Abstract
Investigations of biological invasions focus on patterns and processes that are related to introduction, establishment, spread and impacts of introduced species. This review focuses on the ecological interactions operating during invasions by the most prominent group of insect vectors of disease, mosquitoes. First, we review characteristics of non-native mosquito species that have established viable populations, and those invasive species that have spread widely and had major impacts, testing whether biotic characteristics are associated with the transition from established non-native to invasive. Second, we review the roles of interspecific competition, apparent competition, predation, intraguild predation and climatic limitation as causes of impacts on residents or as barriers to invasion. We concentrate on the best-studied invasive mosquito, Aedes albopictus, evaluating the application of basic ecological theory to invasions by Aedes albopictus. We develop a model based on observations of Aedes albopictus for effects of resource competition and predation as barriers to invasion, evaluating which community and ecosystem characteristics favour invasion. Third, we evaluate the ways in which invasive mosquitoes have contributed to outbreaks of human and animal disease, considering specifically whether invasive mosquitoes create novel health threats, or modify disease transmission for existing pathogen–host systems.
Keywords: Aedes albopictus, apparent competition, Culicidae, disease ecology, disease vectors, interspecific competition, intraguild predation, invasion, local adaptation, predation
INTRODUCTION
Invasion biology focuses on patterns and processes related to introduction, establishment, spread and impacts of non-native species (Williamson 1996; Davis & Thompson 2000; Lounibos 2002). We will apply the term ‘invasive’ to introduced species that have increased and spread, creating the potential for impacts on native species and ecosystems, or on human activities (agriculture, conservation). We refer to species that have become established, but have neither spread widely nor had important impacts as ‘non-native’. Invasive species produce impacts on other species and ecosystems primarily via their biotic interactions, including predation and parasitism, interspecific competition, or ecosystem engineering (Williamson 1996). Also among the potential biotic impacts of some invasive species are effects on human and animal health (Lounibos 2002). Invasive pathogens may affect health directly, and invasive vectors may alter the transmission cycles of native or non-native pathogens (McMichael & Bouma 2000). This review focuses on the ecological interactions that produce impacts associated with invasions by the most prominent group of insect vectors of disease, mosquitoes (Diptera, Culicidae). We are specifically interested in two kinds of impacts of invasive mosquitoes: effects – usually detrimental – on (i) resident species or ecosystems and (ii) human or vertebrate animal health. The first represents a class of effects that could apply to any invasive species, so that what we learn from investigations of impacts of mosquitoes may serve as a paradigm for investigations of any invasion. The second represents a class of impacts relevant primarily to vectors. For mosquitoes, different life cycle stages are likely to be central to the two kinds of impacts. The aquatic larval phase is most likely to interact with and to impact other species, whereas the terrestrial adult phase is the cause of threats to human health. In examining both kinds of effects, we wish to identify the characteristics of the invasive species and the biological interactions that are associated with these impacts.
For mosquitoes, there is one well studied invasive species (Asian tiger mosquito, Aedes albopictus Skuse) for which multiple hypotheses concerning the ecological processes operating during its invasions have been tested in several locations. For other species there is very little information on the processes that operated during invasions. Although a broad review of the processes involved in invasions by mosquitoes would be desirable, relevant data are not available. In some cases (e.g. Ochlerotatus japonicus) the invasions are recent and research on ecological processes is ongoing. In other cases the invasions occurred sufficiently far in the past that the potential to investigate establishment and spread is long gone. We therefore begin with a simple review of the characteristics of the major invasive mosquitoes, evaluating which traits are associated with invasive potential. We then concentrate our review of processes primarily on Aedes albopictus, with a summary of what little is known or postulated about ecological processes involved in other invasions by mosquitoes. This portion of our review will enable us to test basic ecological theory as it applies to mosquitoes, and may thus improve our understanding of not only invasive mosquitoes, but of invasive species in general, and of the roles of biotic interactions that may apply to any invasive species.
We divide the ecological processes into: (i) species interactions that may influence invasions, either by affecting resident species, or by serving as barriers to invasions and (ii) effects of climate that may foster invasion success, act as barriers to invasion, alter the impacts of invasions, or act as agents of natural selection on invasive mosquitoes. Inter-specific competition, predation and apparent competition via shared pathogens are the species interactions that have been best studied. We will review some models of these interactions and empirical data on the roles of these processes in invasions by Aedes albopictus. We also review data on known effects of climate on invasions by Aedes albopictus.
There are data on the health impacts of most invasions (Lounibos 2002), and we finish with a review of the disease ecology of invasive mosquitoes. We do this by posing questions about the origins of disease organisms (e.g. non-native, resident), and whether introduction of a new vector initiates or alters outbreaks of important diseases. We hope that this portion of our review will provide a paradigm for investigations necessary to understand which non-native species constitute the greatest hazard to human health.
CHARACTERISTICS OF INVASIVE MOSQUITOES
The list of successful invasive and non-native culicids (Table 1) is limited, hence we can only address simple questions about species characteristics, but they are questions deemed generally important by those working in the field of invasion biology (e.g. Sakai et al. 2001). First, we can ask what characteristics are associated with becoming an established non-native. Desiccation-resistant eggs, which enhance survival in inhospitable environments, occur in 14 of 31 invasive or non-native species (Table 1). Across the Culicidae, strongly desiccation-resistant eggs are confined to members of the genera Aedes, Ochlerotatus, Psorophora, Haemagogus and Opifex (Clements 1992), totalling some 1012 species, or about 29% of c. 3491 mosquito species (Walter Reed Biosystematics Unit 2005). Thus, desiccation-resistant eggs are strongly associated with becoming an introduced non-native species (Fisher’s exact test, P = 0.0024), perhaps because they increase probability of successful transport. A second question we can ask is what characteristics are associated with making the transition from non-native to invasive (Sakai et al. 2001). Desiccation resistance (yes, no) and status (invasive, non-invasive) are not significantly associated (Fisher’s exact test, P = 0.693), indicating that species with desiccation-resistant eggs are not more likely to become invasive. Development in small man-made containers, tree holes, bromeliads, or rock pools is also common among invasive and non-native mosquitoes (17 of 31 total species; Table 1), but again, larval habitat (container, non-container) and status (invasive, non-native) are not significantly associated (Fisher’s exact test, P = 0.132). Occupying human-dominated habitats (i.e. urban, suburban, domestic) is significantly (Fisher’s exact test, P = 0.028) more common among invasive (six of nine) than among non-native (three of 14) mosquitoes (Table 1). Association of invasion with human disturbance in some form (e.g. Moyle & Light 1996; Richardson et al. 2000) has been observed in other groups. There are at least two broad hypotheses explaining this association: human commensals may have traits (e.g. a high maximum rate of population increase) that make them more likely to become invasive; or expanding human population and urbanization may create new, relatively under exploited, macrohabitat suitable for human commensal specialists. Autogeny and diapause are not significantly associated with invasive status, but data are absent for many species (Table 1). Mode of introduction (natural dispersal vs. human transport) is uncertain for many of these mosquitoes, but hitchhiking on ships is probably common (Lounibos 2002).
Table 1.
Established invasive and non-native mosquitoes and important natural history traits
| Species (origin) | Invaded/colonized region | Macrohabitat preference | Larval habitats | Desiccation- resistant eggs | Autogeny* | Diapause | References |
|---|---|---|---|---|---|---|---|
| Invasive species | |||||||
| Aedes aegypti (Africa) | Cosmotropical | Urban, domestic† | Man-made containers | Yes | Rarely | None | Christophers (1960) |
| Aedes albopictus (temperate and tropical Asia) | Americas, Europe, Africa | Urban, suburban | Phytotelmata‡, man-made containers | Yes | Rarely | Egg§ | Hawley (1988) |
| Ochlerotatus atropalpus (E. N. America) | W. N. America, Europe | Riparian | Rock pools, man-made containers | Yes | Yes | Egg | Lounibos (2002) |
| Ochlerotatus japonicus (temperate Asia) | North America | Rural, sylvan | Rock pools, man-made containers | Yes | No | Egg | Lounibos (2002) |
| Ochlerotatus notoscriptus (Australia) | New Zealand | Urban, suburban, rural | Tree holes, rock pools | Yes | No | Egg, larva | Weinstein et al. (1997) |
| Culex pipiens (Old World) | North America | Urban, domestic, suburban | Man-made containers, subterranean, small groundwater pools | No | Yes | Adult | Vinogradova (2000) |
| Culex quinquefasciatus (Africa) | Americas, Asia, New Zealand, southern Europe | Urban, domestic, suburban | Man-made containers, small groundwater pools | No | Some | None | Vinogradova (2000) |
| Anopheles darlingi (neotropics, especially eastern Amazonia) | Peru | Rural | River margins and lagoons | No | No | None | Lounibos (2002) |
| Anopheles gambiae complex¶ (Africa) | Brazil, Mauritius | Domestic | Groundwater pools | No | No | None | Ross (1911); Soper & Wilson (1943) |
| Non-native, non-invasive species | |||||||
| Aedes neopandani (Saipan and Tinian) | Guam | Phytotelmata | Yes | Ward (1984) | |||
| Aedes rotanus (Rota) | Guam | Phytotelmata | Yes | Ward (1984) | |||
| Aedes saipanensis (Saipan) | Guam | Phytotelmata | Yes | Ward (1984) | |||
| Aedes vexans (Cosmopolitan) | Guam | Ground pools | Yes | Egg§ | Ward (1984) | ||
| Ochlerotatus australis (Australia) | New Zealand | Rocky marine shore | Rock pools | Yes | Yes | Larva | Weinstein et al. (1997) |
| Ochlerotatus camptorhynchus (Australia) | New Zealand | Suburban, rural | Salt marsh, brackish swamps | Yes | Weinstein et al. (1997) | ||
| Ochlerotatus vigilax (New Caledonia) | Fiji | Coastal | Salt marsh | Yes | Yes | Joyce (1961) | |
| Ochlerotatus bahamensis (Bahamas) | Southern North America | Urban, suburban, rural | Tree holes, man-made containers | Yes | Yes | No | O’Meara et al. (1989) |
| Ochlerotatus togoi (temperate Asia) | North America | Rocky marine shoreline | Rock pools | Yes | Yes | Larva§ | Sota (1994) |
| Aedeomyia catasticta (Oriental Region) | Guam | Rural? | Ponds with macrophytes | No | No | Ward (1984) | |
| Mansonia uniformis (Old World tropics) | Guam | Swamps | Ponds with macrophytes | No | No | Ward (1984) | |
| Culex fuscanus (Oriental Region) | Guam | Rural? | Ponds, streams, ditches | No | No | Ward (1984) | |
| Culex fuscocephala (tropical Asia) | Guam | Rural | Rice fields and ponds with vegetation | No | No | Ward (1984) | |
| Culex tritaeniorhynchus (Old World) | Guam | Variable, sometimes domestic | Groundwater pools, rice fields | No | Adult§ | Ward (1984) | |
| Culex sitiens (Old World tropics) | Guam | Coastal | Brackish pools, holes | No | No | Ward (1984) | |
| Anopheles indefinitus (Oriental Region) | Guam | Coastal | Marshes, ground pools | No | No | Ward (1984) | |
| Anopheles barbirostris (Vietnam) | Guam | Rural | River margins, pools, rice paddies, swamps | No | No | Ward (1984) | |
| Anopheles litoralis (Philippines, Borneo) | Guam | Coastal? | Artificial containers | No | No | Ward (1984) | |
| Anopheles subpictus (Oriental Region) | Guam | Fresh and saltwater ground pools | No | No | Ward (1984) | ||
| Anopheles vagus (Oriental Region) | Guam | Pools, ditches, containers | No | No | Ward (1984) | ||
| Armigeres subalbatus (Oriental Region) | Guam | Containers with foul water | No | No | Ward (1984) | ||
| Wyeomyia mitchellii (Caribbean & Florida) | Hawaii | Bromeliads | No | No | No | Shroyer (1981) | |
Blank cells indicate lack of information. Question marks associated with macrohabitat preferences indicate that other habitats may also be used but information is limited. See text for details.
Egg production without a blood meal.
Does not apply to Aedes aegypti formosus, the sylvan morph found only in East Africa.
Phytotelmata are parts of terrestrial plants that hold water and are occupied by a community of inquiline animals; includes tree holes, bromeliads, pitcher plants and bamboo.
Diapause not present in tropical populations.
Only includes the anthropophilic species of the complex, Anopheles gambiae s.s. and Anopheles arabiensis.
ECOLOGICAL PROCESSES
In the context of invasions, effects of competition and predation can be important because they can be impacts of invasive species on native species, and because these processes may act as barriers to invasion. Both competition and predation can, in principle, affect whether invasive mosquitoes alter the potential for disease transmission. An invasive mosquito that replaces a resident species via competition or apparent competition may alter disease transmission if it is either a more or less efficient disease vector. If invasion results in reduced disease transmission via vector replacement, then introduction of a novel mosquito species could be used as biological control of resident vectors via competitive displacement (e.g. Rozeboom 1971; Rosen et al. 1976). This is similar to logic behind attempts to use transgenic, disease-refractory mosquitoes to control disease (O’Brochta 2003). In both cases the goal is to replace a population of disease-competent mosquitoes with a population of disease-refractory mosquitoes (Takken & Boëte 2003). In the former case this replacement occurs via interspecific competitive exclusion. In the case of use of transgenic mosquitoes replacement may occur by intraspecific competition among genotypes or intrasexual competition (i.e. transgenic male mating competitiveness). It is also possible for invasive mosquitoes to alter disease transmission without impacting native vectors, if the invader takes on a novel role in the life cycle of a pathogen. For example, an invasive species that is an effective bridge vector (i.e. capable of transmitting the pathogen outside its enzootic cycle among non-human hosts) may alter disease transmission and may create new human health problems. Likewise, invasive vectors can transport novel pathogens that can spread rapidly in susceptible host populations (see below).
Interspecific competition
In this review, ‘interspecific competition’ encompasses any mechanism that produces negative effects on population growth of the focal species, excluding any mechanisms that involve predation or parasitism on the focal species. Thus, we include resource competition (negative effects via depletion of shared resources; Grover 1997), chemical or physical interference (negative effects via toxins, waste products, or aggression; Sunahara & Mogi 2002), and mating interference (negative effects via interspecific mating depressing reproductive output; Ribiero & Spielman 1986), but exclude intraguild predation and apparent competition (negative effects via a shared predator or parasite; Holt & Lawton 1994), which we consider forms of predation (see below).
Superiority in interspecific competition is often listed as a characteristic of non-native species that enhances the likelihood of becoming invasive (Williamson 1996; Sakai et al. 2001). But superiority in competition is only necessary for invasion and spread if the invader encounters similar species and if resources are limiting, or if interference competition dominates in the invaded community. Some invasive species may expand into new areas by filling an ‘ empty niche’; i.e. occupying previously unoccupied (or unsaturated) habitat or exploiting a previously unused resource (Williamson 1996). Thus, the first line of evidence that superiority in competition is important in an invasion is often that the invasion results in declines or elimination of ecologically similar species (Juliano 1998). Conversely, some non-native species may not spread beyond a limited area because they are not effective competitors, with competition from residents presumably limiting their spread. In either case, interspecific competition plays a role in determining the outcome of an introduction. Among mosquitoes, both declines of residents (see below) and failure of non-native species to spread (Rosen et al. 1976; O’Meara et al. 1989, 1995b) have been observed. Further, historical and natural history data on Aedes aegypti (L.) (whose domestic form larvae preferentially occupy man-made containers, Christophers 1960) may be interpreted as indicating that at least some invasions by mosquitoes have proceeded without much evidence for impacts of competition, suggesting that some invasive mosquitoes may have invaded ‘empty niches’. Unfortunately, there have been very few experimental investigations of competitive interactions involving invasive or other non-native mosquitoes, and we therefore have a reasonable understanding of the roles of interspecific competition only for the recent invasions of North and South America by Aedes albopictus.
Aedes albopictus
Resource competition
Introductions of A. albopictus to North and South America (Forattini 1986; Hawley 1988; O’Meara et al. 1995a,b; Moore 1999), Europe (Romi et al. 1999) and Africa (Fontenille & Toto 2001) in the past two decades are well documented (Lounibos 2002 gives extensive references for this invasion). Concomitant with the spread of A. albopictus in southern North America, there has been a decline, sometimes to local extinction, of the previously resident invader A. aegypti (O’Meara et al. 1995a; Juliano et al. 2004, and references therein) that is consistent with interspecific competition. Aedes aegypti persists in southern and urban areas of the Florida peninsula (O’Meara et al. 1995b; Juliano et al. 2002, 2004), and in other urban areas of the southern USA (e.g. Houston, Savannah, New Orleans), and in these areas it coexists locally with A. albopictus (Juliano et al. 2004; G. F. O’Meara, personal communication; D. M. Wesson personal communication). Coexistence despite interspecific larval competition is one of the major unanswered questions concerning the invasion of North America by A. albopictus. Aedes aegypti remains dominant in Miami (O’Meara et al. 1995a,b) and in the Florida Keys, where A. albopictus is absent on most islands (G. F. O’Meara, personal communication), possibly due to dry conditions (Juliano et al. 2002) or intensive control efforts.
Laboratory experiments in microcosms with plant detritus as a substrate showed that North American A. albopictus are superior in competition with A. aegypti (Barrera 1996; Daugherty et al. 2000). Field experiments in tires showed a similar competitive advantage for A. albopictus (Juliano 1998). Field experiments in cemetery vases demonstrated that interspecific competition among larvae can cause significant reductions in survival of A. aegypti at the typical densities encountered in these habitats (Juliano et al. 2004), providing evidence that interspecific competition is important in nature. Laboratory experiments on competition between larvae of these species have used different detritus resources (e.g. liver powder, yeast, dead insects) and have tended to yield approximate competitive equality, or even an advantage for A. aegypti (Barrera 1996; Daugherty et al. 2000, and references therein). Temperatures between 24 and 30 °C had no significant effect on competition among larvae (Lounibos et al. 2002). Field and laboratory data suggest that habitat drying and resultant mortality of eggs differentially affects A. albopictus (Juliano et al. 2002), and dryer environments can reverse competitive advantage, favouring A. aegypti (K. S. Costanzo, B. Kesavaraju and S. A. Juliano, 2005, personal communication). It is obvious from studies of A. albopictus and A. aegypti that the effects of competition can vary with the environment in which the interaction occurs (condition-specific competition, Taniguchi & Nakano 2000). A more subtle point is that if the abiotic environment has effects on life cycle stages that do not compete (e.g. eggs), impacting one species more than another, those effects may alter the population level consequences of competition in another life cycle stage (e.g. larvae).
In Brazil, A. albopictus was also introduced in the mid-1980s, and it has spread widely throughout that country (Santos 2003). There is some evidence for local reductions of A. aegypti following invasion (Braks et al. 2004), and there are significant negative correlations of abundances of the two species across habitats in southern Brazil (Braks et al. 2003). Despite coexistence of these species in southern Brazil, there is strong evidence from field experiments that A. albopictus from Brazil are also superior to A. aegypti in competition among larvae in man-made containers, just as in North America (Braks et al. 2004), providing one of the few cases in which large-scale geographic variation in the outcome of an invasion has been evaluated. Invasion of Europe by A. albopictus has been suggested to be associated with declines in abundance of A. aegypti (Simberloff & Gibbons 2004), but the decline of A. aegypti in Spain and southern Europe preceded invasions by A. albopictus and probably results from eradication efforts (Eritja et al. 2005).
Laboratory investigations of competition among larvae of A. albopictus and the native North American tree hole mosquito Ochlerotata triseriatus (Say) have consistently shown competitive superiority of A. albopictus (Livdahl & Willey 1991; Novak et al. 1993; Teng & Apperson 2000; Aliabadi & Juliano 2002). In contrast to the case for A. aegypti, there is little evidence for competitive exclusion of O. triseriatus by A. albopictus and declines of O. triseriatus abundance are not apparent (Lounibos et al. 2001). Aedes albopictus and O. triseriatus are more likely to interact in wooded habitats that are both preferred by O. triseriatus and harbour a more diverse community of container-dwelling insects, including prominent predatory species (Lounibos et al. 2001; see below). In more northern, temperate areas like the Midwest, there is also little evidence for displacement of O. triseriatus by A. albopictus (Lancaster 2004), and the more severe detrimental effect of low temperatures on A. albopictus (Teng & Apperson 2000) probably contributes to its more limited invasion success in these habitats.
Invasion of A. albopictus in North America and Europe may also have affected Culex pipiens L. Aedes albopictus is strongly superior to C. pipiens in interspecific larval competition, and co-occurrence of these two species in man-made containers is common in the field in both Europe (Carrieri et al. 2003) and North America (Costanzo et al. 2005). Because C. pipiens larvae inhabit a very wide range of aquatic habitats, many not used by A. albopictus (Vinogradova 2000), the impact of competition on distribution and abundance of C. pipiens has not been obvious. Interaction of A. albopictus with C. quinquefasciatus Say in southern North America and the tropics has not been investigated. The interaction of C. pipiens with A. albopictus in North America is interesting because of the importance of C. pipiens in transmission of West Nile virus (WNV) among birds and to humans. Culex pipiens serves as an enzootic vector (i.e. transmitting WNV among birds) and appears to favour birds as a blood source, although it does feed on humans (Fonseca et al. 2004). The tendency of C. pipiens to feed on birds suggests that a bridge vector, attacking both birds (the amplification host) and humans (a dead end host) could enhance transmission of this disease to humans (Eldridge et al. 2000). Aedes albopictus is potentially such a bridge vector, having wide host range and high competence to transmit WNV (Turell et al. 2001). This complex set of interactions, with two vectors competing as larvae, and the invading potential bridge vector superior in interspecific competition among larvae, provides an opportunity for modelling of the invaded disease system as a means of forecasting the health consequences of this invasion, and more generally, of invasions by vectors with host preferences that differ from those of a native competitor.
Competitors may be a barrier to invasion as well. In some portions of its range, larvae of A. albopictus occur commonly in water-holding plant axils (Chow 1949; Joyce 1961). Within Florida, this species is productive in bromeliad axils only in the north, where native Wyeomyia spp. mosquito occupants of this habitat are absent (O’Meara et al. 1995b). Interspecific interactions, probably through asymmetric larval competition, are responsible for the poor survivorship of A. albopictus in bromeliads co-occupied by larvae of Wyeomyia (Lounibos et al. 2003b). This is a clear example of a resident species limiting the invasive success of a mosquito.
Chemical interference
Competition between A. albopictus and A. aegypti is widely assumed to occur via resource depletion, and manipulating resource levels can alter the impact of competition (Juliano 1998; Daugherty et al. 2000; Braks et al. 2004). However, both species may be affected by interference competition produced by water-borne substances, presumably excretory products (Sunahara & Mogi 2002; Bédhomme et al. 2005). The role of interference competition in invasions by A. albopictus merits further investigation.
Mating interference
Mating interference is another hypothesized mechanism of competition between A. albopictus and A. aegypti (‘satyrization’, Ribiero & Spielman 1986; Harper & Paulson 1994). In the laboratory, male A. albopictus are more aggressive in attempting to mate with A. aegypti females, whereas male A. aegypti are less aggressive in attempting to mate with A. albopictus females (Nasci et al. 1989). This asymmetry can cause detrimental population level effects on the less aggressive species (Ribiero & Spielman 1986). Experiments testing this hypothesis showed that such differences in interspecific mating aggressiveness are not present under more realistic conditions, even in laboratory cages (Harper & Paulson 1994), suggesting that this mechanism is unlikely to explain observed displacement of A. aegypti in North America.
Hatching delays
Hatching delays are yet another mechanism of interference competition hypothesized to contribute to impacts of invading A. albopictus on both A. aegypti and O. triseriatus (Edgerly et al. 1993). Young larvae of A. albopictus, A. aegypti and O. triseriatus hatch from eggs when they are flooded, and all these species respond, to varying degrees, to the presence of older larvae feeding in the water by delaying hatching. Aedes albopictus is both least sensitive to this effect in the egg stage, and more likely as a fourth instar larva to produce this effect than the other species (Edgerly et al. 1993). The importance of this asymmetrical effect in nature is difficult to estimate.
Other invasive mosquitoes
There are relatively few data on competitive effects in invasions and introductions of other mosquito species (Table 2). A role for competition is suggested in the replacement of A. albopictus by A. aegypti in urban Southeast Asia, but definitive tests have not been conducted. The presence or absence of native competitors may have influenced invasion success of C. quinquefasciatus, which may also have affected native North American Culex via competition. The role of competition in determining the invasive vs. non-native status of O. japonicus vs. Ochlerotatus bahamensis should be tested in field and laboratory experiments.
Table 2.
Synopsis of invasive and non-native mosquitoes (excluding Aedes albopictus) for which ecological processes are postulated to produce effects on invasion success and impact
| Species (references) | Location and date of introduction (status) | Possible ecological processes (references) |
|---|---|---|
| Aedes aegypti (Christophers 1960; Tabachnick 1991; Lounibos 2002) | Southern North America 16–17th centuries (invasive, but reduced in distribution as introduction of Aedes albopictus) | Interspecific competition: Aedes aegypti is superior in larval competition to Ochlerotatus triseriatus (Ho et al. 1989) and may have displaced O. triseriatus in man-made containers in domestic areas.
Predation: Aedes aegypti is more vulnerable to predation by Toxorhynchites rutilus than is O. triseriatus (Grill & Juliano 1996), and abundance of T. rutilus in containers in wooded areas may have limited invasion success of Aedes aegypti in these habitats |
| Aedes aegypti (Christophers 1960; Tabachnick 1991; Lounibos 2002) | Southeast Asia 19–20th centuries (invasive) | Interspecific competition: Declines of Aedes albopictus in tropical urban areas may have been caused by invasion of Aedes aegypti (Chan et al. 1971; Sucharit et al. 1978) |
| Culex pipiens (Vinogradova 2000; Lounibos 2002) | North America (16–17th centuries; invasive) | Interspecific competition: Absence of competitors at the time of invasion may have facilitated colonization of urban containers. More recently, competition with invading Aedes albopictus may impact C. pipiens in these habitats (Carrieri et al. 2003; Costanzo et al. 2005) |
| Culex quinquefasciatus (Weinstein et al. 1997) | New Zealand, Hawaii, 19th century (invasive) | Absence of competitors and predators: This may have contributed to invasive success in container habitats (Weinstein et al. 1997) |
| Culex quinquefasciatus | Australia 19th century (invasive) | Interspecific competition, or apparent competition via a gut symbiotic fungus: Asymmetrical interactions with tadpoles may have limited invasive success in pond habitats (Mokany & Shine 2003a,b,c) |
| Culex quinquefasciatus (Vinogradova 2000) | North America 19th century (invasive) | Interspecific competition: Superiority in interspecific competition with native Culex may have contributed to declines of C. tarsalis in California (Smith et al. 1995) |
| Anopheles gambiae complex (Soper & Wilson 1943) | Brazil, 1930s (invasive, but eradicated in 1941) | Intraguild predation: Last instar larvae of this complex are facultatively predaceous on smaller Anopheles larvae (Koenraadt & Takken 2003; Koenraadt et al. 2004), and thus have the potential to impact native Anopheles via intraguild predation.
Interspecific competition: Absence of competitors may have facilitated invasion of man-made habitats |
| Ochlerotatus bahamensis (O’Meara et al. 1989, 1995a) | South Florida (non-native) | Interspecific competition: This with previously introduced Aedes albopictus may limit invasion success of Ochlerotatus bahamensis |
| Ochlerotatus japonicus (Andreadis et al. 2001) | Eastern North America (invasive) | Interspecific competition: This with invasive Ochlerotatus atropalpus and native O. triseriatus may limit invasion success of Ochlerotatus japonicus |
Predation and parasitism
When a non-native species escapes the predators and parasites that attack it in its native range, the likelihood of that species attaining high abundance and spreading can be enhanced. Similarly, the presence of predators or parasites that are capable of attacking non-native species may help to keep those species from becoming invasive, or indeed from succeeding in becoming established. These effects have been documented in several invasions, and form the basis of most biological control efforts directed at invasive species (Williamson 1996), including attempts to control invasive mosquitoes (Focks & Sackett 1985). These kinds of effects of predation or parasitism are straightforward, but there are other more subtle effects of predators and parasites, such as ‘apparent competition’, in which a shared enemy produces effects on prey that mimic effects of interspecific competition (Holt & Lawton 1994).
Aedes albopictus
Apparent competition
Apparent competition caused by shared parasites is an alternative hypothesis for the mechanism of displacement of North American Aedes aegypti by invading Aedes albopictus. Aedes mosquitoes typically harbor protozoan parasites in the genus Ascogregarina, and the species that parasitizes Aedes albopictus [Ascogregarina taiwanensis (Lien & Levine)] can infect Aedes aegypti, and can, under some circumstances, cause high mortality of Aedes aegypti (Munstermann & Wesson 1990; Blackmore et al. 1995). Ascogregarina culicis (Ross), which parasitizes Aedes aegypti, does not infect Aedes albopictus. Thus, these parasites have asymmetrical negative effects on their hosts that could cause declines of Aedes aegypti as observed in North America. However, Aedes albopictus has an advantage over Aedes aegypti even when there is virtually no parasitism of Aedes aegypti (Juliano 1998). Further, parasitism of Aedes aegypti by Ascogregarina taiwanensis is relatively rare in nature, and mortality of Aedes aegypti induced by Ascogregarina taiwanensis at typical intensities of parasitism is approximately equal to that of Aedes albopictus (Garcia et al. 1994). Thus, apparent competition via Ascogregarina taiwanensis may contribute to the declines of Aedes aegypti, but does not seem to be necessary for the negative effect of invading Aedes albopictus on Aedes aegypti.
Intraguild predation
Intraguild predation may limit invasion of A. albopictus into habitats dominated by O. triseriatus, which has fourth instar larvae that are more likely to prey upon newly hatched larvae of conspecifics, A. albopictus, or A. aegypti, and first instar larvae that are less vulnerable to such intraguild predation (Edgerly et al. 1999). In temperate regions, O. triseriatus hatches earlier in the year than does A. albopictus (Teng & Apperson 2000), so that this asymmetry in intraguild predation could favour coexistence of O. triseriatus and A. albopictus, despite the latter’s superiority in resource competition (Novak et al. 1993; Teng & Apperson 2000; Aliabadi & Juliano 2002, S. A. Juliano, unpublished data). This hypothesis remains untested and speculative.
Parasitism
Parasitism by gregarines may affect the interaction of Aedes albopictus and O. triseriatus. The gregarine parasites of O. triseriatus and Aedes albopictus [Ascogregarina barretti (Vavra) and Ascogregarina taiwanensis, respectively] cannot successfully infect each other’s hosts, so apparent competition is unlikely. When Aedes albopictus invades a new geographic area, parasitism by Ascogregarina taiwanensis is low in the new area for about 2 years following colonization (Munstermann & Wesson 1990; Blackmore et al. 1995; Fukuda et al. 1997; Aliabadi & Juliano 2002). Unparasitized Aedes albopictus larvae have slightly, but significantly, greater negative effect on survivorship of competing O. triseriatus larvae (i.e. parasitism reduces competitive ability), and also have more rapid development, than do parasitized Aedes albopictus (Aliabadi & Juliano 2002). Thus, escape from its parasite during the initial phases of colonization of a new site may make Aedes albopictus more competitive and yield increased population growth rate, both of which would improve its ability to become established at a site (Aliabadi & Juliano 2002). Because parasitism rates by Ascogregarina taiwanensis increase following colonization, these effects may not contribute to long-term impacts of Aedes albopictus on the invaded community.
Predation
Predation is a prominent feature of tree holes and tires in forested areas of the southern USA. The predators Toxorhynchites rutilus (Coquillet) and Corethrella appendiculata Grabham produce strong top-down effects on these communities, which are dominated by O. triseriatus after abundances of predators and other mosquito species are reduced by drought (Bradshaw & Holzapfel 1983, 1985; Lounibos 1983, 1985). The failure of A. albopictus to dominate in these habitats suggests a prominent role for predation by native species in limiting invasions (Lounibos et al. 2001). Laboratory tests show that A. albopictus is more vulnerable to predation by these species (Lounibos et al. 2001; Griswold & Lounibos 2005a). One mechanism contributing to this difference is behavioural responses to water-borne cues from predation. Ochlerotatus triseriatus shows reduced movement and foraging, which is associated with reduced risk of predation, in response to water-borne cues from T. rutilus predation, whereas A. albopictus does not show these behavioural changes (Kesavaraju & Juliano 2004).
Other invasive mosquitoes
As with competition, data on the role of predation in other mosquito invasions are rare (Table 2). One intriguing case is the potential role of intraguild predation by invasive members of the Anopheles gambiae complex (Table 2). Field study of this question is no longer possible, but laboratory comparisons of predaceous tendencies of Anopheles gambiae complex and Brazilian Anopheles could yield data on the role of intraguild predation in this invasion.
Modelling combined effects of predation and competition
This review has shown that A. albopictus is a superior competitor to O. triseriatus (Livdahl & Willey 1991; Novak et al. 1993; Teng & Apperson 2000; Aliabadi & Juliano 2002) and that A. albopictus is more vulnerable to predation by T. rutilus (Kesavaraju & Juliano 2004) and by C. appendiculata (Griswold & Lounibos 2005a), which can foster coexistence of A. albopictus and O. triseriatus by selective predation on the competitively dominant invasive species (Griswold & Lounibos 2005a,b). These results suggest that a trade-off between competitive ability and vulnerability to predation may affect invasion success of A. albopictus or the potential for coexistence of A. albopictus and O. triseriatus.
To understand the potential combined effects of inter-specific competition and predation in the invasion of A. albopictus, models of keystone predation and resource competition (Leibold 1996) can be adapted to invasions of container systems (Fig. 1). Although we describe this model for invasion of tree hole systems by A. albopictus, this model could describe the invasions of other prey mosquitoes, assuming relevant predators are present. For tree hole systems, we identify the predator as T. rutilus, although it should be applicable to other predators (e.g. C. appendiculata). In this model, developed using STELLA ® RESEARCH v. 5.1.1, a population of micro-organisms (M) grows on a substrate (C) that enters the system from outside (e.g. plant detritus, stem flow). Micro-organism resource consumption is modelled as a type 2 functional response (Juliano 2001) to substrate abundance (Fig. 1), with a maximal feeding rate (FM) and a half-saturation constant (KM). Micro-organisms are in turn a shared resource for two mosquito species, a resident (i.e. O. triseriatus) and an invader (i.e. A. albopictus) that compete for this resource. Consumption of the micro-organisms also follows a type 2 functional response with two parameters each for resident and invader: maximum consumption rate (FR and FI, respectively) and half saturation constant (KR and KI, respectively). Population growth of the mosquitoes is a function of feeding rate and conversion efficiency (cR and cI, respectively). In this model, interspecific competition occurs only via the shared micro-organism resource. Competitive superiority is determined by the values of half saturation constant (Ks) for the competitors, with the lower K (i.e. able to feed successfully at low food availability) resulting in greater competitive ability (see Grover 1997 for a thorough development of theory behind this statement). These mosquitoes are in turn eaten by a predator (i.e. T. rutilus), with predator feeding a two-prey type 2 functional response with maximal feeding rate (FPR and FPI) and half saturation constant (KPR and KPI) for each prey species. In this context, values of KP for the predator determine the relative vulnerabilities of the prey to predation, with lower KP yielding greater prey vulnerability (i.e. the predator can find and eat prey even when they are scarce).
Figure 1.
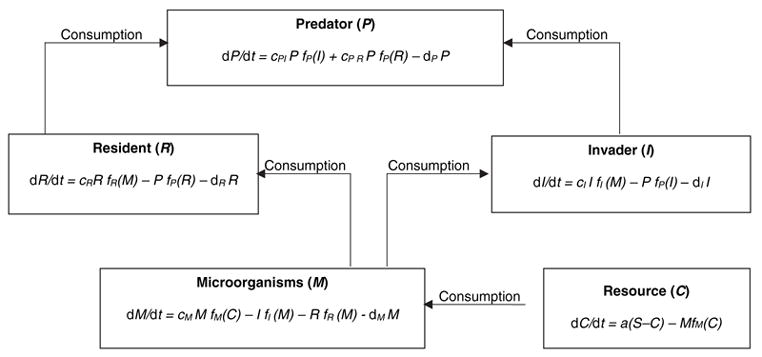
Model of predation after invasion by a superior competitor (I) into a community consisting of a resident prey (R), a predator (P), and micro-organisms (M). This model is an extension of that of Leibold (1996), adding a lower trophic level (M) that is a resource for the competitors, and incorporating type 2 functional responses for competing prey (R, I), P and M. Conversion efficiencies indicate the rate at which consumed food is converted into growth for micro-organisms (cM), invader (cI), resident (cR), and predator feeding on invader (cPI) and resident (cPR). Feeding rates of micro-organisms feeding on the resource [fM (C)], invader and resident feeding on micro-organisms [fI(M) and fR(M), respectively], and predator feeding on invader [fP(I)] and resident [fP(R)] are all hyperbolic functions of the general form: f(X) = F X/(K + X), where X = food abundance, F = maximum feeding rate and K = food abundance yielding one half maximum feeding rate. The two species functional responses for the predator feeding on resident and invader are f(R) = FPR R/(R + KPR + I KPR/KPI) and f(I) = FPI I/(I + KPI + R KPI/KPR), respectively. Lower Ks result in greater competitive ability for the consumer (i.e. ability to feed at low food availability) and greater vulnerability for the prey. Death rates (dP, dR, dI, dM) for each species are density-independent. Resources (C) for micro-organisms (e.g. plant detritus) become available at a rate logistically dependent on their abundance, with a maximum availability of S (supply).
Simulations focused on the effects of vulnerability to predation (KPI) and competitive ability (KI) of the invader, and on overall productivity of the environment (as indicated by S, the maximum nutrient availability). We are specifically interested in the conditions under which a predator can serve as a barrier to invasion, and under which the presence of a predator can result in stable coexistence of the invader and the resident prey. We tested effects of relative vulnerability of the invading mosquito (KPI) across a productivity (S) gradient, and when the invader has a large or a small competitive advantage over the resident (i.e. KI much less than KR vs. KI slightly less than KR, respectively). The invader was always the superior competitor. All other parameters were held constant, and parameters for the two prey mosquitoes (ds, cs, cPs, FPs, and Fs) were equal so that death rates, conversion efficiencies for food, nutritional value to the predator and maximal feeding rates are all the same for the resident and the invader.
In the first set of simulations, the invader has a strong competitive advantage (i.e. KI ≪ KR) over the resident. In the absence of the predator, invasion always results in competitive exclusion of the resident. Further, the invader is always more vulnerable to predation (KPI ≪ KPR). With the predator present, the outcome of the invasion depends on the invader’s relative vulnerability to predation (horizontal axis, Fig. 2a). When the invader has relatively low vulnerability to predation (left in Fig. 2a), it always excludes the resident species, just as it would in the absence of predation. Further, when productivity of the system is low and the invader has low vulnerability to predation (lower left, Fig. 2a), the invader population goes through stable oscillations, and the predator population goes through expanding oscillations resulting in predator extinction following invasion, and extinction of the resident. When the invader is highly vulnerable to predation (right in Fig. 2a), the presence of a predator acts as a barrier to invasion by the superior competitor, particularly at high productivity (Fig. 2a, upper right). Between these extremes are values of predator KPR and S that result in stable coexistence of the three species (Fig. 2a, shaded region). Under these conditions, the predator would foster coexistence of the invader and resident via a keystone predator effect. A greater range of conditions can permit coexistence (i.e. the width of the shaded area in Fig. 2a is greater) when productivity is lower and vulnerability to predation of the invader higher (lower right, Fig. 2a). This result may seem counterintuitive, but it emphasizes that coexistence of competitors is more likely when they are differentially affected and limited by their environment. In less productive environments, with high vulnerability to predation, the invading superior competitor would be relatively free from resource limitation and effects of competition, and almost exclusively limited by predation. Similarly, the resident poorer competitor would be limited almost totally by resource competition, and relatively unaffected by the predator, which disproportionately attacks (and benefits from) the invader.
Figure 2.
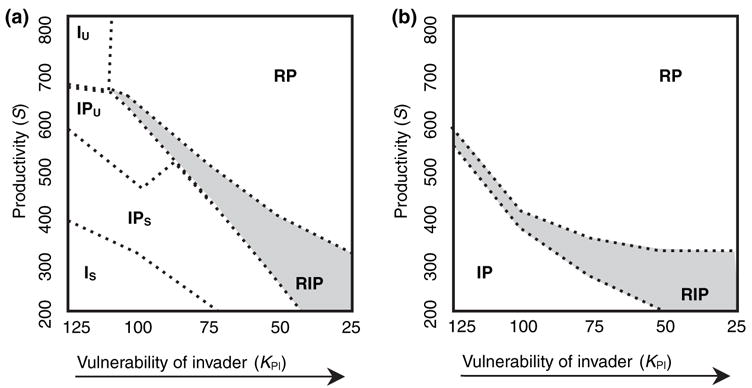
Simulations of invasion of a stable predator–prey system by a superior competitor, illustrating the sensitivity of the outcome to the invader’s vulnerability to predation (increasing vulnerability with decreasing KPI) and environmental productivity (S), the maximal resource availability for the micro-organism population. Simulations ran for 30 000 time steps with the invader invading at time 2000. Parameters are in arbitrary units so results only illustrate the qualitative trends expected in this system. Letters on the graph indicate species combinations attaining stable coexistence (I = invader, R = resident, P = predator, subscript S = stable oscillations, subscript U = unstable expanding oscillations; e.g., RP = resident + predator). Shaded regions indicate combinations of KPI and S where the presence of the predator fosters coexistence of competing resident and invader via a keystone predator effect. (a) Competitive advantage of invader is large (KI = 100, KR = 600). (b) Competitive advantage of the invader is small (KI = 300, KR = 600).
The second set of simulations, in which the competitive advantage of the invader is less (Fig. 2b), also shows that predator-mediated coexistence is more likely when the invader is more vulnerable to predation and when the environment is less productive (Fig. 2b, lower right). When the competitive advantage of the invader is lower, the range of conditions under which the predator acts as a barrier to invasion encompasses a greater proportion of the range of simulated parameters (i.e. the region labelled RP is relatively larger in Fig. 2b than in Fig. 2a). When competitive advantage of the invader is smaller the outcomes of the invasion are less likely to include unstable (expanding) oscillations (Fig. 2b) at least in the range of S and KPI used in these simulations. When the invader is highly effective at foraging for the microbial resource (i.e. Fig. 2a), there is a strong tendency for populations to oscillate, and this often destabilizes the invaded system, particularly as the invader’s vulnerability to the predator decreases (towards the left, Fig. 2a). Under these conditions, as productivity increases, the amplitude of population oscillations for all members of the community increases with invasion, and stochastic extinctions occur, sometimes of all members of the community.
This model predicts that productivity of the environment should affect whether competitive exclusion of the resident (at low productivity), coexistence (at intermediate productivity), or failure of the invasion (at high productivity) should be the result of this invasion. Productivity for container systems would be directly related to the input rate of detritus and nutrients in stem flow. There is some evidence for greater productivity of tree holes compared with man-made containers in forested habitats (e.g. Livdahl & Willey 1991; Aspbury & Juliano 1998), suggesting that invasion success of A. albopictus and displacement of O. triseriatus should be more likely in man-made containers, which is consistent with observations in Florida (Lounibos et al. 2001). Empirical determination of the parameters of this model (mainly half saturation constants for predator and prey, and environmental productivity) would provide a means of making quantitative predictions about whether exclusion, coexistence, or failed invasion is expected in predator-dominated habitats. The model also predicts successful invasion should be less common in predator-dominated habitats, and this prediction should be tested using field observations and experiments.
Climatic limitations on invasions by Aedes albopictus
There is at least one case in which A. albopictus had a competitive advantage over a resident species in laboratory experiments, yet introduction of A. albopictus (which was intentional) failed to result in an established population. On islands in the southern Pacific Ocean, A. polynesiensis Marks is the primary vector of Wuchereria bancrofti, which causes lymphatic filariasis in humans (Rozeboom 1971; Rosen et al. 1976). Cage experiments suggested that A. albopictus, which cannot transmit W. bancrofti, displaces A. polynesiensis in <1 year, probably via interspecific competition among larvae for food, and among adults via satyrization (Rozeboom 1971). Despite this laboratory outcome, and the demonstrated success of A. albopictus as an invader of other parts of the world, deliberate introduction of A. albopictus to an atoll in the Pacific with A. polynesiensis failed to establish populations of A. albopictus (Rosen et al. 1976). Because this investigation focused on the presumed impact of introduced A. albopictus on resident A. polynesiensis, there were no control introductions of A. albopictus without A. polynesiensis, hence it is unknown whether competition from the resident inhibited colonization by the introduced species. Alternative explanations for the failed introduction include the unsuitably dry, hot climate on this island (Rosen et al. 1976; see also Alto & Juliano 2001; Juliano et al. 2002; K. S. Costanzo, B. Kesavaraju and S. A. Juliano, 2005, personal communication), reliance on a single release period, and dispersal by A. albopictus adults off this isolated atoll and into the vast dispersal sink of the ocean (Rosen et al. 1976). This case illustrates how difficult it is to predict whether a non-native species will become invasive, even when there is some background information, and even when the process of introduction is controlled. Further, this field trial shows the limitations of laboratory studies like those of Rozeboom (1971) for predicting invasion outcomes.
Climate limits the ranges of most invasive species, and Nawrocki & Hawley (1987) accurately predicted the northern limits of the distribution of A. albopictus in North America based on the native range of the species in Japan, where North American colonists originated (Hawley et al. 1987). Cold-hardiness in temperate populations of A. albopictus results from egg diapause, which is absent in tropical populations of this species (Hawley et al. 1987, 1989). However, expression of diapause evolved relatively rapid during the spread of invasive populations (Lounibos et al. 2003a). Incidence of this trait decreased as temperate A. albopictus spread into warmer, southerly latitudes of the USA, and diapause appeared at low frequencies in tropical A. albopictus that spread southward from tropical to temperate regions of southern Brazil (Lounibos et al. 2003a). Diapause responses at similar (N vs. S) latitudes in Brazil and the USA have not converged, perhaps because of genetic constraints of different founder populations (tropical vs. temperate). Life history traits of size and age at pupation of North American A. albopictus may also have evolved rapidly, with data suggesting latitudinal differentiation at about 16 years post-introduction (P. Armbruster & J. E. Conn, in preparation). There is a non-significant trend towards increased size at pupation and decreased age at pupation for females from populations from more northern latitudes, suggesting counter gradient selection on growth and development rates of larvae (P. Armbruster & J. E. Conn, in preparation).
The interaction of abiotic and biotic factors in determining invasion success was suggested by field experiments demonstrating that the outcome of interspecific competition between A. albopictus and A. aegypti larvae was independent of whether the experiment was conducted at a site of coexistence or of exclusion of A. aegypti (Juliano et al. 2004). This result implies that differences in the aquatic environments at these types of sites do not account for the heterogeneous patterns of exclusion of A. aegypti or its coexistence with A. albopictus in south Florida cemeteries (Juliano et al. 2004). Cemetery-specific microclimates, which contribute to differential egg survivorship (Juliano et al. 2002; K. S. Costanzo, B. Kesavaraju and S. A. Juliano, 2005, personal communication; L. P. Lounibos, unpublished data) are believed to shift competitive advantage and to determine whether coexistence or exclusion is the outcome of invasion. Thus, climate impacts life cycle stages that are not involved in competition, and these impacts can determine invasion success, effects of invaders and community composition following invasion.
DISEASE OUTBREAKS ASSOCIATED WITH INVASIVE MOSQUITOES
Introduced mosquitoes may affect human health by (i) simultaneous introduction of a novel vector and novel pathogen, (ii) acquisition by an introduced vector of a native pathogen, or (iii) independent introductions of a novel vector and a novel pathogen. Simultaneous introduction of a novel vector and a novel pathogen may be sufficient to create a new public health threat, with potential to cause large outbreaks of disease, particularly because the host population will likely have limited herd immunity (=reduced probability of epidemic transmission of a disease, even among susceptible individuals, which arises because of the presence of a substantial proportion of immune individuals within a population). If an invasive vector takes on a role in an existing disease transmission cycle, it changes (presumably for the worse) the nature of an existing public health threat. In such cases, the risk to public health derives not from the novelty of the disease, but only from an increase in the transmission rate because of the efficiency of the invasive vector. If an invasive vector acquires, sometime after establishment, an association with an independently introduced pathogen, susceptible hosts are exposed to a novel pathogen with a new vector. This situation may be particularly prone to unpredictable disease dynamics. All three paths to mosquito-borne disease outbreaks have occurred (Table 3).
Table 3.
Human and animal disease outbreaks associated with invasive mosquitoes
| Disease | Location | Dates of epidemics | Reference | Invasive mosquito | Source of pathogen |
|---|---|---|---|---|---|
| Yellow fever | Americas | 16–20th centuries | Tabachnick (1991) | Aedes aegypti | Introduced with the mosquito |
| Dengue | Americas | 17–20th centuries | Gubler (1997) | Aedes aegypti | Introduced with the mosquito |
| Dengue | Asia | 19–20th centuries | Gubler (1997) | Aedes aegypti | Native |
| Dengue | Hawaii | 2001 | Mortality and Morbidity Weekly Reports (MMWR) (2002) | Aedes albopictus | Introduced after the mosquito |
| West Nile encephalitis | North America | 1999–present | Kramer & Bernard (2001) | Culex pipiens* | Introduced after the mosquitoes |
| Avian malaria | Hawaii | 20th century | Van Riper et al. (1986) | Culex quinquefasciatus* | Introduced after the mosquito |
| Human malaria | Brazil | 1930–1940 | Soper & Wilson (1943) | Anopheles gambiae complex† | Native |
| Human malaria | Mauritius | 1866–67 | Ross (1911) | Anopheles gambiae complex | Introduced before the mosquito |
| Human malaria | Peru | 1992–1999 | Lounibos (2002) | Anopheles darlingi | Native |
Species of the C. pipiens complex.
Only includes the anthropophilic species of the complex, Anopheles gambiae s.s. and Anopheles arabiensis.
Simultaneous introduction of vector and pathogen
Simultaneous introduction of vector and pathogen appears possible in only two disease outbreaks, and both involve historical introductions of Aedes aegypti and associated viruses. Yellow fever originated in Africa and appears to have been spread initially by the slave trade of the 16–17th centuries (Lounibos 2002). Outbreaks of yellow fever occurred in many North American cities, as far north as New York City, and it is virtually certain that these outbreaks occurred when both the vector and virus arrived via ship. Aedes aegypti is a tropical mosquito, lacking diapause (Christophers 1960), hence it is unlikely that this mosquito became permanently established in these northern cities. Introduction of dengue to South America may also have occurred with the introduction of A. aegypti in the 16–17th centuries, based on reports of ‘Dengue like illnesses’ in the 17th century (Gubler 1997). This implies a West African origin of introduced dengue. Because these simultaneous introductions were associated with ship transport of enslaved Africans, they may present a special case of simultaneous introduction of vector, pathogen and a substantial population of infected hosts. The limited data suggest this situation has not occurred with recent mosquito introductions.
Introduced vectors transmitting native pathogens
Introduced vectors transmitting native pathogens appear to have caused outbreaks in two very different disease systems. Aedes aegypti is the major vector of urban dengue in tropical Asia (Eldridge et al. 2000, Table 2), where dengue is likely native, with all four serotypes of this virus circulating in sylvan cycles in Southeast Asia (Eldridge et al. 2000). Thus, since its invasion of Asia in the late 19th century, A. aegypti became the primary vector of a resident pathogen. Thus, A. aegypti provides examples of both enhanced transmission of a resident disease (dengue in Asia) and transmission of a disease introduced with the vector (yellow fever and dengue in the Americas). A similar case involves invading Anopheles gambiae complex in Brazil and resident pathogens causing malaria (Plasmodium falciparum, P. vivax; Lounibos 2002). This case is unusual in that Anopheles gambiae had a long association with these Plasmodium species in its native range (Lounibos 2002), hence the pathogens were not ‘new’ to the vector. In Brazil, native Anopheles species had been responsible for transmitting malaria, but arrival of a new, more efficient vector resulted in epidemics of the disease.
Independent introductions of vectors and pathogens
Independent introductions of vectors and pathogens that have associations in their native ranges appear to be the most common cause of disease outbreaks (Table 2). For A. albopictus in Hawaii in 2001, it seems likely that dengue virus arrived in Hawaii (probably with infected humans; the viral strain was identical to isolates from Tahiti) sometime after the vector was established (Mortality and Morbidity Weekly Reports (MMWR) 2002). Thus, this case represents a two-step relocation of a vector–pathogen system from Asia to Hawaii. Members of the C. pipiens complex are major vectors of WNV in Eurasia, and appear to have taken on this same role in North America where the pathogen was introduced long after the mosquito (Fonseca et al. 2004). Fonseca et al. (2004) proposed that hybridization of two Old World taxa (bird-biting C. pipiens and mammal-biting C. molestus – the latter which may be a race, subspecies, or separate species) in North America resulted in populations that readily take blood from both kinds of hosts, thus functioning as both enzootic and bridge vectors of WNV (Fonseca et al. 2004). The importance of vector hybrids in the North American WNV life cycle, which includes other vector species, remains speculative. Culex quinquefasciatus became established in the Hawaiian Islands in the early 19th century, well before the arrival of avian malaria (Van Riper et al. 1986). Outbreaks of this disease among native Hawaiian birds have had serious consequences for conservation of endemic avian species, particularly at low elevations (Van Riper et al. 1986). Malaria was almost certainly present among Indian railway workers who immigrated to Mauritius prior to the arrival of Anopheles gambiae (s.l.) by ship from the African mainland or Madagascar (Ross 1911).
WHAT WE LEARN FROM INVASIVE MOSQUITOES
The interactions of invasive species vary in space and time and depend on local conditions and their interacting species. Even for the best-studied invasive mosquito species, A. albopictus, there is no single answer to the question of which ecological processes predominate. Effects of interspecific competition are evident in interactions with A. aegypti and to a lesser extent C. pipiens, but in other invaded systems, the role of predation as a barrier to invasion seems to be paramount. Climatic limitation is evident, but so is evolution of locally adapted life history traits that overcome climatic limitation. Despite the absence of a single dominant pattern in the interactions of this invasive species, the extensive investigations of the ecology of A. albopictus as an invasive species provide us with a guide to the investigation of the mechanisms controlling impacts of and barriers to invasions, not just for other mosquitoes, but more generally for any animal species. For A. albopictus, multiple hypotheses accounting for observed effects on resident species and communities have been tested, and, equally important, similar hypotheses have been tested in the field in multiple locations, with multiple populations, and with reference to multiple interacting native species. These diverse investigations of A. albopictus have yielded a clear understanding of the mechanisms acting during invasion, and how they vary. The extent of ecological investigations of A. albopictus is further justified by its potential for impacts on human health (e.g. Hawley 1988).
Invasion by Aedes albopictus illustrates the importance of monitoring the process from its early phases until it has stabilized for a thorough understanding of the dynamics of effects of invaders. The dynamics of invader and resident populations and communities are likely to be obscured, at best, or obliterated by the passage of time, and in the case of mosquitoes this makes it now impossible to recover information on interactions that took place during historical invasions by Anopheles gambiae complex in Brazil or Mauritius, Aedes aegypti in the Americas and Asia, and members of the C. pipiens complex worldwide (Lounibos 2002). Monitoring alone is insufficient. Experimental tests of alternative hypotheses for mechanisms producing effects of invaders on native populations and communities, for barriers to successful invasion, and in the case of invasive mosquitoes, for effects on transmission of human disease, are necessary.
Acknowledgments
Authors thank L. Blaustein for the invitation to write this review, J.E. Conn, J. Chase, V.A. Borowicz, G. White and three anonymous referees for useful discussion or comments on the manuscript, G.F. O’Meara and P. Armbruster for sharing unpublished data. This work was supported by grants from the National Institute of Allergy and Infectious Disease (R15 AI-51374 and R01 AI-44793).
Footnotes
Editor, Jonathan Chase
References
- Aliabadi BK, Juliano SA. Escape from gregarine parasites affects the competitive impact of an invasive mosquito. Biol Invasions. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Juliano SA. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): implications for range expansion. J Med Entomol. 2001;38:646–656. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera: Culicidae) in Connecticut, USA. J Med Entomol. 2001;38:774–779. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- Aspbury AS, Juliano SA. Negative effects of drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia. 1998;115:137–148. doi: 10.1007/s004420050500. [DOI] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:117–127. [Google Scholar]
- Bédhomme S, Agnew P, Sidbore C, Michalakis Y. Pollution by conspecifics as a component of intraspecific competition among Aedes aegypti larvae. Ecol Entomol. 2005;30:1–7. [Google Scholar]
- Blackmore MS, Scoles GA, Craig GB., Jr Parasitism of Aedes aegypti and Ae. albopictus (Diptera: Culicidae) by Ascogregarina spp. (Apicomplexa: Lecudinidae) in Florida. J Med Entomol. 1995;32:847–852. doi: 10.1093/jmedent/32.6.847. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Predator mediated non-equilibrium coexistence of tree-hole mosquitoes in southeastern North America. Oecologia. 1983;57:249–256. doi: 10.1007/BF00379586. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. The distribution and abundance of treehole mosquitoes in North America: perspectives from north Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, Florida, USA: 1985. pp. 3–23. [Google Scholar]
- Braks MAH, Honório NA, Lourenço-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida, USA. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourenço-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- Carrieri M, Bacchi M, Bellini R, Maini S. On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ Entomol. 2003;32:1313–1321. [Google Scholar]
- Chan KL, Chan YC, Ho BC. Aedes aegypti (L) and Aedes albopitus (Skuse) in Singapore city: 4. Competition between species. Bull World Health Organ. 1971;44:64. [PMC free article] [PubMed] [Google Scholar]
- Chow CY. Observations on mosquitoes breeding in plant systems in Yunnan. Ann Entomol Soc Am. 1949;42:465–470. [Google Scholar]
- Christophers R. Its Life History, Bionomics and Structure. Cambridge University Press; London, UK: 1960. Aedes aegypti (L.) the Yellow Fever Mosquito. [Google Scholar]
- Clements AN. Development, Nutrition and Reproduction. I. Chapman and Hall; London, UK: 1992. The Biology of Mosquitoes. [Google Scholar]
- Costanzo KS, Mormann K, Juliano SA. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae) J Med Entomol. 2005;42 doi: 10.1093/jmedent/42.4.559. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty MP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Thompson K. Eight ways to be a colonizer; two ways to be an invader: a proposed nomenclature scheme for invasion ecology. Bull Ecol Soc Am. 2000;81:226–230. [Google Scholar]
- Edgerly JS, Willey MS, Livdahl T. The community ecology of Aedes hatching: implications for a mosquito invasion. Ecol Entomol. 1993;18:123–128. [Google Scholar]
- Edgerly JS, Willey MS, Livdahl T. Intraguild predation among larval treehole mosquitoes Aedes albopictus, Ae. aegypti, and Ae. triseriatus (Diptera: Culicidae), in laboratory microcosms. J Med Entomol. 1999;36:394–399. doi: 10.1093/jmedent/36.3.394. [DOI] [PubMed] [Google Scholar]
- Eldridge BF, Scott TW, Day JF, Tabachnick WJ. Arbovirus diseases. Medical Entomology. In: Eldridge BF, Edman JD, editors. A Textbook on Public Health and Veterinary Problems Caused by Arthropods. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. pp. 415–460. [Google Scholar]
- Eritja R, Escosa R, Lucientes J, Marquès E, Molina R, Roiz D, et al. Worldwide invasion of vector mosquitoes: present European distribution and challenges for Spain. Biol Invasions. 2005;7:85–87. [Google Scholar]
- Focks DA, Sackett SR. Some factors affecting interaction of Toxorhynchites amboinensis with Aedes and Culex in an urban environment. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, Florida, USA: 1985. pp. 55–64. [Google Scholar]
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, et al. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Fontenille D, Toto JC. Aedes (Stegomyia) albopictus (Skuse), a potential new dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7:1066–1067. doi: 10.3201/eid0706.010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forattini OP. Identificação de Aedes (Stegomyia) albopictus (Skuse) no Brasil. Rev Saude Publica. 1986;20:244–245. doi: 10.1590/s0034-89101986000300009. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Osborne RW, Barnard DR. Parasites of the Asian tiger mosquito and other container-inhabiting mosquitoes (Diptera: Culicidae) in northern Florida. J Med Entomol. 1997;34:226–233. doi: 10.1093/jmedent/34.2.226. [DOI] [PubMed] [Google Scholar]
- Garcia JJ, Fukuda T, Becnel JJ. Seasonality, prevalence and pathogenicity of the gregarine Ascogregarina taiwanensis (Apicomplexa: Lecudinidae) in mosquitoes from Florida. J Am Mosq Cont Assoc. 1994;10:413–418. [PubMed] [Google Scholar]
- Grill CP, Juliano SA. Predicting species interactions based on behaviour: predation and competition in container-dwelling mosquitoes. J Anim Ecol. 1996;65:63–76. [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol Entomol. 2005a;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2005b;86 doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover JP. Resource Competition. Chapman & Hall; London, UK: 1997. [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhagic Fever. CABI International; New York, NY, USA: 1997. pp. 1–22. [Google Scholar]
- Harper JP, Paulson SL. Reproductive isolation between Florida strains of Aedes aegypti and Aedes albopictus. J Am Mosq Cont Assoc. 1994;10:88–92. [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Cont Assoc. 1988;4(Suppl):1–40. [PubMed] [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB. Aedes albopictus in North America: probable introduction in used tires from Northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Hawley WA, Pumpuni CB, Brady RH, Craig GB. Overwintering survival of Aedes albopictus (Diptera: Culicidae) eggs in Indiana. J Med Entomol. 1989;26:122–129. doi: 10.1093/jmedent/26.2.122. [DOI] [PubMed] [Google Scholar]
- Ho BC, Ewert A, Chew L. Interspecific competition among Aedes aegypti, Ae. albopictus, and Ae. triseriatus (Diptera: Culicidae): larval development in mixed cultures. J Med Entomol. 1989;26:615–623. doi: 10.1093/jmedent/26.6.615. [DOI] [PubMed] [Google Scholar]
- Holt RD, Lawton JH. The ecological consequences of shared natural enemies. Ann Rev Ecol Syst. 1994;25:495–520. [Google Scholar]
- Joyce CP. Potentialities for accidental establishment of exotic mosquitoes in Hawaii. Proc Haw Entomol Soc. 1961;17:403–413. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA. Non-linear curve fitting: predation and functional response curves. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. 2. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in south Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenraadt CJM, Takken W. Cannibalism and predation among larvae of the Anopheles gambiae complex. Med Vet Entomol. 2003;17:61–66. doi: 10.1046/j.1365-2915.2003.00409.x. [DOI] [PubMed] [Google Scholar]
- Koenraadt CJM, Majambere S, Hemerik L, Takken W. The effects of food and space on the occurrence of cannibalism and predation among larvae of Anopheles gambiae s.l. Entomol Exp Appl. 2004;112:125–134. [Google Scholar]
- Kramer LD, Bernard KA. West Nile virus in the western hemisphere. Curr Opin Infect Dis. 2001;14:519–525. doi: 10.1097/00001432-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Lancaster MJ. Dissertation. University of Illinois; Urbana/Champaign, IL, USA: 2004. Aedes albopictus in Peoria, an invasion by an exotic mosquito vector into an urban La Crosse virus endemic area in Illinois. [Google Scholar]
- Leibold MA. A graphical model of keystone predators in food webs: trophic regulation of abundance, incidence, and diversity patterns in communities. Am Nat. 1996;147:784–812. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. The mosquito community of tree holes in subtropical Florida. In: Frank JH, Lounibos LP, editors. Phytotelmata: Terrestrial Plants as Hosts for Aquatic Insect Communities. Plexus, Medford; New Jersey, USA: 1983. pp. 223–246. [Google Scholar]
- Lounibos LP. Interactions influencing production of tree hole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, Florida, USA: 1985. pp. 65–77. [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Ann Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, et al. Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Invasions. 2001;3:151–166. [Google Scholar]
- Lounibos LP, Suárez S, Menéndez Z, Nishimura N, Escher RL, O’Connell SM, et al. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- Lounibos LP, Escher RL, Lourenço-de-Oliveira R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Ann Entomol Soc Am. 2003a;96:512–518. [Google Scholar]
- Lounibos LP, O’Meara GF, Nishimura N, Escher RL. Interactions with native mosquito larvae regulate the production of Aedes albopictus from bromeliads in Florida. Ecol Entomol. 2003b;28:551–558. [Google Scholar]
- McMichael AJ, Bouma MJ. Global changes, invasive species and human health. In: Mooney HA, Hobbs RJ, editors. Invasive Species in a Changing World. Island Press; Washington, DC, USA: 2000. pp. 191–210. [Google Scholar]
- Mokany A, Shine R. Competition between tadpoles and mosquito larvae. Oecologia. 2003a;135:615–620. doi: 10.1007/s00442-003-1215-6. [DOI] [PubMed] [Google Scholar]
- Mokany A, Shine R. Biological warfare in the garden pond: tadpoles suppress the growth of mosquito larvae. Ecol Entomol. 2003b;28:102–108. [Google Scholar]
- Mokany A, Shine R. Oviposition site selection by mosquitoes is affected by cues from conspecific larvae and anuran tadpoles. Aust Ecol. 2003c;28:33–37. [Google Scholar]
- Moore CG. Aedes albopictus in the United States: current status and prospects for further spread. J Am Mosq Control Assoc. 1999;15:221–227. [PubMed] [Google Scholar]
- Mortality and Morbidity Weekly Reports (MMWR) Imported Dengue United States, 1999 and 2000. 2002;51:281–283. [PubMed] [Google Scholar]
- Moyle PB, Light T. Biological invasions of fresh water: empirical rules and assembly theory. Biol Conserv. 1996;78:149–162. [Google Scholar]
- Munstermann LE, Wesson DM. First record of Ascogregarina taiwanensis (Apiomplexa: Lecudinidae) in North American Aedes albopictus. J Am Mosq Cont Assoc. 1990;6:235–243. [PubMed] [Google Scholar]
- Nasci RS, Hare SG, Willis FS. Interspecific mating between Louisiana strains of Aedes albopictus and Aedes aegypti in the field and laboratory. J Am Mosq Cont Assoc. 1989;5:416–421. [PubMed] [Google Scholar]
- Nawrocki SJ, Hawley WA. Estimation of the northern limits of distribution of Aedes albopictus in North America. J Am Mosq Cont Assoc. 1987;3:314–317. [PubMed] [Google Scholar]
- Novak MG, Higley LG, Christianssen CA, Rowley WA. Evaluating larval competition between Aedes albopictus and A. triseriatus (Dipera: Culicidae) through replacement series. Environ Entomol. 1993;22:311–318. [Google Scholar]
- O’Brochta DA. Transgenic mosquitoes: the state of the art. In: Takken W, Scott TW, editors. Ecological Aspects for Application of Genetically Modified Mosquitoes. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2003. pp. 15–24. [Google Scholar]
- O’Meara GF, Larson VL, Mook D, Latham MD. Aedes bahamensis: its invasion of south Florida and association with Aedes aegypti. J Am Mosq Control Assoc. 1989;5:1–5. [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995a;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Patteson AW. Exotic tank bromeliads harboring immature Aedes albopictus and Aedes bahamensis (Diptera: Culicidae) in Florida. J Vector Ecol. 1995b;20:216–224. [Google Scholar]
- Ribiero JMC, Spielman A. The satyr effect: a model predicting parapatry and species extinction. Am Nat. 1986;128:513–528. [Google Scholar]
- Richardson DM, Bond WJ, Dean WRJ, Higgins SI, Midgely GF, Milton SJ, et al. Invasive alien species and global change: a South African perspective. In: Mooney HA, Hobbs RJ, editors. Invasive Species in a Changing World. Island Press; Washington, DC, USA: 2000. pp. 303–349. [Google Scholar]
- Romi R, Di Luca M, Majori G. Current status of Aedes albopictus and Aedes atropalpus in Italy. J Am Mosq Cont Assoc. 1999;15:425–442. [PubMed] [Google Scholar]
- Rosen L, Rozeboom LE, Reeves WC, Saugrain J, Gubler DJ. A field trial of competitive displacement of Aedes polynesiensis by Aedes albopictus on a Pacific atoll. Am J Trop Med Hyg. 1976;25:906–913. doi: 10.4269/ajtmh.1976.25.906. [DOI] [PubMed] [Google Scholar]
- Ross R. The Prevention of Malaria. John Murray; London, UK: 1911. [Google Scholar]
- Rozeboom LE. Relative densities of freely breeding populations of Aedes (S) polynesiensis Marks and Aedes (S) albopictus Skuse: a large cage experiment. Am J Trop Med Hyg. 1971;20:356–362. doi: 10.4269/ajtmh.1971.20.356. [DOI] [PubMed] [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J. The population biology of invasive species. Ann Rev Ecol Syst. 2001;32:305–332. [Google Scholar]
- Santos R, La C. Updating of the distribution of Aedes albopictus in Brazil (1997–2002) Rev Saude Publica São Paulo. 2003;37:671–673. [PubMed] [Google Scholar]
- Shroyer DA. Establishment of Wyeomyia mitchelli on the island of Oahu, Hawaii. Mosq News. 1981;41:805–806. [Google Scholar]
- Simberloff D, Gibbons L. Now you see them, now you don’t! – population crashes of established introduced species. Biol Invasions. 2004;6:161–172. [Google Scholar]
- Smith PT, Reisen WK, Cowles DA. Interspecific competition between Culex tarsalis and Culex quinquefasciatus. J Vector Ecol. 1995;20:139–146. [Google Scholar]
- Soper FL, Wilson DB. Anopheles gambiae in Brazil 1930–1940. The Rockefeller Foundation; NY, USA: 1943. [Google Scholar]
- Sota T. Larval diapause, size and autogeny in the mosquito Aedes togoi (Diptera, Culicidae) from tropical to subarctic zones. Can J Zool. 1994;72:1462–1468. [Google Scholar]
- Sucharit S, Tumrasvin W, Vutikes S, Viraboonchai S. Interaction between larvae of Aedes aegypti and Aedes albopictus in mixed experimental populations. Southeast Asian J Trop Med Publ Health. 1978;9:93–97. [PubMed] [Google Scholar]
- Sunahara T, Mogi M. Priority effects of bamboo-stump mosquito larvae: influences of water exchange and leaf litter input. Ecol Entomol. 2002;27:346–354. [Google Scholar]
- Tabachnick WJ. Evolutionary genetics and arthropod-borne disease. The yellow fever mosquito. Am Entomol. 1991;37:14–24. [Google Scholar]
- Takken W, Boëte C. An introduction to the ecological challenges concerning the use of genetically-modified mosquitoes for disease control. In: Takken W, Scott TW, editors. Ecological Aspects for Application of Genetically Modified Mosquitoes. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2003. pp. 9–14. [Google Scholar]
- Taniguchi Y, Nakano S. Condition-dependent competition: implications for the distributions of stream fishes. Ecology. 2000;81:2027–2039. [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperatures. J Med Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Van Riper C, Van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr. 1986;56:327–344. [Google Scholar]
- Vinogradova EB. Culex pipiens Mosquitoes; Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control. Pensoft, Sofia; Bulgaria: 2000. [Google Scholar]
- Walter Reed Biosystematics Unit. 2001 Systematic Catalog of Culicidae. Smithsonian Institution; Washington, DC, USA: 2005. [accessed 21 March 2005]. http://www.mosquitocatalog.org/main.asp. [Google Scholar]
- Ward RA. Mosquito fauna of Guam: case history of an introduced fauna. In: Laird M, editor. Commerce and the Spread of Pests and Disease Vectors. Praeger; New York, USA: 1984. pp. 143–162. [Google Scholar]
- Weinstein P, Laird M, Browne G. Exotic and Endemic Mosquitoes in New Zealand as Potential Arbovirus Vectors. Occas. Pap. Minist. Health; Wellington, New Zealand: 1997. p. 16. [Google Scholar]
- Williamson M. Biological Invasions. Chapman & Hall; New York, USA: 1996. p. 244. [Google Scholar]


