27CK− retinae showing the pattern of Pax6 immunoreactive cells. Lower levels of Pax6 are consistent with retinal progenitor cells as compared with the high levels seen in differentiated amacrine cells in the INL. Ectopic Pax6 immunopositive cells were present at the apical edge of the retina (magnification in I) as well as the basal edge of the ONL (magnification in J). (K,L) p27-null retinae do not show this pattern of Pax6 expression associated with the mild form of retinal dysplasia seen in these retinae. (M) Chx10 is expressed in retinal progenitor cells and differentiated bipolar cells. (N,O) In the p27CK− retinae, Chx10 is expressed in the bipolar cells as well as ectopic cells extending apically to the outer limiting membrane (olm). (P) A magnified view of the cells indicated by the open arrowhead in O. The ectopic Chx10-expressing cells are not found in the p27-null retinae (shown in M). (Q–U) Adult retinae were labeled for 1 h with 10 μM BrdU, and then retinal sections were immunostained for BrdU along with dissociated retinal cells from the same animal (shown in U). (Q) Wild-type adult retinae show now BrdU immunopositive nuclei. (R,S) However, p27CK− retinae had BrdU immunopositive nuclei associated with the retinal dysplasia located through the ONL (open arrowhead). (T) A higher-magnification view of the BrdU immunopositive cells shown in R and S. (V–X) Transmission electron microscopy of wild-type (V), p27-null (W), and p27CK− (X) retinae. (V) In the wild-type retina, the outer limiting membrane (olm) separates the photoreceptor cell bodies from the photoreceptor inner segments (is) and outer segments (os). (W) In the p27-deficient retinae, there is an occasional cell that has been displaced apically beyond the outer limiting membrane (olm; arrow). These cells have the morphological features of rod photoreceptors. Such displaced photoreceptors are also associated with cellular debris in the dysplastic lesion (*). There is also evidence for photoreceptor cell death in the inner segments of photoreceptors in and around the retinal dysplasia in p27-null retinae (open arrowhead). (X) In the p27CK− retinae, hyperplastic lesions of cells with morphological features of progenitor cells (p) migrate apically and separate the photoreceptors (dashed line) from their overlying RPE cells. It is likely that this leads to photoreceptor cell death (open arrowheads). (r) Rod photoreceptor, (p) progenitor cell, (is) inner segments, (os) outer segments, (RPE) retinal pigment epithelium, (ONL) outer nuclear layer, (INL) inner nuclear layer, (GCL) ganglion cell layer, (olm) outer limiting membrane. Bars: A,B, 50 μm; all others, 10 μm.
The p27-null retinae show mild retinal dysplasia (Nakayama et al. 1996) characterized by protrusion of the photoreceptors through lesions in the outer limiting membrane caused by Müller glial cell reactive gliosis (Dyer and Cepko 2000). There is no evidence for the presence of immature proliferating cells in the adult p27-null retinae. In contrast, immature proliferating cells are found in the adult p27CK−/CK− retina. These cells expand from the INL into the ONL and lead to severe disruption of retinal lamination. They also cause retinal degeneration because the photoreceptor outer segments become detached from the overlying RPE as a result of the expansion of immature cells into the ONL. The modest retinal dysplasia previously observed in p27-null retinae is caused by Müller glial cell reactive gliosis. We found that p27CK− and p27-null retinae had similar proportions and patterns of reactive Müller glia (Supplementary Fig. 1D–J), indicating that the more severe phenotype observed in the p27CK−/CK− retinae is due to the expansion of immature cells and not reactive gliosis.
Our data suggested that the dysplasia and degeneration in the p27CK− retinae was due to ectopic proliferation of retinal progenitor cells that normally reside in the INL. In the normal retina, Pax6 is expressed in progenitor cells (Marquardt et al. 2001) and dissociated cell scoring established that Pax6 immunopositive cells were increased in the p27CK− retinae but not the p27-null retinae (Fig. 2D,E). Immunostaining showed that Pax6-expressing cells were abundant in regions with mild or pronounced laminar disruption and could extend all the way to the apical surface of the p27CK− retinae but not the p27-null retinae (Fig. 2F–L). Chx10, which is also expressed in retinal progenitor cells (Liu et al. 1994; Burmeister et al. 1996), showed the same pattern of expression unique to the p27CK− retinae (Fig. 2M–P). Real-time RT–PCR confirmed that retinal progenitor cell markers (Pax6 and cyclin D1) (Sicinski et al. 1995; Ma et al. 1998) were increased in the p27CK− retinae but not the p27-null retinae (Supplementary Fig. 1K,L). Taken together, the increase in Pax6, cyclin D1, and Chx10 expression combined with the disruption in retinal lamination (Fig. 2B,C) suggested that expansion of immature retinal cells from the INL contributed to the phenotype of the p27CK− retinae. To directly test for ectopic progenitor cell proliferation, we cultured the P30 retinae in BrdU for 1 h. Anti-BrdU immunostaining of retinal sections and dissociated cells confirmed that adult p27CK− retinae contained ectopically dividing cells in the regions where lamination was disrupted (Fig. 2Q–U). In addition, TUNEL analysis demonstrated that there was an increase in apoptotic cells in the p27CK− retinae and these dying cells were localized to the regions with ectopic proliferation and increased Pax6 and Chx10 immunopositive cells (data not shown). Ectopic proliferation was not found in p27-null retina at similar stages of development in our studies (Fig. 2U) or previously published experiments. Transmission electron micrographs provided additional evidence that immature proliferating cells persisted in the adult p27CK− retinae but not the p27-null or wild-type control retinae (Fig. 2V–X; Supplementary Fig. 1M–R). There were occasional dying rod photoreceptor cells in the p27-null retinae associated with the dysplastic lesions, but overall the rod outer segments and RPE were normal (Supplementary Fig. 2A–D). In the p27CK− retinae, there were areas completely lacking photoreceptor inner and outer segments and the RPE was juxtaposed to the ONL (Supplementary Fig. 2E). In other regions, when present, photoreceptor inner and outer segments were disorganized and degenerative in appearance (Supplementary Fig. 2F).
The p27CK− allele causes spontaneous lung tumors
In the pure 129S4 background, ∼26% of p27CK−/CK− mice developed lung adenomas and/or adenocarcinomas by 6 mo of age; this incidence was slightly lower in the mixed background (Table 1B). The p27CK−/CK− and p27+/CK− mice that developed tumors in the lung (n = 31) had an average of 1.69 tumors per animal. In contrast, spontaneous lung tumors were very infrequent in p27-null mice. A previously reported cohort of 34 p27-null mice had a zero incidence of lung tumors when sacrificed at ≥7 mo of age (Chien et al. 2006). Similarly to adrenal hyperplasia, ovarian hyperplasia, and T-cell lymphomagenesis, the lung tumor phenotype was genetically dominant, as 46% of p27+/CK− also developed lung adenomas and carcinomas by 12 mo of age (Table 1B). Genotyping of eight lung tumors from p27CK− heterozygous mice did not show any loss of heterozygosity of the p27 gene, thus confirming that the oncogenic action of the p27CK− allele is genetically dominant (data not shown). Note that p27CK− protein levels are elevated only marginally in p27+/CK− compared with p27+/+ tissues (data not shown), making it unlikely that the tumor-prone phenotype of these animals was due to overexpression of the mutant protein.
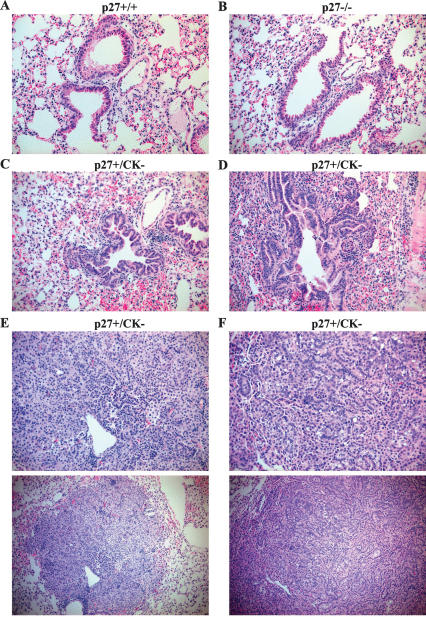
Histological analysis revealed that the lungs of p27CK− mice had an overall increased cellularity compared with wild-type and p27−/− lungs (Fig. 3). The lung tumors developing in these mice arose from the bronchioalveolar epithelium, and were positive for cytokeratins by immunohistochemistry using a pan-keratin antibody (data not shown). Indeed, morphological changes in the bronchioalveolar epithelium could be readily observed, with a progression from hyperplastic to dysplastic and to the development of adenomas and then tumors with the features of adenocarcinomas (Fig. 3C–F, respectively).
Figure 3.
Spontaneous lung tumor formation in p27CK− mice. Representative images of H&E-stained lung sections of wild-type (A), p27−/− (B), and p27+/CK− (C–F) mice (magnification, 20×); in the bottom panels in E and F, magnification is 4×. Panels C–F show the progressive changes occurring in the bronchioalveolar epithelium of p27CK− mice as follows: hyperplasia (C), early dysplastic changes (D), lung adenoma (E), and adenocarcinoma (F).
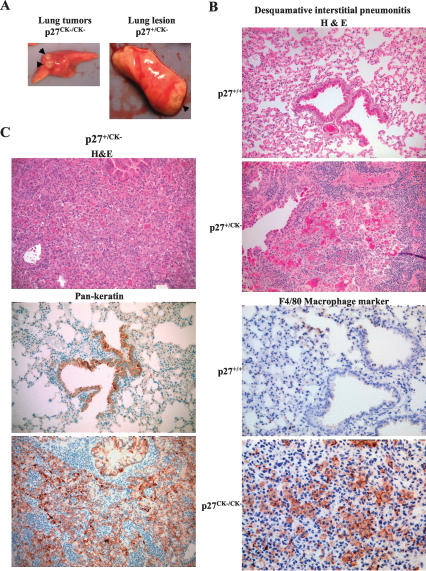
In addition to these nodular adenomas or adenocarcinomas, diffuse lesions on the lung were apparent in some cases but could not readily be classified grossly as tumors or as inflammatory lesions (Fig. 4A). Histological and immunohistochemical analyses were necessary to diagnose these lesions (Fig. 4B,C), and their incidence is reported in Table 1B. Histologically, these lesions presented the features of desquamative interstitial pneumonitis (DIP), with thickening of the alveolar epithelium and the presence of macrophages, neutrophils, and infiltrating lymphocytes, which were absent in wild-type or p27-null mice (Fig. 4B). In some cases, these histopathological features were confirmed by immunohistochemical staining for a macrophage marker (Fig. 4B, bottom panels). However, in other cases, similar lesions were negative for the macrophage marker (data not shown), and cytokeratin immunohistochemistry revealed the presence of diffusely infiltrating carcinomas with abundant lymphocytes (Fig. 4C, bottom panels). The middle panel in Figure 4C is a different field of the same lung section showing cytokeratin staining of the bronchioalveolar epithelium. Lung adenocarcinomas displaying the histopathology of DIP have been observed previously in humans, and were differentiated using cytokeratin markers (Mutton et al. 1998). Out of 14 mice with DIP-like lesions examined, six (42.8%) had keratin-positive carcinomas. Thus, our estimation of lung tumor incidence in p27CK− mice is underestimated due to the ambiguous appearance of the DIP-like lesions (Table 1B).
Figure 4.
DIP and adenocarcinomas masquerading as DIP in p27CK− mice. (A) Pictures of lungs dissected from p27CK/CK− and p27+/CK− mice showing the typical discrete adenomas or adenocarcinomas (left panel) and the diffuse lesions caused by the DIP or DIP-like adenocarcinomas (right panel). (B, top panels) H&E-stained sections showing normal lung structure in a wild-type and DIP in a p27+/CK− animal (magnification, 20×). (Bottom panels) Immunohistochemistry with the pan-macrophage marker F4/80 showing the absence of staining in the wild-type lung and abundant macrophage infiltration in the p27CK−/CK− lung (magnification, 40×). (C) The top panel shows a DIP-like lesion in a p27+/CK− lung section stained with H&E. The bottom panels show a consecutive section of the same lung stained with a pan-keratin antibody to stain the bronchioalveolar epithelium, as shown in a healthy portion of the section (middle picture). (Bottom picture) The DIP-like region of the section revealed a keratin-positive carcinoma (magnification, 20×).
Amplification of the BASC pool in p27CK− mice
The p27 protein is frequently mislocalized to the cytoplasm of human tumor cells, and there is now considerable evidence that cyclin–CDK-independent functions of p27 are likely to be mediated by the cytoplasmic pool of the protein (see Introduction). We therefore investigated the subcellular localization of the p27CK− protein in lung epithelial cells. Using p27−/− lung as a negative control, we found that wild-type p27 was expressed in the epithelium and localized largely to nuclei (Fig. 5A). p27CK− protein levels were increased (Besson et al. 2006), and the protein was abundantly expressed in both the nuclei and cytoplasm; likewise, in the tumors arising in p27CK− animals, p27 protein expression was both nuclear and cytoplasmic (Fig. 5A). Similarly, p27CK− protein could be readily detected in the cytoplasm of serum stimulated primary MEFs (Supplementary Fig. 3) and was abundantly found in the cytoplasm in the corpora lutea, which become abnormal in p27CK− animals (Supplementary Fig. 4).
Figure 5.
Cytoplasmic localization of p27 and dysregulated proliferation in lung epithelium in mice carrying the p27CK− allele. (A) Paraffin-embedded lung sections from p27−/−, p27+/+, p27CK−/CK−, and p27+/CK− mice were stained for p27. All images were acquired using the same exposure time and settings, with a 60× lens. (B–D). Sections of paraffin-embedded lungs from 5-mo-old wild-type (B), p27−/− (C), and p27CK−/CK− (D) mice were stained with a rabbit monoclonal antibody to Ki-67.
In an effort to determine the cell type of origin of the lung tumors arising in p27CK− animals, we performed immunohistochemical analyses with the proliferation marker Ki-67. In age-matched mice (5 mo), there was only marginal Ki-67 staining throughout the lungs of wild-type and p27−/− mice (Fig. 5B,C; Supplementary Fig. 5A,B); however, the lungs of p27CK−/CK− mice commonly presented abundant proliferation, especially in the bronchioalveolar epithelium (Fig. 5D; Supplementary Fig. 5C). Similar observations were made in p27+/CK− mice, but often at a later age (12 mo) (Supplementary Fig. 5D). Abundant proliferation in a lung adenoma is also shown in a p27+/CK− animal (Supplementary Fig. 5D).
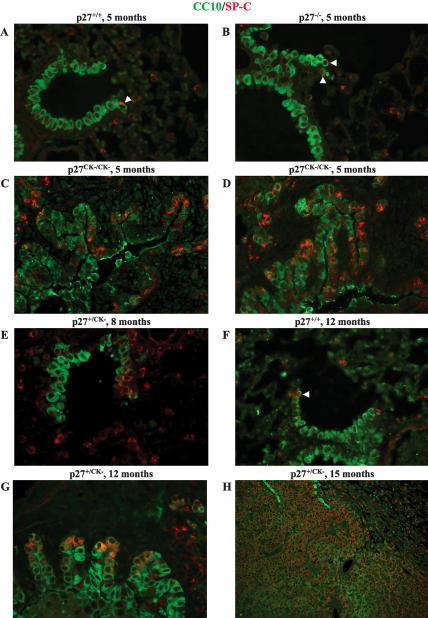
Several lines of evidence indicated that the lung tumors arising in p27CK− mice originated from the aberrant proliferation of the bronchioalveolar epithelium. Kim et al. (2005) recently identified a pool of BASCs. These cells can be identified by their simultaneous expression of protein markers specific for either the bronchiolar or alveolar epithelium (the Clara Cell-Specific Protein [CCSP or CC10] and the type II pneumocyte Surfactant protein-C [SP-C], respectively), and by their specific localization to the epithelial junction between terminal bronchioles (TBs) and alveoli. Furthermore, it was found that the number of BASCs was increased in mice expressing an activated K-Ras allele in the lung, suggesting that lung tumors in these mice arise from the dysregulated proliferation of these stem cells (Johnson et al. 2001; Kim et al. 2005). Following the terminology used previously (Kim et al. 2005; Pei et al. 2007), we refer to the SP-C/CCSP double-positive cells as BASCs, although the ability of these cells to self-renew and give rise to all lineages of lung epithelia in vivo has not been formally shown. To determine whether the BASC pool was disturbed in p27CK− mice, we carried out double immunofluorescence staining for CCSP and SP-C in the lungs. The lungs of 5-mo-old wild-type and p27−/− mice typically showed zero to two BASCs per TB (Fig. 6A,B; Supplementary Fig. 6A,B), as described previously (Kim et al. 2005). In contrast, in 5-mo-old p27CK−/CK− mice, there was an increase in the BASC number in most bronchioles, and some bronchioles exhibited a clear amplification of BASCs, and also displayed signs of hyperplasia (two individual mice) (Fig. 6C,D; Supplementary Figs. 6C,D, 7). Similar changes could be observed in p27+/CK− mice, albeit at a later age, first with localized increase of BASCs at the bronchioalveolar junction and then throughout the bronchioalveolar epithelium (8 and 12 mo old) (Fig. 6E,G; Supplementary Fig. 6E,G, respectively). The number of BASCs in wild-type mice did not change over time (Fig. 6F; Supplementary Figs. 6F, 7A,B). Figure 6H and Supplementary Fig. 6H show a lung tumor with positive staining for SP-C, despite its originating in the bronchioalveolar epithelium, and weak CCSP staining. The progressive loss of CCSP as lung tumors gain an aggressive phenotype has been reported previously (Linnoila et al. 2000; Ji et al. 2006).
Figure 6.
p27CK− causes amplification of the BASC pool. Paraffin-embedded lung sections were stained for CCSP/CC10 (goat anti-CC10, green) and SP-C (rabbit anti-SP-C, red) and merged images are shown. All images were acquired using a 60× objective, except for the image in H, which was acquired with a 20× lens. In A, B, and F, BASCs are indicated with an arrowhead. The individual images as well as the fields with Hoescht-stained nuclei for each panel are provided in Supplementary Figure 7A–H.
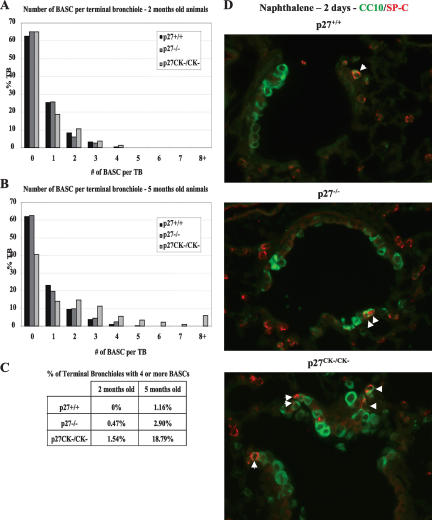
Quantitative analyses revealed a significant age-dependent increase in BASC number per TB in p27CK−/CK− mice compared with wild-type and p27−/− mice. For this, BASCs were counted in three animals per genotype per age, and a minimum of 170 and 240 TBs were counted at 2 mo and 5 mo of age, respectively, for each genotype. At 2 mo of age there were no significant difference in BASC numbers between genotypes (Fig. 7A). However, at 5 mo of age distribution in BASC cells numbers had altered dramatically (Fig. 7B). There was a significant increase (P < 0.001) in the percent of TBs that had four or more BASC cells in p27CK−/CK− mice (18.79%), compared with wild-type and p27−/− mice (1.16% and 2.9%, respectively) (Fig. 7C).
Figure 7.
Increased BASC number in p27CK−/CK− mice. (A–C) The number of BASC per TB was determined in 2-mo-old (A) and 5-mo-old (B) wild-type, p27−/−, and p27CK−/CK− mice. (C) Summary of the percentage of TB having four or more BASCs for each genotype. (D) BASCs are resistant to naphthalene injury. Immunofluorescence for CCSP/CC10 (green) and SP-C (red) of lung sections collected 48 h post-naphthalene injection. Images were acquired with a 60× lens. The individual images as well as the fields with Hoescht-stained nuclei for each panel are provided in Supplementary Figure 8A–C.
To provide further evidence that the CCSP/CC10 and SP-C double-positive cells we observed were bronchioalveolar stem cells, we treated mice with naphthalene, which selectively kills mature Clara cells in the lung epithelium, while sparing BASCs (Kim et al. 2005). Forty-eight hours post-naphthalene injection, there was extensive airway damage manifest by depletion of the Clara cell population, whereas the double-positive cells were unaffected, in both the wild-type and p27CK− mice (Fig. 7D; Supplementary Fig. 8A–C). The numbers of BASCs appeared to be increased in all genotypes at 48 and 72 h following exposure to Naphthalene (Kim et al. 2005; data not shown). Thus, it appears that p27 regulates, independently of its role in cell cycle regulation, the proliferation and fate of resident stem cells in the lung.
Discussion
p27Kip1 has functions in addition to its well defined role as a cyclin–CDK inhibitor and tumor suppressor (Sherr and Roberts 1999). It controls organization of the actin cytoskeleton and cell migration through the regulation of RhoA signaling both in vitro and in vivo, and also regulates neuronal differentiation during development through an interaction with Neurogenin-2 (Besson et al. 2004a, b; Crean et al. 2006; Kawauchi et al. 2006; Nguyen et al. 2006; Wu et al. 2006). Here, we have compared the phenotype of the p27CK− mice (Besson et al. 2006) with the p27 null (Fero et al. 1996) in order to determine the significance of the CDK-independent functions of p27 in vivo. The p27CK− mutation acts like a null mutation with respect to cyclin–CDK interaction and regulation. Consistent with this, p27CK− did not interact with cyclin–CDK complexes (Fig. 1A; Besson et al. 2006), and CDK-associated activity was equally elevated in both p27CK−/CK− and p27−/− tissues. A similar compensatory increase in the amount of p21 bound to cyclin A or CDK4 was observed in p27CK− and p27−/− cells compared with wild-type cells. Also, p27CK− had no effect on the ability of the wild-type p27 protein remaining in p27+/CK− cells to interact with cyclin–CDK complexes. Therefore, p27CK− did not act as a “dominant-negative” toward other CKIs. This was consistent with the fact that p27+/CK− have a size increase similar to but not greater than that observed in p27+/− animals (Fero et al. 1996; Besson et al. 2006). In addition, double or triple CKI deletions have been reported, and their phenotypes do not resemble that described in the present study (Malumbres et al. 2000). Our main findings were that unlike p27−/− mice, which only spontaneously develop pituitary gland adenomas, p27CK− mice developed hyperplastic lesions and tumors in multiple tissues. Moreover, the neoplastic lesions in both the lung and retina were associated with amplification of stem/progenitor cell populations.
Our results show that specific loss of the CDK inhibitory function of p27 uncovers a potent oncogenic activity resident elsewhere in the protein. Indeed, in heterozygous mice the p27CK− allele was dominant to wild-type p27 in causing tumorigenesis. This oncogenic activity of p27 helps to explain several earlier, puzzling observations: (1) In mammary and prostate tumor models in mice, the deletion of one p27 allele rendered the animals more susceptible to tumor development than homozygous deletion of p27, suggesting that partial down-regulation of the cyclin/CDK inhibitory activity but maintenance of other roles is selected for in the context of tumorigenesis (Muraoka et al. 2002; Gao et al. 2004). (2) The p27S10A knock-in mice are partially resistant to urethane-induced tumorigenesis, despite having levels of p27 protein similar to that of p27+/− mice (Besson et al. 2006). This was attributed to the fact that p27S10A, unlike wild-type p27, remained largely nuclear in the tumors where it binds to and inhibits cyclin–CDK complexes. Our data suggest that the confinement of this mutant protein to the nucleus may also diminish if not eliminate its oncogenic activity. (3) p27 is an unconventional tumor suppressor since mutation or silencing of its gene is extremely rare in tumors. During the course of tumorigenesis, there evidently is a selective advantage to preserving partial p27 expression, thus harnessing its ability to provide a growth advantage to the neoplasm (Kawamata et al. 1995; Morosetti et al. 1995; Pietenpol et al. 1995; Ponce-Castaneda et al. 1995; Spirin et al. 1996; Fero et al. 1998). (4) Clinical studies investigating the subcellular localization of p27 in tumors suggest that while the loss of nuclear p27 is commonly observed, mislocalization of p27 to the cytoplasm of tumor cells is correlated with high tumor grade and poor prognosis (Nakasu et al. 1999; Saez et al. 1999; Sanchez-Beato et al. 1999; Slingerland and Pagano 2000; Philipp-Staheli et al. 2001; Kouvaraki et al. 2002; Liang et al. 2002; Besson et al. 2004a; Rosen et al. 2005; Qi et al. 2006).
To date, activation of the Ras and Akt/PKB signaling pathways, which are commonly deregulated in human neoplasms, have been found to induce the cytoplasmic localization of p27 by inducing its phosphorylation on distinct residues (Liu et al. 2000; Kouvaraki et al. 2002; Liang et al. 2002; Kfir et al. 2005; Besson et al. 2006). We found that significant amounts of p27CK− localized in the cytoplasm in the lung epithelium, luteal cells, and primary fibroblasts, as well as in lung tumors. Thus, there is a correlation between the cytoplasmic localization of p27 and the abnormalities arising in the corresponding tissues, suggesting that the oncogenic role played by p27 likely depends on the cytoplasmic localization of the protein.
One function of cytoplasmic p27 that may be related to its oncogenic action is regulation of Rho activity (Besson et al. 2004b). p27 binds directly to RhoA, interfering with the interaction between RhoA and Rho-GEFs and thereby decreasing the amount of active Rho-GTP. Changes in the cytoplasmic abundance of p27 therefore alter signaling downstream from Rho, causing changes in the assembly of the actin cytoskeleton and focal adhesion complexes. Altered Rho signaling is oncogenic, possibly through the effects that these changes may have on cell motility, cell polarity, cell proliferation, and mitosis (Gomez del Pulgar et al. 2005; Wang et al. 2007). We have shown that p27CK− MEFs do not have the changes in the actin cytoskeleton, focal adhesions, and cell motility seen in p27-null MEFs, confirming that the p27CK− retains its ability to interact with Rho (data not shown; see also Besson et al. 2004b). Moreover, in preliminary experiments we have used the phosphorylation state of cofilin as a biomarker for Rho activity, and have observed misregulation of the Rho pathway in vivo in the lung epithelial cells of p27CK− mice (data not shown).
We point out that there may be a requirement for the N terminus of p27 to mediate its cyclin–CDK independent functions, perhaps to regulate its subcellular localization via phosphorylation on Ser10 (McAllister et al. 2003; Besson et al. 2004b). Thus a “p27-null” mouse lacking only the 51 N-terminal amino acids of the protein (Δ51) shares most of the phenotypes resulting from complete deletion of the cdkn1b gene, but does not recapitulate the p27CK− phenotype (Kiyokawa et al. 1996).
A major finding of our experiments was that the p27CK− protein caused the expansion of BASCs, thus providing further insight into the mechanism of p27’s oncogenic function. We propose that deregulated proliferation and/or differentiation of BASCs progressively gave rise to the histological changes observed in the lungs of p27CK− mice, namely hyperplasia and dysplasia of the bronchioalveolar epithelium, followed by the development of adenomas and adenocarcinomas. In contrast to the p27CK− mouse, overt alteration in BASC numbers was not observed in p27−/− mice. Nevertheless, Ras-induced tumorigenesis in the lung, which is thought to arise from BASC expansion and transformation (Kim et al. 2005), occurred at a higher rate in p27−/− mice compared with wild-type controls (Besson et al. 2006). Thus, at least in the context of Ras-dependent tumorigenesis, p27 deficiency may affect stem cell dynamics in the lung, suggesting that loss of the cyclin–CDK inhibitory function of p27 is likely to contribute to stem cell expansion. Indeed, complete deletion of the cdkn1b gene has previously been linked to modest alterations in stem cell dynamics. In p27−/− mice, there is expansion of committed progenitor cells in the hematopoietic and glial lineages, although pluripotent hematopoietic stem cells were not affected (Fero et al. 1996; Ohnuma et al. 1999; Cheng et al. 2000). Increased proliferation of progenitor cells in the sensory epithelia of the inner ear is also increased in p27−/− mice (Chen and Segil 1999; Levine et al. 2000; Dyer and Cepko 2001).
In the retina, we also observed a much greater effect of the p27CK− mutation on progenitor cell proliferation than deletion of the p27 gene. Based on the similar extent of Müller glial cell reactive gliosis in p27-null and p27CK− retinae, we concluded that the CDK inhibitory activity of p27 was required for maintaining these cells in their quiescent state in the absence of neuronal stress or injury. However, there was no evidence for hyperplasia in the adult p27-null retina nor was there any evidence for a failure of retinal neurons or glia to differentiate normally in the absence of p27. In contrast, the adult p27CK− mice displayed massive retinal dysplasia that was associated with the presence of proliferating immature retinoblasts, and a dramatic reduction in mature rod photoreceptors. The immature cells expressed progenitor cell markers such as Pax6 and Chx10, incorporated BrdU, and retained an immature morphology as determined from transmission electron microscopy. Importantly, this phenotype was strikingly similar to early stage lesions in Rb and Rb;p107-null retinae. However, similar to Rb-null retinae, the p27CK− mutation alone was not sufficient to cause progression to advanced retinoblastoma. These observations suggested that p27 had a CDK-independent function in the developing retina, which could lead to severe hyperplasia and subsequent retinal degeneration when it was uncoupled from CDK inhibition. The independent effects of p27 on Müller glia and immature retinoblasts clearly distinguished the unique domains and functions of p27 in the developing retina.
In the two tissues examined in detail here, retina and lung, pathological progenitor/stem cell expansion was greatly exacerbated in p27CK− mice compared with the p27 null. We have not directly compared hematopoietic progenitors in the p27CK− and p27−/− mice. However, the fact that p27CK− animals developed lymphomas, DIP in the lung, and histiocytomas in the spleen at a substantially higher rate than p27−/− mice suggests a possible deregulation of hematopoietic progenitors in these tissues as well. The strikingly more severe phenotype of the p27CK− mutation compared with the p27 null suggests that stem cell expansion and tumorigenesis in the p27CK− mouse likely arose from the complementary effects of losing the CDK inhibitory, tumor-suppressive function of p27 while retaining the other functions mediated by the remainder of the protein.
Another important phenotype of p27CK− mice was the development of DIP. DIP is a disease of unknown etiology characterized by diffuse alveolar infiltration of macrophages displaying an epithelial morphology and other immune cells. Interestingly, 90% of cases of DIP are linked to tobacco smoking, and exposure to organic or inorganic dusts (such as asbestos) is also a risk factor to the development of DIP in humans (Ryu et al. 2001). Exposure to these agents is also commonly associated with the development of lung cancers, and there is evidence to suggest that DIP may facilitate lung tumorigenesis (Genereux and Merriman 1973; Ayvazian 1974; Mutton et al. 1998). We are currently testing whether the onset of DIP may be a precursor to the development of lung tumors in our model.
Overall, a model taking into account our current knowledge of p27 biology is that p27 suppresses tumorigenesis by inhibiting cyclin/CDK activity in the nucleus, but also has other functions in the cytoplasm, including the regulation of RhoA signaling, that are potentially oncogenic. While both roles may be important for homeostasis in normal cells, inactivation of the nuclear function and maintenance (or even exacerbation) of the cytoplasmic functions may be selected for during tumor progression.
Materials and methods
Mice
p27+/− mice in the 129S4 background were bred to generate the p27−/− mice used in this study and were genotyped as described previously (Fero et al. 1996). p27+/CK− mice in the 129S4 or 129S4/C57BL6 backgrounds were bred to generate the p27+/+, p27+/CK−, and p27CK−/CK− mice and were genotyped as described previously (Besson et al. 2006). The historical p27−/− mice mentioned in this study were kept in the same facility and in similar conditions as the other mice used here. At the indicated age, mice were sacrificed and dissected. Tissues were examined for apparent lesions and photographed with a Nikon Coolpix 5700 digital camera, and lung tumors were counted and their size measured. Tissues were fixed overnight in formalin, embedded in paraffin, and sectioned (5-μm thickness) for histochemistry with hematoxylin and eosin. The mice were maintained and procedures were performed in accordance with federal and institutional regulations.
Western blotting, immunoprecipitations, and kinase assays
These techniques were performed as described previously (Besson and Yong 2000; Besson et al. 2006), except that tissues were lysed in 1% NP-40 lysis buffer, dounced, and sonicated. Antibodies used were as follows: monoclonal antibodies to p21 (BD-Pharmingen), CDK2 (D12), cyclin D1 (A12), p27 (F8) from Santa Cruz Biotechnology, Grb2 (BD-Transduction Laboratories), and CDK4 (Ab6, Neomarker); polyclonal antibodies to CDK2 (M2), cyclin A (H432), p27 (C19), cyclin D1 (H295), CDK4 (C22) from Santa Cruz Biotechnology, phospho-Ser780 Rb (Cell Signaling).
Immunohistochemistry
Paraffin sections were deparaffinized. Immunostainings using the monoclonal antibodies F4/80 pan-macrophage marker (Serotec, BM8), pan-keratin (4,5,6,8,13,18), and Ki67 (SP6, Neomarker) were performed according to the methods provided in the ImmunoCruz Staining System Kit (Santa Cruz Biotechnology). Briefly, hydrated tissue sections were steamed for 30 min in 0.1% citrate acid and blocked in serum for 20 min at room temperature. Samples were subsequently incubated with the indicated antibody for 1 h and with biotinylated secondary antibody for 30 min, and were visualized using the chromogen 3′3′-diaminobenzidine (DAB). Slides were counterstained with Mayer’s hematoxylin for 20 sec and rinsed abundantly in H2O before dehydration and mounting.
BASC and p27 immunofluorescence
This procedure was adapted from Kim et al. (2005). Briefly, paraffin sections were deparaffinized. Antigens were unmasked by heat treatment for 30 min at 95°C with sections covered with 10 mM sodium citrate. Slides were washed 5 min each in ddH2O twice, twice in PBS/0.2% Triton X-100, and once in PBS. Slides were then incubated for 30 min with Image-it FX signal enhancer (Invitrogen), washed twice in PBS/0.2% Triton X-100, and once in PBS, and incubated 30 min in block solution (PBS/0.2% Triton X-100/3% BSA complemented with 10% donkey serum [Sigma]). The block solution was removed and the slides were incubated overnight at 4°C with primary antibodies diluted in block solution (goat polyclonal anti-CC10, T18, sc9772, Santa Cruz Biotechnology at 1/50 dilution; rabbit polyclonal anti-SP-C, AB 3786, Chemicon, at 1/250 dilution). For p27 immunofluorescence, the polyclonal p27 (C19, Santa Cruz Biotechnology) antibody was used at 1/100. Slides were washed three times in PBS/0.2% Triton X-100, and incubated for 30 min at 37°C with secondary antibodies (donkey anti-goat Cy2 and donkey anti-rabbit Cy3, at 1/250 dilution). Slides were washed three times in PBS, and cellular DNA was stained with Hoescht diluted in the first wash. Slides were mounted and images were acquired on a Nikon E800 microscope using a Spot CCD camera. For BASC quantification, a minimum of 170 and 240 TBs were counted for each genotype at 2 mo and 5 mo, respectively, and at least three mice per age per genotype were counted. We performed a χ2 analysis to determine whether genotype had a significant effect on the number of BASCs observed per TB at 5 mo of age. We compared all three genotypes (p27+/+, p27−/−, and p27CK−/CK−) using a 3 × 9 table. We then compared the distribution of BASC per TB in p27+/+ versus p27−/− mice using a 2 × 9 table. Finally, we compared the distribution of BASC per TB in p27CK−/CK− with the other two genotypes by summing the observations in the p27+/+ and p27−/− mice.
Antibodies, immunostaining, BrdU, and TUNEL studies
We immunolabeled retinal vibratome sections and dissociated retinae (500 cells per sample in triplicate) as previously described. To label S-phase retinal progenitor cells, we incubated freshly dissected retinae in 1 mL of explant culture medium containing 10 μM BrdU for 1 h at 37°C. BrdU detection was carried out as described previously. For apoptosis analysis, the colorimetric TUNEL apoptosis system (Promega) was used on vibratome sections per the manufacturer’s instructions; however, for detection, we used tyramide-Cy3 (NEN) rather than the colorimetric substrate.
Real-time RT–PCR
Real-time RT–PCR experiments were performed using the ABI 7900 HT Sequence Detection System (Applied Biosystems). Primers and probes were designed using Primer Express software (Applied Biosystems). TaqMan probes were synthesized with 5′-FAM and 3′-BHQ. RNA was prepared using Trizol, and cDNA was synthesized using the SuperScript system (Invitrogen). Samples were analyzed in duplicate and normalized to Gapdh, Gpi1, and Mmt2 expression levels.
Transmission electron microscopy
Animals were anesthetized with avertin until a loss of deep tendon reflexes. Transcardial perfusion was performed with carboxygenated Ames Medium supplemented with 40 mM glucose to clear the vasculature, followed by perfusion with Sorenson’s phosphate buffer (pH 7.2) with 2% EM-grade paraformaldehyde and 1% EM-grade glutaraldehyde. Eyes were then harvested, a slit was made in the cornea to aid in diffusion, and the tissue was placed in 3% glutaraldehyde in Sorenson’s phosphate buffer overnight. Tissue was washed with 0.2 M cacodylate buffer in 5% sucrose, post-fixed in 1% OsO4, embedded, sectioned, and viewed by transmission electron microscopy.
Acknowledgments
We thank Drs. Carla Kim and Tyler Jacks (MIT) for technical advice. We thank Dr. Julie Randolph-Habecker and the members of the Experimental Histopathology shared resource laboratory at the Fred Hutchinson Cancer Research Center. We thank Sharon Frase of the Integrated Microscopy Center at the University of Memphis for assistance with EM analysis. We thank Dr. Hilary Coller (Princeton University) for performing the statistical analyses. We thank Dr. David Madtes for his help with the naphthalene experiment. We thank members of the Roberts, Clurman, Kemp, and Fero laboratories for helpful discussions. A.B. is a Leukemia and Lymphoma Society Special Fellow. S.L.D. is supported by grants from Fight for Sight and the Gerwin Fellowship Foundation. This work was supported by grants from the NIH, Cancer Center Support from the National Cancer Institute, the American Cancer Society, Research to Prevent Blindness, the Pearle Vision Foundation, the International Retinal Research Foundation, and the American Lebanese Syrian Associated Charities (ALSAC) to M.A.D. M.A.D. is a Pew Scholar. This work was supported by NIH grants 1K08HL073670-01 (to H.C.H), R01084069 (to B.E.C.), and 1R01CA118043 (to J.M.R.).
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1556607
References
- Ayvazian L.F. Desquamative interstitial pneumonia. A systemic disease and precancerous lesion? J. Med. Soc. N. J. 1974;71:115–119. [PubMed] [Google Scholar]
- Besson A., Yong V.W., Yong V.W. Involvement of p21(Waf1/Cip1) in protein kinase C α-induced cell cycle progression. Mol. Cell. Biol. 2000;20:4580–4590. doi: 10.1128/mcb.20.13.4580-4590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A., Assoian R.K., Roberts J.M., Assoian R.K., Roberts J.M., Roberts J.M. Regulation of the cytoskeleton: An oncogenic function for CDK inhibitors? Nat. Rev. Cancer. 2004a;4:948–955. doi: 10.1038/nrc1501. [DOI] [PubMed] [Google Scholar]
- Besson A., Gurian-West M., Schmidt A., Hall A., Roberts J.M., Gurian-West M., Schmidt A., Hall A., Roberts J.M., Schmidt A., Hall A., Roberts J.M., Hall A., Roberts J.M., Roberts J.M. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes & Dev. 2004b;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A., Gurian-West M., Chen X., Kelly-Spratt K.S., Kemp C.J., Roberts J.M., Gurian-West M., Chen X., Kelly-Spratt K.S., Kemp C.J., Roberts J.M., Chen X., Kelly-Spratt K.S., Kemp C.J., Roberts J.M., Kelly-Spratt K.S., Kemp C.J., Roberts J.M., Kemp C.J., Roberts J.M., Roberts J.M. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization and tumor suppression. Genes & Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M., Novak J., Liang M.Y., Basu S., Ploder L., Hawes N.L., Vidgen D., Hoover F., Goldman D., Kalnins V.I., Novak J., Liang M.Y., Basu S., Ploder L., Hawes N.L., Vidgen D., Hoover F., Goldman D., Kalnins V.I., Liang M.Y., Basu S., Ploder L., Hawes N.L., Vidgen D., Hoover F., Goldman D., Kalnins V.I., Basu S., Ploder L., Hawes N.L., Vidgen D., Hoover F., Goldman D., Kalnins V.I., Ploder L., Hawes N.L., Vidgen D., Hoover F., Goldman D., Kalnins V.I., Hawes N.L., Vidgen D., Hoover F., Goldman D., Kalnins V.I., Vidgen D., Hoover F., Goldman D., Kalnins V.I., Hoover F., Goldman D., Kalnins V.I., Goldman D., Kalnins V.I., Kalnins V.I., et al. Ocular retardation mouse caused by Chx10 homeobox null allele: Impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- Catzavelos C., Bhattacharya N., Ung Y.C., Wilson J.A., Roncari L., Sandhu C., Shaw P., Yeger H., Morava-Protzner I., Kapusta L., Bhattacharya N., Ung Y.C., Wilson J.A., Roncari L., Sandhu C., Shaw P., Yeger H., Morava-Protzner I., Kapusta L., Ung Y.C., Wilson J.A., Roncari L., Sandhu C., Shaw P., Yeger H., Morava-Protzner I., Kapusta L., Wilson J.A., Roncari L., Sandhu C., Shaw P., Yeger H., Morava-Protzner I., Kapusta L., Roncari L., Sandhu C., Shaw P., Yeger H., Morava-Protzner I., Kapusta L., Sandhu C., Shaw P., Yeger H., Morava-Protzner I., Kapusta L., Shaw P., Yeger H., Morava-Protzner I., Kapusta L., Yeger H., Morava-Protzner I., Kapusta L., Morava-Protzner I., Kapusta L., Kapusta L., et al. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: Prognostic implications in primary breast cancer. Nat. Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- Chen P., Segil N., Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Cheng T., Rodrigues N., Dombkowski D., Stier S., Scadden D.T., Rodrigues N., Dombkowski D., Stier S., Scadden D.T., Dombkowski D., Stier S., Scadden D.T., Stier S., Scadden D.T., Scadden D.T. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat. Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- Chien W.M., Rabin S., Macias E., de Miliani Marval P.L., Garrison K., Orthel J., Rodriguez-Puebla M., Fero M.L., Rabin S., Macias E., de Miliani Marval P.L., Garrison K., Orthel J., Rodriguez-Puebla M., Fero M.L., Macias E., de Miliani Marval P.L., Garrison K., Orthel J., Rodriguez-Puebla M., Fero M.L., de Miliani Marval P.L., Garrison K., Orthel J., Rodriguez-Puebla M., Fero M.L., Garrison K., Orthel J., Rodriguez-Puebla M., Fero M.L., Orthel J., Rodriguez-Puebla M., Fero M.L., Rodriguez-Puebla M., Fero M.L., Fero M.L. Genetic mosaics reveal both cell-autonomous and cell-nonautonomous function of murine p27Kip1. Proc. Natl. Acad. Sci. 2006;103:4122–4127. doi: 10.1073/pnas.0509514103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S., Flanagan W.M., Nourse J., Roberts J.M., Flanagan W.M., Nourse J., Roberts J.M., Nourse J., Roberts J.M., Roberts J.M. Requirements of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Crean J.K., Furlong F., Mitchell D., McArdle E., Godson C., Martin F., Furlong F., Mitchell D., McArdle E., Godson C., Martin F., Mitchell D., McArdle E., Godson C., Martin F., McArdle E., Godson C., Martin F., Godson C., Martin F., Martin F. Connective tissue growth factor/CCN2 stimulates actin disassembly through Akt/protein kinase B-mediated phosphorylation and cytoplasmic translocation of p27Kip-1. FASEB J. 2006;20:1712–1714. doi: 10.1096/fj.05-5010fje. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A., De Acetis M., Koff A., Cordon-Cardo C., Pandolfi P.P., De Acetis M., Koff A., Cordon-Cardo C., Pandolfi P.P., Koff A., Cordon-Cardo C., Pandolfi P.P., Cordon-Cardo C., Pandolfi P.P., Pandolfi P.P. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat. Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- Dyer M.A., Cepko C.L., Cepko C.L. Control of Muller glial cell proliferation and activation following retinal injury. Nat. Neurosci. 2000;3:873–880. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- Dyer M.A., Cepko C.L., Cepko C.L. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J. Neurosci. 2001;21:4259–4271. doi: 10.1523/JNEUROSCI.21-12-04259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fero M.L., Rivkin M., Tasch M., Porter P., Carow C.E., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Rivkin M., Tasch M., Porter P., Carow C.E., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Tasch M., Porter P., Carow C.E., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Porter P., Carow C.E., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Carow C.E., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Tsai L.H., Broudy V., Perlmutter R.M., Broudy V., Perlmutter R.M., Perlmutter R.M., et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Fero M.L., Randel E., Gurley K.E., Roberts J.M., Kemp C.J., Randel E., Gurley K.E., Roberts J.M., Kemp C.J., Gurley K.E., Roberts J.M., Kemp C.J., Roberts J.M., Kemp C.J., Kemp C.J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin D.S., Godfrey V.L., O’Brien D.A., Deng C., Xiong Y., Godfrey V.L., O’Brien D.A., Deng C., Xiong Y., O’Brien D.A., Deng C., Xiong Y., Deng C., Xiong Y., Xiong Y. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol. Cell. Biol. 2000;20:6147–6158. doi: 10.1128/mcb.20.16.6147-6158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Ouyang X., Banach-Petrosky W., Borowsky A.D., Lin Y., Kim M., Lee H., Shih W.J., Cardiff R.D., Shen M.M., Ouyang X., Banach-Petrosky W., Borowsky A.D., Lin Y., Kim M., Lee H., Shih W.J., Cardiff R.D., Shen M.M., Banach-Petrosky W., Borowsky A.D., Lin Y., Kim M., Lee H., Shih W.J., Cardiff R.D., Shen M.M., Borowsky A.D., Lin Y., Kim M., Lee H., Shih W.J., Cardiff R.D., Shen M.M., Lin Y., Kim M., Lee H., Shih W.J., Cardiff R.D., Shen M.M., Kim M., Lee H., Shih W.J., Cardiff R.D., Shen M.M., Lee H., Shih W.J., Cardiff R.D., Shen M.M., Shih W.J., Cardiff R.D., Shen M.M., Cardiff R.D., Shen M.M., Shen M.M., et al. A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. 2004;101:17204–17209. doi: 10.1073/pnas.0407693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genereux G.P., Merriman J.E., Merriman J.E. Desquamative interstitial pneumonia: Progression to the end-stage lung and the unusual complication of alveolar cell carcinoma: Case report. J. Can. Assoc. Radiol. 1973;24:144–149. [PubMed] [Google Scholar]
- Georgitsi M., Raitila A., Karhu A., van der Luijt R.B., Aalfs C.M., Sane T., Vierimaa O., Makinen M.J., Tuppurainen K., Paschke R., Raitila A., Karhu A., van der Luijt R.B., Aalfs C.M., Sane T., Vierimaa O., Makinen M.J., Tuppurainen K., Paschke R., Karhu A., van der Luijt R.B., Aalfs C.M., Sane T., Vierimaa O., Makinen M.J., Tuppurainen K., Paschke R., van der Luijt R.B., Aalfs C.M., Sane T., Vierimaa O., Makinen M.J., Tuppurainen K., Paschke R., Aalfs C.M., Sane T., Vierimaa O., Makinen M.J., Tuppurainen K., Paschke R., Sane T., Vierimaa O., Makinen M.J., Tuppurainen K., Paschke R., Vierimaa O., Makinen M.J., Tuppurainen K., Paschke R., Makinen M.J., Tuppurainen K., Paschke R., Tuppurainen K., Paschke R., Paschke R., et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J. Clin. Endocrinol. Metab. 2007 doi: 10.1210/jc.2006-2843. [DOI] [PubMed] [Google Scholar]
- Gomez del Pulgar T., Benitah S.A., Valeron P.F., Espina C., Lacal J.C., Benitah S.A., Valeron P.F., Espina C., Lacal J.C., Valeron P.F., Espina C., Lacal J.C., Espina C., Lacal J.C., Lacal J.C. Rho GTPase expression in tumourigenesis: Evidence for a significant link. Bioessays. 2005;27:602–613. doi: 10.1002/bies.20238. [DOI] [PubMed] [Google Scholar]
- Ji H., Houghton A.M., Mariani T.J., Perera S., Kim C.B., Padera R., Tonon G., McNamara K., Marconcini L.A., Hezel A., Houghton A.M., Mariani T.J., Perera S., Kim C.B., Padera R., Tonon G., McNamara K., Marconcini L.A., Hezel A., Mariani T.J., Perera S., Kim C.B., Padera R., Tonon G., McNamara K., Marconcini L.A., Hezel A., Perera S., Kim C.B., Padera R., Tonon G., McNamara K., Marconcini L.A., Hezel A., Kim C.B., Padera R., Tonon G., McNamara K., Marconcini L.A., Hezel A., Padera R., Tonon G., McNamara K., Marconcini L.A., Hezel A., Tonon G., McNamara K., Marconcini L.A., Hezel A., McNamara K., Marconcini L.A., Hezel A., Marconcini L.A., Hezel A., Hezel A., et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25:2105–2112. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- Johnson L., Mercer K., Greenbaum D., Bronson R.T., Crowley D., Tuveson D.A., Jacks T., Mercer K., Greenbaum D., Bronson R.T., Crowley D., Tuveson D.A., Jacks T., Greenbaum D., Bronson R.T., Crowley D., Tuveson D.A., Jacks T., Bronson R.T., Crowley D., Tuveson D.A., Jacks T., Crowley D., Tuveson D.A., Jacks T., Tuveson D.A., Jacks T., Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- Kawamata N., Morosetti R., Miller C.W., Park D., Spirin K.S., Nakamaki T., Takeuchi S., Hatta Y., Simpson J., Wilcyznski S., Morosetti R., Miller C.W., Park D., Spirin K.S., Nakamaki T., Takeuchi S., Hatta Y., Simpson J., Wilcyznski S., Miller C.W., Park D., Spirin K.S., Nakamaki T., Takeuchi S., Hatta Y., Simpson J., Wilcyznski S., Park D., Spirin K.S., Nakamaki T., Takeuchi S., Hatta Y., Simpson J., Wilcyznski S., Spirin K.S., Nakamaki T., Takeuchi S., Hatta Y., Simpson J., Wilcyznski S., Nakamaki T., Takeuchi S., Hatta Y., Simpson J., Wilcyznski S., Takeuchi S., Hatta Y., Simpson J., Wilcyznski S., Hatta Y., Simpson J., Wilcyznski S., Simpson J., Wilcyznski S., Wilcyznski S., et al. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 1995;55:2266–2269. [PubMed] [Google Scholar]
- Kawauchi T., Chihama K., Nabeshima Y., Hoshino M., Chihama K., Nabeshima Y., Hoshino M., Nabeshima Y., Hoshino M., Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat. Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- Kfir S., Ehrlich M., Goldshmid A., Liu X., Kloog Y., Henis Y.I., Ehrlich M., Goldshmid A., Liu X., Kloog Y., Henis Y.I., Goldshmid A., Liu X., Kloog Y., Henis Y.I., Liu X., Kloog Y., Henis Y.I., Kloog Y., Henis Y.I., Henis Y.I. Pathway- and expression level-dependent effects of oncogenic N-Ras: p27Kip1 mislocalization by the Ral–GEF pathway and Erk-mediated interference with Smad signaling. Mol. Cell. Biol. 2005;25:8239–8250. doi: 10.1128/MCB.25.18.8239-8250.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.F., Jackson E.L., Woolfenden A.E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T., Jackson E.L., Woolfenden A.E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T., Woolfenden A.E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T., Vogel S., Crowley D., Bronson R.T., Jacks T., Crowley D., Bronson R.T., Jacks T., Bronson R.T., Jacks T., Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H., Kineman R.D., Manova-Todorova K.O., Soares V.C., Hoffman E.S., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A., Kineman R.D., Manova-Todorova K.O., Soares V.C., Hoffman E.S., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A., Manova-Todorova K.O., Soares V.C., Hoffman E.S., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A., Soares V.C., Hoffman E.S., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A., Hoffman E.S., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A., Khanam D., Hayday A.C., Frohman L.A., Koff A., Hayday A.C., Frohman L.A., Koff A., Frohman L.A., Koff A., Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Kouvaraki M., Gorgoulis V.G., Rassidakis G.Z., Liodis P., Markopoulos C., Gogas J., Kittas C., Gorgoulis V.G., Rassidakis G.Z., Liodis P., Markopoulos C., Gogas J., Kittas C., Rassidakis G.Z., Liodis P., Markopoulos C., Gogas J., Kittas C., Liodis P., Markopoulos C., Gogas J., Kittas C., Markopoulos C., Gogas J., Kittas C., Gogas J., Kittas C., Kittas C. High expression levels of p27 correlate with lymph node status in a subset of advanced invasive breast carcinomas. Cancer. 2002;94:2454–2465. doi: 10.1002/cncr.10505. [DOI] [PubMed] [Google Scholar]
- Levine E.M., Close J., Fero M., Ostrovsky A., Reh T.A., Close J., Fero M., Ostrovsky A., Reh T.A., Fero M., Ostrovsky A., Reh T.A., Ostrovsky A., Reh T.A., Reh T.A. p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Dev. Biol. 2000;219:299–314. doi: 10.1006/dbio.2000.9622. [DOI] [PubMed] [Google Scholar]
- Liang J., Zubovitch J., Petrocelli T., Kotchetkov R., Connor M.K., Han K., Lee J.H., Ciarallo S., Catzavelos C., Beniston R., Zubovitch J., Petrocelli T., Kotchetkov R., Connor M.K., Han K., Lee J.H., Ciarallo S., Catzavelos C., Beniston R., Petrocelli T., Kotchetkov R., Connor M.K., Han K., Lee J.H., Ciarallo S., Catzavelos C., Beniston R., Kotchetkov R., Connor M.K., Han K., Lee J.H., Ciarallo S., Catzavelos C., Beniston R., Connor M.K., Han K., Lee J.H., Ciarallo S., Catzavelos C., Beniston R., Han K., Lee J.H., Ciarallo S., Catzavelos C., Beniston R., Lee J.H., Ciarallo S., Catzavelos C., Beniston R., Ciarallo S., Catzavelos C., Beniston R., Catzavelos C., Beniston R., Beniston R., et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- Linnoila R.I., Szabo E., DeMayo F., Witschi H., Sabourin C., Malkinson A., Szabo E., DeMayo F., Witschi H., Sabourin C., Malkinson A., DeMayo F., Witschi H., Sabourin C., Malkinson A., Witschi H., Sabourin C., Malkinson A., Sabourin C., Malkinson A., Malkinson A. The role of CC10 in pulmonary carcinogenesis: From a marker to tumor suppression. Ann. N. Y. Acad. Sci. 2000;923:249–267. doi: 10.1111/j.1749-6632.2000.tb05534.x. [DOI] [PubMed] [Google Scholar]
- Liu I.S., Chen J.D., Ploder L., Vidgen D., van der Kooy D., Kalnins V.I., McInnes R.R., Chen J.D., Ploder L., Vidgen D., van der Kooy D., Kalnins V.I., McInnes R.R., Ploder L., Vidgen D., van der Kooy D., Kalnins V.I., McInnes R.R., Vidgen D., van der Kooy D., Kalnins V.I., McInnes R.R., van der Kooy D., Kalnins V.I., McInnes R.R., Kalnins V.I., McInnes R.R., McInnes R.R. Developmental expression of a novel murine homeobox gene (Chx10): Evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Liu X., Sun Y., Ehrlich M., Lu T., Kloog Y., Weinberg R.A., Lodish H.F., Henis Y.I., Sun Y., Ehrlich M., Lu T., Kloog Y., Weinberg R.A., Lodish H.F., Henis Y.I., Ehrlich M., Lu T., Kloog Y., Weinberg R.A., Lodish H.F., Henis Y.I., Lu T., Kloog Y., Weinberg R.A., Lodish H.F., Henis Y.I., Kloog Y., Weinberg R.A., Lodish H.F., Henis Y.I., Weinberg R.A., Lodish H.F., Henis Y.I., Lodish H.F., Henis Y.I., Henis Y.I. Disruption of TGF-β growth inhibition by oncogenic ras is linked to p27Kip1 mislocalization. Oncogene. 2000;19:5926–5935. doi: 10.1038/sj.onc.1203991. [DOI] [PubMed] [Google Scholar]
- Loda M., Cukor B., Tam S.W., Lavin P., Fiorentino M., Draetta G.F., Jessup J.M., Pagano M., Cukor B., Tam S.W., Lavin P., Fiorentino M., Draetta G.F., Jessup J.M., Pagano M., Tam S.W., Lavin P., Fiorentino M., Draetta G.F., Jessup J.M., Pagano M., Lavin P., Fiorentino M., Draetta G.F., Jessup J.M., Pagano M., Fiorentino M., Draetta G.F., Jessup J.M., Pagano M., Draetta G.F., Jessup J.M., Pagano M., Jessup J.M., Pagano M., Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- Ma C., Papermaster D., Cepko C.L., Papermaster D., Cepko C.L., Cepko C.L. A unique pattern of photoreceptor degeneration in cyclin D1 mutant mice. Proc. Natl. Acad. Sci. 1998;95:9938–9943. doi: 10.1073/pnas.95.17.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M., Ortega S., Barbacid M., Ortega S., Barbacid M., Barbacid M. Genetic analysis of mammalian cyclin-dependent kinases and their inhibitors. Biol. Chem. 2000;381:827–838. doi: 10.1515/BC.2000.105. [DOI] [PubMed] [Google Scholar]
- Marquardt T., Ashery-Padan R., Andrejewski N., Scardigli R., Guillemot F., Gruss P., Ashery-Padan R., Andrejewski N., Scardigli R., Guillemot F., Gruss P., Andrejewski N., Scardigli R., Guillemot F., Gruss P., Scardigli R., Guillemot F., Gruss P., Guillemot F., Gruss P., Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- McAllister S.S., Becker-Hapak M., Pintucci G., Pagano M., Dowdy S.F., Becker-Hapak M., Pintucci G., Pagano M., Dowdy S.F., Pintucci G., Pagano M., Dowdy S.F., Pagano M., Dowdy S.F., Dowdy S.F. Novel p27kip1 C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol. Cell. Biol. 2003;23:216–228. doi: 10.1128/MCB.23.1.216-228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosetti R., Kawamata N., Gombart A.F., Miller C.W., Hatta Y., Hirama T., Said J.W., Tomonaga M., Koeffler H.P., Kawamata N., Gombart A.F., Miller C.W., Hatta Y., Hirama T., Said J.W., Tomonaga M., Koeffler H.P., Gombart A.F., Miller C.W., Hatta Y., Hirama T., Said J.W., Tomonaga M., Koeffler H.P., Miller C.W., Hatta Y., Hirama T., Said J.W., Tomonaga M., Koeffler H.P., Hatta Y., Hirama T., Said J.W., Tomonaga M., Koeffler H.P., Hirama T., Said J.W., Tomonaga M., Koeffler H.P., Said J.W., Tomonaga M., Koeffler H.P., Tomonaga M., Koeffler H.P., Koeffler H.P. Alterations of the p27KIP1 gene in non-Hodgkin’s lymphomas and adult T-cell leukemia/lymphoma. Blood. 1995;86:1924–1930. [PubMed] [Google Scholar]
- Muraoka R.S., Lenferink A.E., Law B., Hamilton E., Brantley D.M., Roebuck L.R., Arteaga C.L., Lenferink A.E., Law B., Hamilton E., Brantley D.M., Roebuck L.R., Arteaga C.L., Law B., Hamilton E., Brantley D.M., Roebuck L.R., Arteaga C.L., Hamilton E., Brantley D.M., Roebuck L.R., Arteaga C.L., Brantley D.M., Roebuck L.R., Arteaga C.L., Roebuck L.R., Arteaga C.L., Arteaga C.L. ErbB2/Neu-induced, cyclin D1-dependent transformation is accelerated in p27-haploinsufficient mammary epithelial cells but impaired in p27-null cells. Mol. Cell. Biol. 2002;22:2204–2219. doi: 10.1128/MCB.22.7.2204-2219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutton A., Hasleton P., Curry A., Bishop P., Egan J., Carroll K., Hanley S., Hasleton P., Curry A., Bishop P., Egan J., Carroll K., Hanley S., Curry A., Bishop P., Egan J., Carroll K., Hanley S., Bishop P., Egan J., Carroll K., Hanley S., Egan J., Carroll K., Hanley S., Carroll K., Hanley S., Hanley S. Differentiation of desquamative interstitial pneumonitis (DIP) from pulmonary adenocarcinoma by immunochemistry. Histopathology. 1998;33:129–135. doi: 10.1046/j.1365-2559.1998.00463.x. [DOI] [PubMed] [Google Scholar]
- Nakasu S., Nakajima M., Handa J., Nakajima M., Handa J., Handa J. Anomalous p27Kip1 expression in a subset of malignant gliomas. Brain Tumor Pathol. 1999;16:17–21. doi: 10.1007/BF02478897. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D.Y., Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D.Y., Nakayama K., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D.Y., Nakayama K., Inomata A., Inoue T., Shishido N., Horii I., Loh D.Y., Nakayama K., Inoue T., Shishido N., Horii I., Loh D.Y., Nakayama K., Shishido N., Horii I., Loh D.Y., Nakayama K., Horii I., Loh D.Y., Nakayama K., Loh D.Y., Nakayama K., Nakayama K. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Nguyen L., Besson A., Heng J., Schurrmans C., Teboul L., Philpott A., Roberts J.M., Guillemot F., Besson A., Heng J., Schurrmans C., Teboul L., Philpott A., Roberts J.M., Guillemot F., Heng J., Schurrmans C., Teboul L., Philpott A., Roberts J.M., Guillemot F., Schurrmans C., Teboul L., Philpott A., Roberts J.M., Guillemot F., Teboul L., Philpott A., Roberts J.M., Guillemot F., Philpott A., Roberts J.M., Guillemot F., Roberts J.M., Guillemot F., Guillemot F. p27Kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes & Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma S., Philpott A., Wang K., Holt C.E., Harris W.A., Philpott A., Wang K., Holt C.E., Harris W.A., Wang K., Holt C.E., Harris W.A., Holt C.E., Harris W.A., Harris W.A. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell. 1999;99:499–510. doi: 10.1016/s0092-8674(00)81538-x. [DOI] [PubMed] [Google Scholar]
- Pei X.H., Bai F., Smith M.D., Xiong Y., Bai F., Smith M.D., Xiong Y., Smith M.D., Xiong Y., Xiong Y. p18Ink4c collaborates with Men1 to constrain lung stem cell expansion and suppress non-small-cell lung cancers. Cancer Res. 2007;67:3162–3170. doi: 10.1158/0008-5472.CAN-06-4517. [DOI] [PubMed] [Google Scholar]
- Pellegata N.S., Quintanilla-Martinez L., Siggelkow H., Samson E., Bink K., Hofler H., Fend F., Graw J., Atkinson M.J., Quintanilla-Martinez L., Siggelkow H., Samson E., Bink K., Hofler H., Fend F., Graw J., Atkinson M.J., Siggelkow H., Samson E., Bink K., Hofler H., Fend F., Graw J., Atkinson M.J., Samson E., Bink K., Hofler H., Fend F., Graw J., Atkinson M.J., Bink K., Hofler H., Fend F., Graw J., Atkinson M.J., Hofler H., Fend F., Graw J., Atkinson M.J., Fend F., Graw J., Atkinson M.J., Graw J., Atkinson M.J., Atkinson M.J. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc. Natl. Acad. Sci. 2006;103:15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp-Staheli J., Payne S.R., Kemp C.J., Payne S.R., Kemp C.J., Kemp C.J. p27Kip1: Regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp. Cell Res. 2001;264:148–168. doi: 10.1006/excr.2000.5143. [DOI] [PubMed] [Google Scholar]
- Pietenpol J.A., Bohlander S.K., Sato Y., Papadopoulos N., Liu B., Friedman C., Trask B.J., Roberts J.M., Kinzler K.W., Rowley J.D., Bohlander S.K., Sato Y., Papadopoulos N., Liu B., Friedman C., Trask B.J., Roberts J.M., Kinzler K.W., Rowley J.D., Sato Y., Papadopoulos N., Liu B., Friedman C., Trask B.J., Roberts J.M., Kinzler K.W., Rowley J.D., Papadopoulos N., Liu B., Friedman C., Trask B.J., Roberts J.M., Kinzler K.W., Rowley J.D., Liu B., Friedman C., Trask B.J., Roberts J.M., Kinzler K.W., Rowley J.D., Friedman C., Trask B.J., Roberts J.M., Kinzler K.W., Rowley J.D., Trask B.J., Roberts J.M., Kinzler K.W., Rowley J.D., Roberts J.M., Kinzler K.W., Rowley J.D., Kinzler K.W., Rowley J.D., Rowley J.D., et al. Assignment of the human p27Kip1 gene to 12p13 and its analysis in leukemias. Cancer Res. 1995;55:1206–1210. [PubMed] [Google Scholar]
- Polyak K., Kato J., Solomon M.J., Sherr C.J., Massague J., Roberts J.M., Koff A., Kato J., Solomon M.J., Sherr C.J., Massague J., Roberts J.M., Koff A., Solomon M.J., Sherr C.J., Massague J., Roberts J.M., Koff A., Sherr C.J., Massague J., Roberts J.M., Koff A., Massague J., Roberts J.M., Koff A., Roberts J.M., Koff A., Koff A. p27Kip1, a cyclin–CDK inhibitor, links transforming growth factor β and contact inhibition to cell cycle arrest. Genes & Dev. 1994a;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M.H., Erdjument-Bromage H., Koff A., Roberts J.M., Tempst P., Massague J., Lee M.H., Erdjument-Bromage H., Koff A., Roberts J.M., Tempst P., Massague J., Erdjument-Bromage H., Koff A., Roberts J.M., Tempst P., Massague J., Koff A., Roberts J.M., Tempst P., Massague J., Roberts J.M., Tempst P., Massague J., Tempst P., Massague J., Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994b;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Ponce-Castaneda M.V., Lee M.H., Latres E., Polyak K., Lacombe L., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J., Lee M.H., Latres E., Polyak K., Lacombe L., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J., Latres E., Polyak K., Lacombe L., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J., Polyak K., Lacombe L., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J., Lacombe L., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J., Mathew S., Krauter K., Sheinfeld J., Massague J., Krauter K., Sheinfeld J., Massague J., Sheinfeld J., Massague J., Massague J. p27Kip1: Chromosomal mapping to 12p12–12p13.1 and absence of mutations in human tumors. Cancer Res. 1995;55:1211–1214. [PubMed] [Google Scholar]
- Porter P.L., Malone K.E., Heagerty P.J., Alexander G.M., Gatti L.A., Firpo E.J., Daling J.R., Roberts J.M., Malone K.E., Heagerty P.J., Alexander G.M., Gatti L.A., Firpo E.J., Daling J.R., Roberts J.M., Heagerty P.J., Alexander G.M., Gatti L.A., Firpo E.J., Daling J.R., Roberts J.M., Alexander G.M., Gatti L.A., Firpo E.J., Daling J.R., Roberts J.M., Gatti L.A., Firpo E.J., Daling J.R., Roberts J.M., Firpo E.J., Daling J.R., Roberts J.M., Daling J.R., Roberts J.M., Roberts J.M. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat. Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- Qi C.F., Xiang S., Shin M.S., Hao X., Lee C.H., Zhou J.X., Torrey T.A., Hartley J.W., Fredrickson T.N., Morse H.C., Xiang S., Shin M.S., Hao X., Lee C.H., Zhou J.X., Torrey T.A., Hartley J.W., Fredrickson T.N., Morse H.C., Shin M.S., Hao X., Lee C.H., Zhou J.X., Torrey T.A., Hartley J.W., Fredrickson T.N., Morse H.C., Hao X., Lee C.H., Zhou J.X., Torrey T.A., Hartley J.W., Fredrickson T.N., Morse H.C., Lee C.H., Zhou J.X., Torrey T.A., Hartley J.W., Fredrickson T.N., Morse H.C., Zhou J.X., Torrey T.A., Hartley J.W., Fredrickson T.N., Morse H.C., Torrey T.A., Hartley J.W., Fredrickson T.N., Morse H.C., Hartley J.W., Fredrickson T.N., Morse H.C., Fredrickson T.N., Morse H.C., Morse H.C. Expression of the cyclin-dependent kinase inhibitor p27 and its deregulation in mouse B-cell lymphomas. Leuk. Res. 2006;30:153–163. doi: 10.1016/j.leukres.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Rosen D.G., Yang G., Cai K.Q., Bast R.C., Gershenson D.M., Silva E.G., Liu J., Yang G., Cai K.Q., Bast R.C., Gershenson D.M., Silva E.G., Liu J., Cai K.Q., Bast R.C., Gershenson D.M., Silva E.G., Liu J., Bast R.C., Gershenson D.M., Silva E.G., Liu J., Gershenson D.M., Silva E.G., Liu J., Silva E.G., Liu J., Liu J. Subcellular localization of p27Kip1 expression predicts poor prognosis in human ovarian cancer. Clin. Cancer Res. 2005;11:632–637. [PubMed] [Google Scholar]
- Russo A.A., Jeffrey P.D., Patten A.K., Massague J., Pavletich N.P., Jeffrey P.D., Patten A.K., Massague J., Pavletich N.P., Patten A.K., Massague J., Pavletich N.P., Massague J., Pavletich N.P., Pavletich N.P. Crystal structure of the p27Kip1 cyclin dependent kinase inhibitor bound to the cyclin A–Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- Ryu J.H., Colby T.V., Hartman T.E., Vassallo R., Colby T.V., Hartman T.E., Vassallo R., Hartman T.E., Vassallo R., Vassallo R. Smoking-related interstitial lung diseases: A concise review. Eur. Respir. J. 2001;17:122–132. doi: 10.1183/09031936.01.17101220. [DOI] [PubMed] [Google Scholar]
- Saez A., Sanchez E., Sanchez-Beato M., Cruz M.A., Chacon I., Munoz E., Camacho F.I., Martinez-Montero J.C., Mollejo M., Garcia J.F., Sanchez E., Sanchez-Beato M., Cruz M.A., Chacon I., Munoz E., Camacho F.I., Martinez-Montero J.C., Mollejo M., Garcia J.F., Sanchez-Beato M., Cruz M.A., Chacon I., Munoz E., Camacho F.I., Martinez-Montero J.C., Mollejo M., Garcia J.F., Cruz M.A., Chacon I., Munoz E., Camacho F.I., Martinez-Montero J.C., Mollejo M., Garcia J.F., Chacon I., Munoz E., Camacho F.I., Martinez-Montero J.C., Mollejo M., Garcia J.F., Munoz E., Camacho F.I., Martinez-Montero J.C., Mollejo M., Garcia J.F., Camacho F.I., Martinez-Montero J.C., Mollejo M., Garcia J.F., Martinez-Montero J.C., Mollejo M., Garcia J.F., Mollejo M., Garcia J.F., Garcia J.F., et al. p27Kip1 is abnormally expressed in diffuse large B-cell lymphomas and is associated with an adverse clinical outcome. Br. J. Cancer. 1999;80:1427–1434. doi: 10.1038/sj.bjc.6690539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Beato M., Camacho F.I., Martinez-Montero J.C., Saez A., Villuendas R., Sanchez-Verde L., Garcia J.F., Piris M.A., Camacho F.I., Martinez-Montero J.C., Saez A., Villuendas R., Sanchez-Verde L., Garcia J.F., Piris M.A., Martinez-Montero J.C., Saez A., Villuendas R., Sanchez-Verde L., Garcia J.F., Piris M.A., Saez A., Villuendas R., Sanchez-Verde L., Garcia J.F., Piris M.A., Villuendas R., Sanchez-Verde L., Garcia J.F., Piris M.A., Sanchez-Verde L., Garcia J.F., Piris M.A., Garcia J.F., Piris M.A., Piris M.A. Anomalous high p27Kip1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27Kip1–cyclin D3 colocalization in tumor cells. Blood. 1999;94:765–772. [PubMed] [Google Scholar]
- Sherr C.J., Roberts J.M., Roberts J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes & Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Donaher J.L., Parker S.B., Li T., Fazeli A., Gardner H., Haslam S.Z., Bronson R.T., Elledge S.J., Weinberg R.A., Donaher J.L., Parker S.B., Li T., Fazeli A., Gardner H., Haslam S.Z., Bronson R.T., Elledge S.J., Weinberg R.A., Parker S.B., Li T., Fazeli A., Gardner H., Haslam S.Z., Bronson R.T., Elledge S.J., Weinberg R.A., Li T., Fazeli A., Gardner H., Haslam S.Z., Bronson R.T., Elledge S.J., Weinberg R.A., Fazeli A., Gardner H., Haslam S.Z., Bronson R.T., Elledge S.J., Weinberg R.A., Gardner H., Haslam S.Z., Bronson R.T., Elledge S.J., Weinberg R.A., Haslam S.Z., Bronson R.T., Elledge S.J., Weinberg R.A., Bronson R.T., Elledge S.J., Weinberg R.A., Elledge S.J., Weinberg R.A., Weinberg R.A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Singh S.P., Lipman J., Goldman H., Ellis F.H., Aizenman L., Cangi M.G., Signoretti S., Chiaur D.S., Pagano M., Loda M., Lipman J., Goldman H., Ellis F.H., Aizenman L., Cangi M.G., Signoretti S., Chiaur D.S., Pagano M., Loda M., Goldman H., Ellis F.H., Aizenman L., Cangi M.G., Signoretti S., Chiaur D.S., Pagano M., Loda M., Ellis F.H., Aizenman L., Cangi M.G., Signoretti S., Chiaur D.S., Pagano M., Loda M., Aizenman L., Cangi M.G., Signoretti S., Chiaur D.S., Pagano M., Loda M., Cangi M.G., Signoretti S., Chiaur D.S., Pagano M., Loda M., Signoretti S., Chiaur D.S., Pagano M., Loda M., Chiaur D.S., Pagano M., Loda M., Pagano M., Loda M., Loda M. Loss or altered subcellular localization of p27 in Barrett’s associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- Slingerland J., Pagano M., Pagano M. Regulation of the CDK inhibitor p27 and its deregulation in cancer. J. Cell. Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Spirin K.S., Simpson J.F., Takeuchi S., Kawamata N., Miller C.W., Koeffler H.P., Simpson J.F., Takeuchi S., Kawamata N., Miller C.W., Koeffler H.P., Takeuchi S., Kawamata N., Miller C.W., Koeffler H.P., Kawamata N., Miller C.W., Koeffler H.P., Miller C.W., Koeffler H.P., Koeffler H.P. p27/Kip1 mutation found in breast cancer. Cancer Res. 1996;56:2400–2404. [PubMed] [Google Scholar]
- Tan P., Cady B., Wanner M., Worland P., Cukor B., Magi-Galluzzi C., Lavin P., Draetta G., Pagano M., Loda M., Cady B., Wanner M., Worland P., Cukor B., Magi-Galluzzi C., Lavin P., Draetta G., Pagano M., Loda M., Wanner M., Worland P., Cukor B., Magi-Galluzzi C., Lavin P., Draetta G., Pagano M., Loda M., Worland P., Cukor B., Magi-Galluzzi C., Lavin P., Draetta G., Pagano M., Loda M., Cukor B., Magi-Galluzzi C., Lavin P., Draetta G., Pagano M., Loda M., Magi-Galluzzi C., Lavin P., Draetta G., Pagano M., Loda M., Lavin P., Draetta G., Pagano M., Loda M., Draetta G., Pagano M., Loda M., Pagano M., Loda M., Loda M. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res. 1997;57:1259–1263. [PubMed] [Google Scholar]
- Tong W., Kiyokawa H., Soos T.J., Park M.S., Soares V.C., Manova K., Pollard J.W., Koff A., Kiyokawa H., Soos T.J., Park M.S., Soares V.C., Manova K., Pollard J.W., Koff A., Soos T.J., Park M.S., Soares V.C., Manova K., Pollard J.W., Koff A., Park M.S., Soares V.C., Manova K., Pollard J.W., Koff A., Soares V.C., Manova K., Pollard J.W., Koff A., Manova K., Pollard J.W., Koff A., Pollard J.W., Koff A., Koff A. The absence of p27Kip1, an inhibitor of G1 cyclin-dependent kinases, uncouples differentiation and growth arrest during the granulosa → luteal transition. Cell Growth Differ. 1998;9:787–794. [PubMed] [Google Scholar]
- Vlach J., Hennecke S., Amati B., Hennecke S., Amati B., Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Eddy R., Condeelis J., Eddy R., Condeelis J., Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F.Y., Wang S.E., Sanders M.E., Shin I., Rojo F., Baselga J., Arteaga C.L., Wang S.E., Sanders M.E., Shin I., Rojo F., Baselga J., Arteaga C.L., Sanders M.E., Shin I., Rojo F., Baselga J., Arteaga C.L., Shin I., Rojo F., Baselga J., Arteaga C.L., Rojo F., Baselga J., Arteaga C.L., Baselga J., Arteaga C.L., Arteaga C.L. Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res. 2006;66:2162–2172. doi: 10.1158/0008-5472.CAN-05-3304. [DOI] [PubMed] [Google Scholar]