Abstract
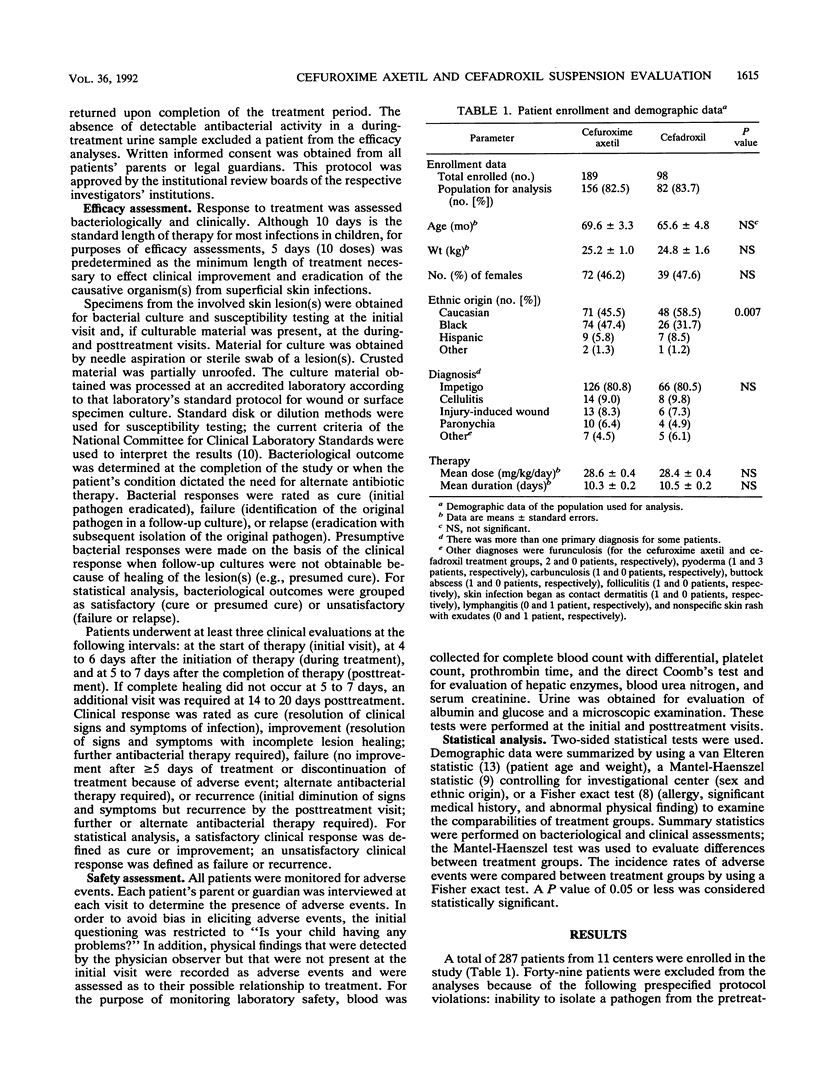
A randomized, single-blind, multicenter study was conducted to evaluate the safety and efficacy of cefuroxime axetil and cefadroxil suspensions for the treatment of skin or skin structure infections in 287 children. Each drug was given at a dosage of 30 mg/kg of body weight per day in two divided doses. Staphylococcus aureus and Streptococcus pyogenes, or a combination of the two, were the primary pathogens isolated from infected skin lesions. A satisfactory bacteriological response (cure or presumed cure) was obtained in 97.1 and 94.3% of children in the cefuroxime axetil and cefadroxil groups, respectively (P greater than 0.05). Satisfactory clinical responses (cure or improvement) were more likely to occur in cefuroxime axetil recipients than in cefadroxil recipients (97.8 versus 90.3%; P less than 0.05). Both regimens were equally well tolerated, with adverse events occurring in 7.9 and 6.1% of cefuroxime axetil and cefadroxil recipients, respectively. There were more patients who refused to take cefuroxime axetil (7 of 189) than there were who refused to take cefadroxil (0 of 98), but the difference was not statistically significant (P = 0.1). In this study, cefuroxime axetil was at least as effective as cefadroxil in resolving skin and skin structure infections in children.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballantyne F. N. Comparative efficacy of cefadroxil and cefaclor in the treatment of skin and soft-tissue infections. Clin Ther. 1985;7(4):487–491. [PubMed] [Google Scholar]
- Dornbusch K., Kronvall G., Göransson E., Mörtsell E. In-vitro activity of FCE 22101 against respiratory tract pathogens with reference to production of beta-lactamases. J Antimicrob Chemother. 1989 Mar;23 (Suppl 100):31–41. doi: 10.1093/jac/23.suppl_c.31. [DOI] [PubMed] [Google Scholar]
- Finn A., Straughn A., Meyer M., Chubb J. Effect of dose and food on the bioavailability of cefuroxime axetil. Biopharm Drug Dispos. 1987 Nov-Dec;8(6):519–526. doi: 10.1002/bdd.2510080604. [DOI] [PubMed] [Google Scholar]
- Gooch W. M., 3rd, Kaminester L., Cole G. W., Binder R., Morman M. R., Swinehart J. M., Wisniewski M., Yilmaz H. M., Collins J. J. Clinical comparison of cefuroxime axetil, cephalexin and cefadroxil in the treatment of patients with primary infections of the skin or skin structures. Dermatologica. 1991;183(1):36–43. doi: 10.1159/000247629. [DOI] [PubMed] [Google Scholar]
- Gudgeon A. C., Vandenburg M. J., Wight L. J., Griffiths G. K., Kelsey M. Is oral cefuroxime axetil suitable for the treatment of unidentified bacterial infection of skin and soft tissue? Br J Clin Pract. 1987 Oct;41(10):954–956. [PubMed] [Google Scholar]
- Hains C. S., Johnson S. E., Nelson K. G. Once daily cefadroxil therapy for pyoderma. Pediatr Infect Dis J. 1989 Sep;8(9):648–649. doi: 10.1097/00006454-198909000-00018. [DOI] [PubMed] [Google Scholar]
- Harding S. M., Williams P. E., Ayrton J. Pharmacology of Cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob Agents Chemother. 1984 Jan;25(1):78–82. doi: 10.1128/aac.25.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- Parish L. C., Cocchetto D. M., Werner K., Jungkind D. L., Witkowski J. Cefuroxime axetil in the treatment of cutaneous infections. Int J Dermatol. 1987 Jul-Aug;26(6):389–393. doi: 10.1111/j.1365-4362.1987.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Powell D. A., James N. C., Ossi M. J., Nahata M. C., Donn K. H. Pharmacokinetics of cefuroxime axetil suspension in infants and children. Antimicrob Agents Chemother. 1991 Oct;35(10):2042–2045. doi: 10.1128/aac.35.10.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]



