Abstract
In previous in vivo voltammetry studies, acute nicotine administration increased striatal dopamine clearance. The current study aimed to determine whether nicotine also increases [3H]dopamine uptake across the time course of the previous voltammetry studies and whether dopamine transporter trafficking to the cell surface mediates the nicotine-induced augmentation of dopamine clearance in striatum. Rats were administered nicotine (0.32 mg/kg, s.c.); striatal synaptosomes were obtained 5, 10, 40 or 60 min later. Nicotine increased (25%) the Vmax of [3H]dopamine uptake at 10 and 40 min. To determine whether the increase in Vmax was due to an increase in dopamine transporter density, [3H]GBR 12935 (1-(2-[bis(4-fluorophenyl)methoxy]ethyl)-4-(3-phenylpropyl)piperazine dihydrochloride) binding was performed using rat striatal membranes; no differences were found between nicotine and saline control groups at 5, 10 or 40 min post-injection, indicating that nicotine did not increase striatal dopamine transporter density; however, [3H]GBR 12935 binding assays determine both cell surface and intracellular dopamine transporter. Changes in cellular dopamine transporter localization in striatum were determined using biotinylation and subfractionation approaches; no differences between nicotine and saline control groups were observed at 10 and 40 min post-injection. These results suggest that the nicotine-induced increase in dopamine uptake and clearance in striatum may occur via a trafficking-independent mechanism.
Keywords: Nicotine, Dopamine transporter, Trafficking
1. Introduction
Nicotine is accepted to be the alkaloid in tobacco primarily responsible for nicotine dependence (Clarke, 1987; Pomerleau and Pomerleau, 1992). Activation of nicotinic receptors by nicotine results in an increase in the extracellular concentration of dopamine, which is thought to mediate the rewarding effects of nicotine and maintain tobacco use in dependent individuals (Corrigall et al., 1992; Koob, 1992; Stolerman and Jarvis, 1995). Acute administration of nicotine increases dopamine release from its presynaptic terminals in the striatum in a concentration-dependent manner (Andersson et al., 1981; Dwoskin et al., 1999; Kaiser et al., 1998; Ksir et al., 1995; Pontieri et al., 1996; Rowell et al., 1987; Vezina et al., 1992; Westfall et al., 1983). Synaptic dopamine concentrations are regulated by the dopamine transporter, which transports extracellular dopamine into the presynaptic terminal. The dopamine transporter is a major target for psychostimulant drugs, e.g., cocaine and amphetamine (Horn, 1990). The dopamine transporter has been shown to be regulated by constitutive internalization and recycling (i.e., trafficking) involving transporter phosphorylation and protein-protein interactions (Kahlig and Galli, 2003; Loder and Melikian, 2003; Melikian, 2004; Torres et al., 2003; Zahniser and Doolen, 2001).
Psychostimulant drugs and second messengers alter dopamine transporter function and trafficking (Garcia et al., 2005; Gnegy et al., 2004; Kahlig et al., 2004, 2006; Holton et al., 2005; Sorkina et al., 2005). For example, data from in vivo voltammetry studies indicate that cocaine and amphetamine act at the dopamine transporter to decrease dopamine clearance (Cass et al., 1993; Zahniser et al., 1999). In contrast, data from in vivo voltammetry studies show that nicotine increases the clearance of exogenously applied dopamine in rat striatum, nucleus accumbens and medial prefrontal cortex (Hart and Ksir, 1996; Middleton et al., 2004). Dopamine transporter surface expression in cell expression systems is also acutely sensitive to amphetamine and cocaine, which decreases and increases dopamine transporter surface levels, respectively (Daws et al., 2002; Kahlig et al., 2004, 2006; Little et al., 2002; Saunders et al., 2000; Zahniser and Sorkin, 2004). Nicotine and amphetamine both evoke [3H]dopamine release from superfused rat striatal slices, albeit via different mechanisms. (Corrigall et al., 1992; Koob, 1992; Stolerman and Jarvis, 1995). Previous results show that in vitro exposure to nicotine does not alter [3H]dopamine uptake into striatal synaptosomes (Carr et al., 1989; Zhu et al., 2003); however, the effect of systemic nicotine administration on [3H]dopamine uptake into striatal synaptosomes and on dopamine transporter trafficking has not been determined.
Thus, the present study used cell surface biotinylation and subfractionation approaches to determine whether systemic administration of nicotine alters [3H]dopamine uptake into rat striatal synaptosomes and alters the cellular localization of the dopamine transporter in striatum. The ability of nicotine to modulate dopamine transporter function, and thereby extracellular dopamine concentration, may have physiological importance with regards to nicotine enhancement of cognitive processes such as attention, learning and memory, as well as important clinical relevance with respect to schizophrenia and drug abuse. Therefore, understanding nicotine-induced regulation of dopamine transporter function may provide further insight into the mechanism of nicotine action.
2. Materials and methods
2.1. Materials
Antibodies recognizing rat dopamine transporter (sc-1433; goat polyclonal antibody), calnexin (sc-11397; rabbit polyclonal antibody); β-actin (sc-7210; rabbit polyclonal antibody) and protein phosphatase 2A (PP2A; sc-6110; goat polyclonal antibody) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-goat antibody was purchased from Dako Cytomation (#Z 0454; Carpinteria, CA). Horseradish peroxidase (HRP) conjugated goat anti-rabbit antibody was purchased from Bio-Rad (Hercules, CA). (+)Methamphetamine hydrochloride, GBR 12909 (1-(2-[bis(4-fluorophenyl)methoxy]ethyl)-4-(3-phenylpropyl)piperazine dihydrochloride), nicotine ditartrate and nomifensine maleate were purchased from Sigma Chemical Co. (St. Louis, MO). Sulfosuccinimidobiotin (sulfo-NHS-biotin) and immunoPure immobilized monomeric avidin gel were purchased from Pierce Biotechnology, Inc. (Rockford, IL). [3H]Dopamine (3,4-ethyl-2 [N-3H] dihydroxyphenylethylamine; specific activity 31 Ci/mmol) and [3H]GBR 12935 ([propylene-2,3-3H] (1-[2-diphenylmethoxy)ethyl]-4-(3-phenylpropyl)-piperazine); specific activity 43.5 Ci/mmol) were purchased from New England Nuclear (Boston, MA). D-Glucose was purchased from Aldrich Chemical Co, Inc. (Milwaukee, WI). All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA).
2.2. Animals
Male Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN) and were housed with free access to food and water in a colony room in the Division of Laboratory Animal Resources at the University of Kentucky. Animal handling procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky and were performed in accordance with the 1996 version of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.3. Synaptosomal [3H]dopamine uptake
[3H]Dopamine uptake assays were conducted using a previously published method (Zhu et al. 2003). Separate groups of rats were injected with nicotine (0.32 mg/kg, free base weight, s.c.) or saline. Nicotine dose was chosen based on previous studies from our laboratory showing that nicotine (0.32 mg/kg, s.c.) increased dopamine clearance (~60%) in striatum in vivo (Middleton et al., 2004). In the current study, separate groups of rats (treatment and control) were killed at 5, 10, 40 or 60 min post-injection. Thus, striata from the saline control groups were time-matched to the nicotine injection for the nicotine treatment groups. Striata were homogenized in 20 ml of ice-cold 0.32 M sucrose solution containing 5 mM sodium bicarbonate (pH 7.4) with 16 passes of a Teflon pestle homogenizer (clearance, 0.015 in). Homogenates were centrifuged (2,000 g, 4 °C, 10 min), and resulting supernatants were centrifuged (20,000 g, 4 °C, 15 min). Pellets were resuspended in 2.4 ml of ice-cold assay buffer (125 mM 10 mM glucose, 25 NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, mM N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid] (HEPES), 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.1 mM pargyline and 0.1 mM L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4). Striatal synaptosomes (20 μg protein in 50 μl) were incubated in an oxygenated environment for 5 min at 34 °C. Subsequently, one of ten [3H]dopamine concentrations (1 nM – 5 μM) was added to each tube. Total assay volume was 500 μl. Nonspecific [3H]dopamine uptake was determined in the presence of 10 μM nomifensine. Incubation continued for 10 min at 34 °C and was terminated by the addition of 3 ml of ice-cold assay buffer containing pyrocatechol (1 mM), followed by immediate filtration through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 h). Filters were washed 3 times with 3 ml ice-cold buffer containing 1 mM pyrocatechol using a Brandel cell harvester (Model MP-43RS; Biochemical Research and Development Laboratories Inc., Gaithersburg, MD). Radioactivity was determined by liquid scintillation spectrometry (Model B1600TR, Perkin-Elmer Life Sciences, Downers Grove, IL). Protein concentrations were determined with bovine serum albumin as the standard (Bradford, 1976). At each of the time points after nicotine administration, aliquots of synaptosomes were incubated with the same range of [3H]dopamine concentrations for 10 min, and then [3H]dopamine uptake was terminated by dilution and filtration. Thus, the duration of the in vitro uptake assay was consistent across the time points studied. Kinetic parameters (Vmax and Kt ) for [3H]dopamine uptake were determined using GraphPad Prism software (GraphPad Prism, version 3.0; GraphPad Software, San Diego, CA).
2.4. [3H]GBR 12935 binding
[3H]GBR 12935 binding assays were performed using striata obtained from groups of rats injected with nicotine (0.32 mg/kg, s.c.) or saline and killed 5, 10 or 40 min post-injection. Thus, striata from the saline control groups were time-matched to the nicotine injection for the nicotine treatment groups. Striata were obtained and stored at −70 °C until assay. Striata were homogenized with a polytron homogenizer (setting 40; Tekmar, Cincinnati, OH), in 10 volumes of ice-cold assay buffer (118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, and 1.2 mM MgSO4, pH 7.5). Homogenates were incubated at 37 °C for 5 min, placed on ice and centrifuged (25,000 g, 20 min, 4 °C). Pellets were resuspended in 10 volumes of ice-cold Milli-Q water, incubated at 37 °C for 5 min and centrifuged (25,000 g, 20 min, 4 °C). Pellets were resuspended in 10 volumes of ice-cold 10% assay buffer and incubation, and centrifugation steps were repeated twice. Final pellets were stored in 10% assay buffer at −70 °C. Upon assay, pellets were resuspended in 20 ml assay buffer. Samples (250 μl) consisting of 100 – 140 μg of membrane protein and a range of [3H]GBR 12935 concentrations (0.1–35 nM) in assay buffer containing 50 mM Tris were incubated for 90 min at 4 °C. Nonspecific binding was determined in the presence of 10 μM GBR 12909. Reactions were terminated by dilution of the samples with 3 ml of ice-cold 20 mM Krebs-HEPES buffer followed by immediate filtration through Whatman GF/B glass fiber filters presoaked for 2 h in 0.5% polyethylenimine using the Brandel harvester. Filters were rinsed 3 times with 3 ml of ice-cold 20 mM buffer and transferred to vials, and scintillation cocktail (4 ml) was added. Radioactivity was determined using a Tri-Carb 2100 TR liquid scintillation analyzer (PerkinElmer Life Sciences). Protein concentrations were determined as previously described. Kinetic parameters (Bmax and Kd) of [3H]GBR 12935 binding were determined using GraphPad Prism software, version 3.0.
2.5. Biotinylation and immunoblotting assay
For the determination of cell surface and intracellular levels of dopamine transporter protein in striatal synaptosomes, surface biotinylation and immunoblot analysis was performed as described previously (Apparsundaram et al., 1998; Melikian et al., 1996; Ramamoorthy et al., 1998; Zhu et al., 2005). To provide a positive control for the current study, dopamine transporter cell surface expression was determined in a separate group of rats administered methamphetamine (5 mg/kg, salt weight, s.c.) or saline. Striata were obtained 30 min following injection of methamphetamine or saline, and synaptosomes were prepared as described above for the [3H]dopamine uptake assays. To determine the ability of nicotine to alter cell surface and intracellular levels of dopamine transporter protein, assays were performed using striata obtained from groups of rats injected with nicotine (0.32 mg/kg, s.c.) or saline and killed 10 or 40 min post-injection. The previous study showed that Vmax for [3H]dopamine uptake was increased at these time points. As in the previous experiments, striata from the saline control groups were time-matched to the nicotine injection for the nicotine treatment groups.
Impermeant biotinylation reagent, sulfo-NHS-biotin, was used for the isolation of plasma membrane proteins. Dopamine transporter protein was identified using polyclonal dopamine transporter antibody (Taubenblatt et al., 1999; Salvatore et al., 2003; Vaughan et al., 1997). Samples of striatal synaptosomes (500 μg total protein) were incubated for 1 h at 4 °C with continual shaking in 500 μl of 1.5 mg/ml sulfo-NHS-biotin in phosphate buffered saline/Ca/Mg buffer (138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 9.6 mM Na2HPO4, 1 mM MgCl2, 0.1 mM CaCl2, pH 7.3). After incubation, samples were centrifuged (8,000 g, 4 min, 4 °C). To remove free biotinylation reagent, the resulting pellet was resuspended in 1 ml of ice-cold 100 mM glycine in phosphate buffered saline/Ca/Mg buffer and centrifuged (8,000 g, 4 min, 4 °C). Resuspension and centrifugation steps were repeated. Final pellets were resuspended in 1 ml of ice-cold 100 mM glycine in phosphate buffered saline/Ca/Mg buffer and incubated with continual shaking for 30 min at 4 °C. Samples were centrifuged (8,000 g, 4 min, 4 °C), and resulting pellets were resuspended in 1 ml ice-cold phosphate buffered saline/Ca/Mg buffer and centrifuged again. Resuspension and centrifugation steps were repeated twice to remove excess glycine. Final pellets were lysed by sonication for 2–4 s in 300 μl Triton X-100 buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1.0% Triton X-100, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μM pepstatin, 250 μM phenylmethysulfonyl fluoride) followed by incubation and continual shaking for 20 min at 4 °C. Lysates (300 μl) were centrifuged (21,000 g, 20 min, 4 °C). Remaining supernatant was incubated with continuous shaking in the presence of monomeric avidin beads in Triton-X100 buffer (100 μl/tube) for 1 h at 22–24 °C. Samples were centrifuged (17,000 g, 4 min, 4 °C), and supernatants containing non-biotinylated proteins (intracellular) were stored at −20 °C. The resulting pellets containing biotinylated proteins (cell-surface) were resuspended in 1 ml of 1.0% Triton X-100 buffer and centrifuged (17,000 g, 4 min, 4 °C), and the pellets were resuspended and centrifuged two times. Final pellets consisted of biotinylated proteins adsorbed to monomeric avidin beads. Biotinylated proteins were eluted by incubating with 50 μl Laemmli buffer (62.5 mM Tris-HCl, 20% glycerol, 2% sodium dodecyl sulfate, 0.05% β-mercaptoethanol and 0.05% bromophenol blue, pH 6.8) for 20 min at 22–24 °C. Intracellular and cell surface fractions were stored at −20 °C.
Samples (intracellular and cell surface fractions) were thawed and subjected to gel electrophoresis and Western blotting as previously described (Melikian et al. 1994; Salvatore et al. 2003; Zhu et al., 2005). Briefly, proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 90 min at 150 V and transferred to Immobilon-P transfer membranes (Cat # IPVH00010, 0.45 μm pore size; MILLIPORE Co., Bedford, MA) in transfer buffer (50 mM Tris, 250 mM glycine, 3.5 mM sodium dodecyl sulfate) using a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad Laboratories Ltd., Hercules, CA) for 110 min at 72 V. Transfer membranes were incubated with blocking buffer (5% milk powder in phosphate buffered saline containing 0.5% Tween 20) for 1 h at 22–24 °C, followed by incubation with goat polyclonal dopamine transporter antibody (1 μg/ml in blocking buffer) overnight at 4 °C. Transfer membranes were washed 5 times with wash buffer (phosphate buffered saline containing 0.5% Tween 20) at 22–24 °C and then incubated with rabbit anti-goat antibody (1:2500 dilution in blocking buffer) for 1 h at 22–24 °C. Transfer membranes were then washed and incubated with peroxidase-conjugated goat anti-rabbit antibody (diluted 1:5000) for 1 h at 22–24 °C. Protein bands were detected using enhanced chemiluminescence and developed on Hyperfilm (ECL-plus; Amersham Biosciences UK Ltd., Little Chalfont Buckinghamshire, UK). After detection and quantification of dopamine transporter protein, each blot was stripped using Tris buffer (62.5 mM Tris-HCl with 2% sodium dodecyl sulfate and 100 mM β-mercaptoethanol, pH 6.8) and probed for detection of protein phosphatase 2A and calnexin. Protein phosphatase 2A, an intracellular protein (Janssens and Goris, 2001), served as a control protein to monitor the efficiency of biotinylation of cell surface proteins. Protein phosphatase 2A was detected using goat polyclonal protein phosphatase 2A antibody (1:500). Calnexin, an endoplasmic reticular protein (Hochstenbach et al., 1992; Krijnse-Locker et al., 1995; Rajagopalan et al., 1994), was detected using rabbit polyclonal calnexin antibody (1:5000) to monitor biotinylation of intracellular proteins. β-Actin was quantified to normalize for protein loading across samples.
Immunoreactive bands were quantified by densitometric scanning using Scion image software (Scion Corp., Frederick, MD). Band density measurements were used to calculate levels of dopamine transporter in non-biotinylated and biotinylated fractions. Specifically, dopamine transporter levels in the non-biotinylated fractions were calculated as density of dopamine transporter-immunoreactive bands in an aliquot of supernatant post-avidin incubation multiplied by the total volume of the extract and divided by the volume of supernatant subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In preliminary studies, quantification of protein phosphatase 2A revealed a maximum 10% contamination of intracellular proteins in the plasma membrane biotinylated fraction. Immunoreactive bands were quantified and were found to be within the linear range of detection.
2.6. Subcellular fractionation
To verify the results obtained using the biotinylation approach, a subcellular fractionation strategy was adapted from previously described methods using presynaptic vesicular proteins (Clift-O’Grady et al., 1990; Huttner et al., 1983). Briefly, rat striata were homogenized in 0.32 M sucrose buffer containing 5 mM HEPES-NaOH (pH 7.3) using a Wheaton Instruments Potter Elvejhem homogenizer (10 strokes) and centrifuged (1000 g, 10 min, 4°C). Supernatants were centrifuged (13,000 g, 17 min, 4°C) to yield a crude synaptosomal pellet (P2). Synaptosomes in this P2 fraction (1 mg) were lysed by homogenization (5 strokes) in ice-cold 5 mM HEPES-NaOH (pH 7.4) plus protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μM pepstatin, 250 μM phenylmethysulfonyl fluoride). Synaptic plasma membranes (LP1) and other large membranes were separated at 15,000 g for 20 min. The vesicle-enriched LP2 pellet was obtained following centrifugation of the resulting supernatant (LS1 Fraction; 200,000 g, 30 min, 4°C). Proteins were extracted from each fraction with 1% sodium dodecyl sulfate, 5 mM HEPES-KOH (pH 7.3), 1 mM EDTA, 1 mM EGTA and protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μM pepstatin, 250 μM phenylmethysulfonyl fluoride). Protein concentrations were determined using the bicinchoninic acid method (Pierce, Rockford, IL). Each fraction (50 μg) was subjected to immunoblot analysis with antibodies against dopamine transporter, protein phosphatase 2A, calnexin and β-actin. Dopamine transporter-immunoreactive bands were detected using goat polyclonal anti-dopamine transporter antibody (1 μg/ml) in blocking buffer (5% milk in phosphate buffered saline containing 0.05% Tween 20, pH 7.4) followed by rabbit polyclonal anti-goat antibody (1:2500 dilution in blocking buffer) and goat alkaline phosphatase conjugated polyclonal anti-rabbit antibody (1:10,000 dilution). Protein bands present in the transfer membrane were measured using enhanced chemifluorescence (GE Healthcare, Piscataway, NJ) with a Typhoon Imaging System (GE Healthcare) and Imagequant TL software (GE Healthcare). Immunoreactive bands were quantified and were within the linear range of detection. β-Actin was quantified to normalize for protein loading across samples. The relative proportion of dopamine transporter in the plasma membrane was expressed by calculating the ratio LP1/LP1+LP2.
2.7. Statistics
[3H]Dopamine uptake data were analyzed using two-way analysis of variance (ANOVA) with drug treatment and time as between-subjects factors. One-way ANOVA was performed on the [3H]dopamine uptake data from the saline control groups across the different time points. Tukey’s post hoc analysis was used to determine differences between the nicotine treatment groups and the pooled saline-control group for the [3H]dopamine uptake data. Tukey’s post hoc analysis was also used to determine differences between the nicotine treatment groups and the time-matched saline control groups for the data from the binding and cellular localization assays. Analyses were performed using the commercially available software, Statistical Packages for the Social Sciences (SPSS; standard version 11.0, Chicago, IL). P < 0.05 was considered statistically significant.
3. Results
3.1. Nicotine administration increases the Vmax for [3H]dopamine uptake in rat striatal synaptosomes
Kinetic analyses of [3H]dopamine uptake performed in synaptosomes obtained 5, 10, 40 or 60 min following administration of nicotine (0.32 mg/kg, s.c.) or saline indicated that [3H]dopamine uptake was not different between the saline control groups across the time points; these saline control data were pooled for statistical analysis and graphical presentation (Fig. 1). While the interaction between treatment and time was not significant, a main effect of nicotine treatment was observed (F1,48 = 4.16; P < 0.05). Compared to the saline control group, nicotine significantly increased (~25%) the Vmax for [3H]dopamine uptake at 10 and 40 min post-injection (P < 0.05). The effect of nicotine on Vmax was not significant at either the 5 or 60 min time points. There was no change in Kt at any of the time points investigated (data not shown).
Fig. 1. Nicotine administration increases the Vmax for [3H]dopamine uptake in striatal synaptosomes.
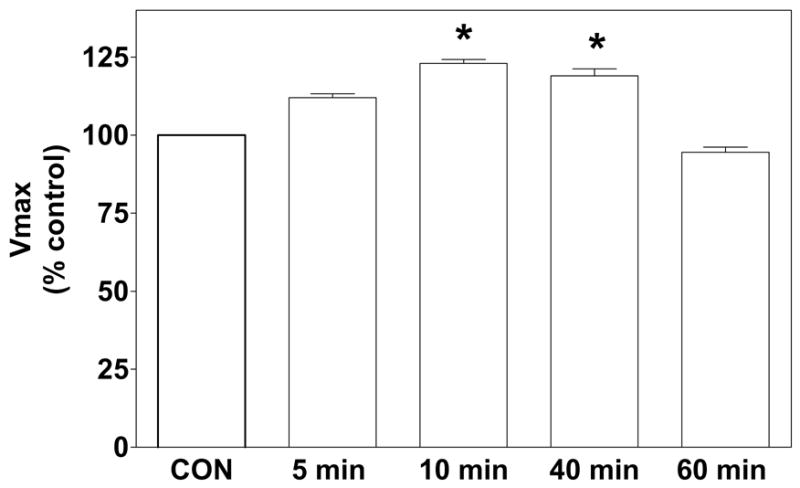
Rats were injected with nicotine (0.32 mg/kg, s.c.; open bars) or saline (control, CON; hatched bar), and synaptosomes were prepared 5, 10, 40 or 60 min post-injection. Specific [3H]dopamine uptake for the 5, 10, 40 and 60 min saline-control groups was 32.8 ± 1.66, 29.7 ± 1.18, 27.8 ± 1.45, and 33.2 ± 1.72 pmol/mg/min, respectively. ANOVA revealed that [3H]dopamine uptake was not different among the saline-control groups across the time points, and thus, these data were pooled for statistical analysis and graphical presentation. Nicotine increased the Vmax for [3H]dopamine uptake at 10 and 40 min following nicotine injection compared to the pooled saline control. Data are expressed as mean ± S.E.M. percentage of the pooled saline-control group. * indicates significant difference from the pooled saline-control group, P < 0.05; n = 6/group.
3.2. Nicotine administration does not alter [3H]GBR 12935 binding to rat striatal membranes
No differences between nicotine (0.32 mg/kg) and saline control groups were observed in either the Bmax or Kd for [3H]GBR 12935 binding at the 5, 10 or 40 min time points (Table 1), suggesting that nicotine pretreatment does not alter the total amount of dopamine transporter protein in striatum.
Table 1.
Nicotine (0.32 mg/kg, s.c.) pretreatment does not alter [3H]GBR 12935 binding to rat striatal membranes.
| Bmax (pmol/mg protein) | Kd (μM) | |||
|---|---|---|---|---|
| Time (min) | SALINE | NICOTINE | SALINE | NICOTINE |
| 5 | 183 ± 22 | 192 ± 33 | 3.3 ± 0.4 | 4.6 ± 1.0 |
| 10 | 290 ± 35 | 195 ± 32 | 5.3 ± 1.0 | 4.4 ± 0.5 |
| 40 | 277 ± 69 | 229 ± 37 | 4.8 ± 1.0 | 5.0 ± 0.7 |
Data are expressed as Mean ± S.E.M, n = 4–9/group.
3.3. Nicotine administration does not alter the cellular localization of dopamine transporter in striatum
Initial experiments determined whether methamphetamine administration alters the cellular localization of the dopamine transporter, as reported previously (Saunders et al., 2000). Pretreatment with methamphetamine (5 mg/kg, s.c.) resulted in a significant decrease in the level of biotinylated dopamine transporter compared with the saline control group (Fig. 2). The current results are in agreement with previous reports (Saunders et al., 2000) and serve to validate the biotinylation method used in the current study.
Fig. 2. Methamphetamine administration decreases the distribution of striatal dopamine transporters to the cell surface.
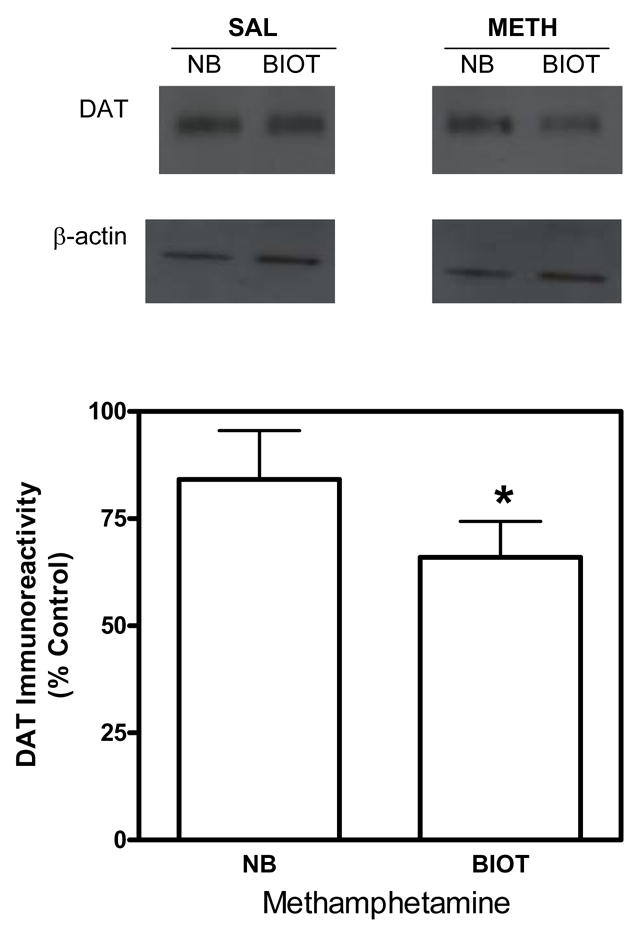
Representative immunoblots (top panel) for dopamine transporters (DAT) and β-actin in non-biotinylated (NB) and biotinylated (BIOT) fractions of striatal synaptosomes obtained 30 min following methamphetamine (METH; 5 mg/kg, s.c.) or saline injection. Group data (mean ± S.E.M, n=6/group) for dopamine transporter immunoreactivity in nonbiotinylated and biotinylated fractions are presented as a percentage of the saline control group (bottom panel). Dopamine transporter immunoreactivity for nonbiotinylated and biotinylated fractions for the saline control group were 200 ± 19.7, and 40.3 ± 3.47 × 103 arbitrary units, respectively. Dopamine transporter immunoreactivity for nonbiotinylated and biotinylated fractions for the methamphetamine group were 173 ± 20.3, and 25.6 ± 4.49 × 103 arbitrary units, respectively. Immunoreactive bands were within the linear range of detection. Biotinylation assays used β-actin as a control for protein loading. * indicates difference from the time-matched saline control group, P < 0.05 (Student’s t test).
To determine whether nicotine pretreatment (0.32 mg/kg) altered cell surface localization of dopamine transporter, rats were administered nicotine or saline and killed 10 or 40 min later. Striatal synaptosomes were prepared for biotinylation and subfractionation assays. For all groups, dopamine transporter bands were observed at 80 kilodaltons, as previously reported (Salvatore et al., 2003; Zhu et al., 2005). Dopamine transporter-immunoreactive bands were detected in both biotinylated and non-biotinylated fractions. Dopamine transporter band density was not different between nicotine and saline control groups at the 10 and 40 min time points (Fig. 3). Also, there were no differences among groups in the levels of control proteins, protein phosphatase 2A, calnexin and β-actin. Consistent with the results from the biotinylation assays, subfractionation experiments also revealed no difference in dopamine transporter levels in total and plasma membrane fractions (LP1). No differences between the nicotine and saline control groups were observed in the ratio of LP1/LP1+LP2 at the 10 min and 40 min time points (Fig. 4). Also, there was no difference in the levels of control proteins, β-actin, protein phosphatase 2A and calnexin, between nicotine and saline-control groups.
Fig. 3. Nicotine does not alter the cellular distribution of the dopamine transporter in striatum 10 or 40 min following nicotine administration, when assessed using the biotinylation assay.
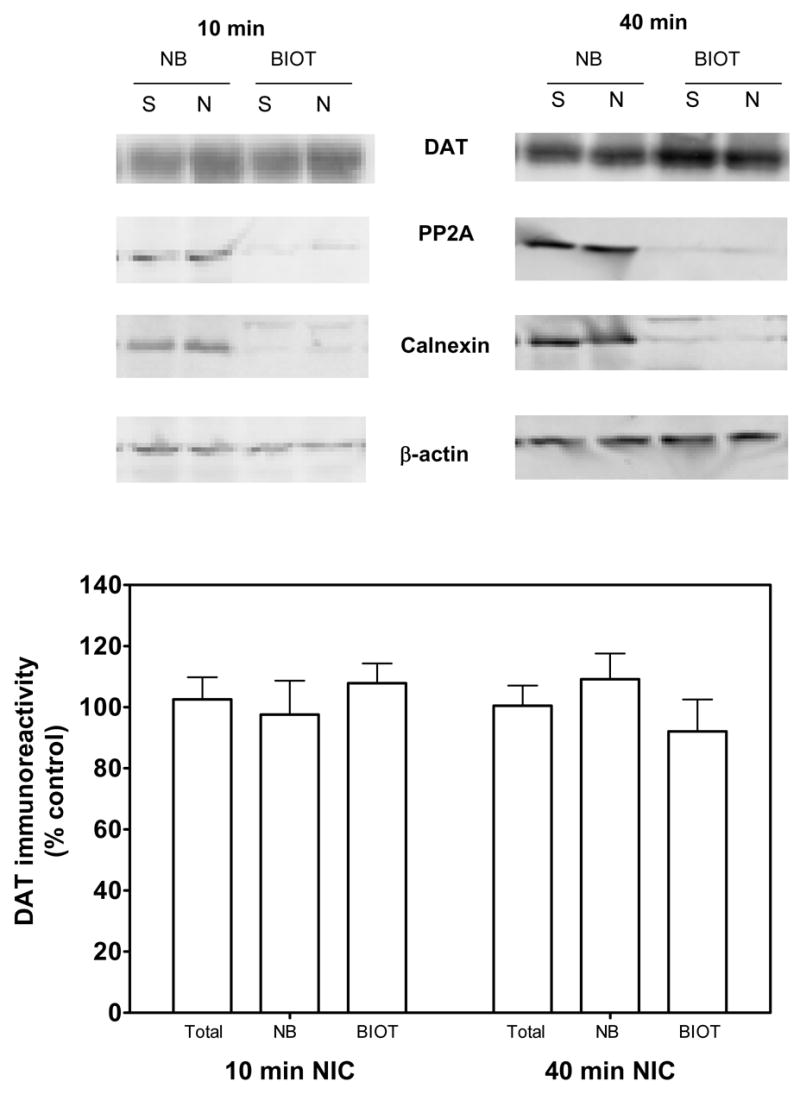
Representative immunoblots (Top panel) for dopamine transporter (DAT), protein phosphatase 2A (PP2A), calnexin and β-actin in nonbiotinylated (NB) and biotinylated (BIOT) fractions of striatal synaptosomes obtained 10 and 40 min following nicotine (0.32 mg/kg; s.c.) or saline (control) injection. Group data (mean ± S.E.M, n = 5/group) for dopamine transporter immunoreactivity in nonbiotinylated and biotinylated fractions are presented as a percentage of the respective, time-matched saline-control group (Bottom panel). Dopamine transporter immunoreactivity for total, nonbiotinylated and biotinylated fractions for the 10 min saline-control group was 8.15 ± 0.46, 4.19 ± 0.24 and 3.95 ± 0.64 arbitrary units × 107, respectively. Dopamine transporter immunoreactivity for total nonbiotinylated and biotinylated fractions for the 40 min saline control group was 8.11 ± 0.60, 3.97 ± 0.13 and 4.13 ± 0.55 arbitrary units × 107, respectively. Immunoreactive bands were within the linear range of detection. Biotinylation assays used β-actin as a control for protein loading. Protein phosphatase 2A and calnexin were used to monitor biotinylation of proteins located in intracellular compartments; these proteins were found predominantly in the nonbiotinylated fractions.
Fig. 4. Nicotine does not alter the cellular distribution of the dopamine transporter in striatum 10 or 40 min following nicotine administration, when assessed using the subfractionation assays.
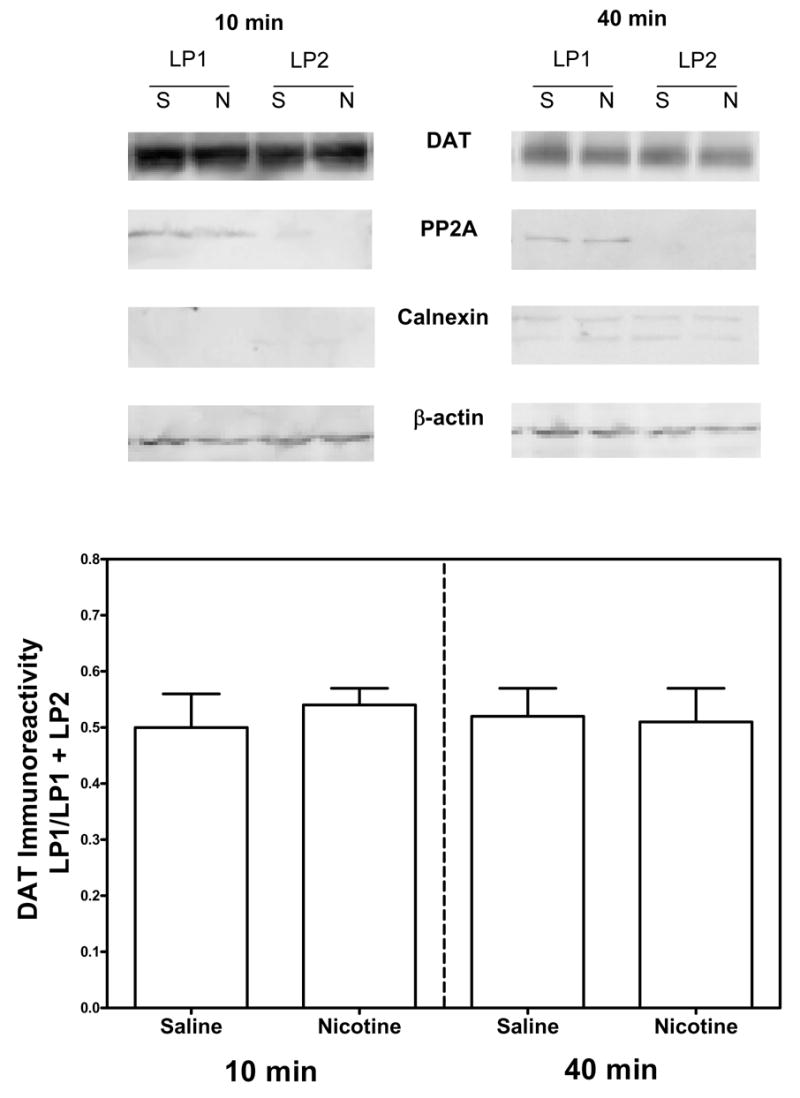
Representative immunoblots of dopamine transporter (DAT), protein phosphatase 2A (PP2A), calnexin and β-actin in total, LP1 (synaptic plasma membrane) and LP2 (vesicular) fractions of striatal synaptosomes from nicotine (0.32 mg/kg; s.c.) or saline-injected rats 10 and 40 min post-injection (Top panel). Group data for dopamine transporter are expressed as the LP1/LP1+LP2 ratio at 10 and 40 min following nicotine or saline injection (Bottom panel). The LP1/LP1+LP2 ratio represents the fraction of dopamine transporter in the plasma membrane as a percent of the dopamine transporter in the plasma membrane plus the vesicular fraction. Data are presented as mean ± S.E.M, n = 5/group. The levels of dopamine transporter-immunoreactivity in total fractions were 16.8 ± 2.37 (NIC, 10 min), 18.0 ± 1.34 (saline, 10 min), 15.2 ± 1.92 (NIC, 40 min) and 13.8 ± 1.36 (saline, 40 min) expressed as arbitrary units × 105. Immunoreactive bands were within the linear range of detection. β-Actin was used as a control for protein loading.
4. Discussion
The current results demonstrate a significant increase (25%) in the Vmax for [3H]dopamine uptake into striatal synaptosomes obtained from rats 10 and 40 min after nicotine (0.32 mg/kg) administration compared to control rats administered saline. These data are consistent with previous findings from our laboratory showing that systemically administered nicotine increases dopamine clearance in vivo in rat striatum (Middleton et al., 2004). The nicotine-induced increase in dopamine clearance observed in vivo is likely due to an increase in Vmax for dopamine transport. The nicotine-induced increase in [3H]dopamine uptake into striatal synaptosomes was not accompanied by an increase in the total amount of dopamine transporter protein, as indicated by [3H]GBR 12935 binding and immunoblotting methods. Furthermore, the biotinylation and subfractionation results revealed no change in the cellular distribution of dopamine transporter in striatum following nicotine administration compared with saline administration. In contrast, methamphetamine decreased cell-surface expression of the dopamine transporter compared with the saline-control group, similar to results reported in previous studies (Kahlig et al., 2004, 2006; Saunders et al., 2000). The results with methamphetamine indicate that the biotinylation and immunoblotting assay utilized in the current study provides an accurate measure of changes in the cellular localization of the dopamine transporter. Taken together, the current results suggest that the nicotine-induced increase in Vmax for [3H]dopamine uptake in vitro and the nicotine-induced increase in dopamine clearance in vivo are not due to alterations in the cellular localization of the dopamine transporter in striatum, but are due to a trafficking-independent mechanism.
Previous research using in vivo voltammetry revealed that nicotine increased exogenous dopamine clearance in striatum in anesthetized rats (Middleton et al., 2004). The nicotine-induced increase in dopamine clearance was dose dependent with the 0.32 mg/kg dose increasing clearance by ~60%. Nicotine increased dopamine clearance 15 min following nicotine administration, and the increase in clearance persisted for one hour post-injection (Middleton et al., 2004). Consistent with the latter study, results from the current study show a 25% increase in Vmax for [3H]dopamine uptake into striatal synaptosomes; however, differences in the results between the two studies are noted. The percent increase in Vmax for [3H]dopamine uptake in the current study was lower than the percent increase in dopamine clearance observed in the previous in vivo voltammetry studies. Furthermore, dopamine transporter function in the current study was increased by nicotine at 10 and 40 min post-injection and was back to saline-control levels by 60 min, whereas the effect of nicotine was still apparent at 60 min in the previous in vivo voltammetry studies. Differences in the magnitude and duration of nicotine effect on dopamine transporter function between the two assays may be explained in part by the fact that dopamine clearance in the in vivo voltammetry assays is measured in localized areas of striatum, whereas the in vitro assay utilizes the entire striatum, potentially diluting localized changes in function. In this regard, results from in vivo voltammetry studies show that the striatum is heterogeneous with respect to dopamine clearance, which may be related to variations in the density of the dopamine transporter (Ciliax et al., 1995). Thus, the smaller magnitude of nicotine effect in the synaptosomal preparation may be due to an averaging of effect across this heterogeneous brain region. Furthermore, differences between the assays, including use of anesthetic and repeated exposure to exogenous dopamine in the in vivo studies, may have contributed to the observed differences in the magnitude and duration of the effect of nicotine on dopamine transporter function.
In the current in vitro studies, the time course showed that nicotine increased [3H]dopamine uptake at the 40-min time point, but not at the 60-min time point. There are several potential explanations for the lack of effect of nicotine at 60 min in the in vitro studies. One possibility is that changes in [3H]dopamine efflux in vitro at the 60 min time (i.e., rundown of the [3H]dopamine) may have contributed to the loss of the nicotine effect on Vmax in the in vitro uptake assay. However, rats were injected with nicotine and after either 5 min, 10 min, 40 min or 60 min, the striatum was obtained and synaptosomes were prepared. In each of the experiments (at each time point), aliquots of the synaptosomes were incubated with the same range of concentrations of [3H]dopamine for the same period of time (10 min), and then [3H]dopamine uptake was terminated by dilution and filtration. Thus, the duration of the in vitro uptake assays was consistent across the groups, such that [3H]dopamine rundown would be expected to be the same at each time point following nicotine administration; consequently, this likely does not explain the lack of effect of nicotine on Vmax at the 60-min time point.
An alternative explanation for the lack of effect of nicotine at the 60-min time point is that changes in endogenous dopamine efflux in response to nicotine may have altered dopamine transporter function. Ahtee and colleagues have reported that striatal dopamine release is increased modestly for an extended period (>60 min) after s.c. nicotine administration in awake rats (Janhunen et al., 2005). Furthermore, nicotine administration (s.c.) increased striatal dopamine transporter function (measured using in vivo voltammetry in anesthetized rats) across a 60-min period (Middleton et al., 2004). Thus, in both dopamine clearance and dopamine release assays performed in vivo, nicotine effects on dopamine transporter function and dopamine efflux are sustained across a 60 min period. Ghosheh et al (1999) demonstrated that peak nicotine brain levels occur by 5 min after peripheral injection and that the half-life of nicotine in rat brain is 52 min. The inability to observe the effect of nicotine at the 60 min time point in the current in vitro study may be due to decreasing levels of nicotine in brain at the 60-min time point in combination with further decrements in nicotine levels resulting from the preparation of synaptosomes for in vitro dopamine uptake. Thus, insufficient levels of nicotine in brain may have contributed to the inability to observe an effect of nicotine dopamine transporter function in vitro at the 60-min time point.
Although in vivo nicotine administration results in an increase in striatal dopamine transporter function as demonstrated using in vivo voltammetry and in vitro synaptosomal [3H]dopamine uptake, previous results show that when striatum is exposed to nicotine in vitro, there is no effect on [3H]dopamine uptake (Carr et al., 1989; Zhu et al., 2003). These contrasting findings may be due to the effect of nicotine at the dopaminergic cell body or upon the neuronal circuitry, which has been interrupted during the preparation of striatal slices or synaptosomes.
Several studies have shown that dopamine transporter undergoes internalization and recycling, which may involve dynamin-clathrin mediated pathways and multiple protein-protein interactions, such as syntaxin-1A, protein phosphatase 2A, protein interacting with C kinase 1 and synuclein (Lee et al. 2004; Melikian, 2004; Torres et al., 2003; Zahniser and Doolen, 2001). Changes in dopamine transporter surface expression have been shown to be induced by psychostimulants or protein kinase C activation, and such changes correlate with alterations in [3H]dopamine uptake in striatum (Chi and Reith, 2003; Copeland et al. 1996; Vaughan et al., 1997) and in cell systems expressing the dopamine transporter (Kahlig et al., 2004, 2006; Little et al., 2002; Pristupa et al. 1998). The previous studies used biotinylation and subfractionation approaches to show drug-induced changes in dopamine transporter cellular localization. One mechanism by which the nicotine-induced increase in dopamine transporter function may occur is via the trafficking of the dopamine transporter to the cell surface. Recent studies have used biotinylation to determine dopamine transporter distribution in total plasma membrane and intracellular fractions in rat striatal synaptosomes (Chi and Reith, 2003; Salvatore et al., 2003; Zhu et al., 2005). To evaluate this potential mechanism, cell surface biotinylation and subfractionation approaches were employed in the current study to assess dopamine transporter cellular localization in striatum. However, the biotinylation assay revealed no differences in dopamine transporter cellular localization between the nicotine-treated and saline-control groups; this finding was further confirmed using the subfractionation approach. Thus, it appears that the nicotine-induced increase in dopamine transporter function in striatum may occur via a trafficking-independent mechanism.
Alterations in transporter function in the absence of changes in transporter trafficking have been reported previously (Apparsundaram et al., 2001; Zhu et al., 2005). Specifically, insulin increases norepinephrine transporter function without a change in transporter cellular localization (Apparsundaram et al., 2001). Similarly, p38 mitogen-activated protein kinase stimulation of the serotonin transporter was also recently shown to occur via a trafficking-independent mechanism (Zhu et al., 2005), suggesting that multiple pathways exist to regulate neurotransmitter transporter function.
Nicotine has been shown to activate several different second messenger pathways. For example, nicotine releases nitric oxide from rat hippocampal slices (Smith et al., 1998). Nicotine-induced nitric oxide release is inhibited by α-bungarotoxin, suggesting the involvement of calcium permeable α7 nicotinic receptors. Also, nicotinic receptor activation induces extracellular signal-regulated kinase phosphorylation in pheochromocytoma PC 12 cells (Nakayama et al., 2001) and alters calmodulin and mitogen-activated protein kinase function (Hu et al., 2002). Additional mechanistic insight is needed because of the fact that Vmax for [3H]dopamine uptake is increased, while total dopamine transporter protein (assessed by binding) and cell surface expression (assessed in biotinylation and subfractionation studies) are not increased. The specific underlying cellular mechanism(s) responsible for the nicotine-induced increase in Vmax for [3H]dopamine uptake may be due to dopamine transporter phosphorylation through a kinase or phosphatase. Recently, the nitric oxide pathway has been implicated in increasing dopamine transporter function in striatum in studies using rotating disk electrode voltammetry (Volz and Schenk, 2004). It is possible that such signaling mechanisms may be involved in the nicotine-induced increase in dopamine transporter function without altering cell surface localization of dopamine transporter.
Acknowledgments
The authors would like to thank Agripina G. Deaciuc and Jackie Huller for their expert technical assistance, and Kathleen Grzech for editing the manuscript. This work was supported by National Institute of Health grants F31DA15292 (LM), R21DA018372 (LD, SA), KO2DA00399 (LD) and a grant from NARSAD (SA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson K, Fuxe K, Agnati LF, Eneroth P. Effects of acute central and peripheral administration of nicotine on ascending dopamine pathways in the male rat brain. Evidence for nicotine induced increases of dopamine turnover in various telencephalic dopamine nerve terminal systems. Med Biol. 1981;59:170–176. [PubMed] [Google Scholar]
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport: II.PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998;287:744–751. [PubMed] [Google Scholar]
- Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carr LA, Rowell PP, Pierce WM. Effects of subchronic nicotine administration on central dopaminergic mechanisms in the rat. Neurochem Res. 1989;14:511–515. doi: 10.1007/BF00964911. [DOI] [PubMed] [Google Scholar]
- Cass WA, Zahniser NR, Flach KA, Gerhardt GA. Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: Role of metabolism and effects of locally applied uptake inhibitors. J Neurochem. 1993;61:2269–2278. doi: 10.1111/j.1471-4159.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB. Nicotine and smoking: a perspective from animal studies. Psychopharmacology. 1987;92:135–143. doi: 10.1007/BF00177905. [DOI] [PubMed] [Google Scholar]
- Clift-O’Grady L, Linstedt AD, Lowe AW, Grote E, Kelly RB. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland BJ, Vogelsberg V, Neff NH, Hadjiconstantinou M. Protein kinase C activators decrease dopamine uptake into striatal synaptosomes. J Pharmacol Exp Ther. 1996;277:1527–1532. [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Wilkins LH, Pauly JR, Crooks PA. Development of a novel class of subtype-selective nicotinic receptor antagonist: pyridine-N-substituted nicotine analogs. Ann N Y Acad Sci. 1999;868:617–619. doi: 10.1111/j.1749-6632.1999.tb11334.x. [DOI] [PubMed] [Google Scholar]
- Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol Pharmacol. 2005;68:102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- Gnegy ME, Khoshbouei H, Berg KA, Javitch JA, Clarke WP, Zhang M, Galli A. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol. 2004;66:137–143. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- Hart C, Ksir C. Nicotine effects on dopamine clearance in rat nucleus accumbens. J Neurochem. 1996;66:216–221. doi: 10.1046/j.1471-4159.1996.66010216.x. [DOI] [PubMed] [Google Scholar]
- Hochstenbach F, David V, Watkins S, Brenner MB. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc Natl Acad Sci. 1992;89:4734–4738. doi: 10.1073/pnas.89.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn AS. Dopamine uptake: a review of progress in the last decade. Prog Neurobiol. 1990;34:387–400. doi: 10.1016/0301-0082(90)90033-d. [DOI] [PubMed] [Google Scholar]
- Hu M, Liu QS, Chang KT, Berg DK. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci. 2002;21:616–625. doi: 10.1006/mcne.2002.1202. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol. 2003;479:153–158. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Javitch JA, Galli A. Amphetamine regulation of dopamine transport. Combined measurements of transporter currents and transporter imaging support the endocytosis of an active carrier. J Biol Chem. 2004;279:8966–8975. doi: 10.1074/jbc.M303976200. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, Galli A. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol Phamacol. 2006;70:542–548. doi: 10.1124/mol.106.023952. [DOI] [PubMed] [Google Scholar]
- Kaiser SA, Soliakov L, Harvey SC, Luetje CW, Wonnacott S. Differential inhibition by alpha-conotoxin-MII of the nicotinic stimulation of [3H]dopamine release from rat striatal synaptosomes and slices. J Neurochem. 1998;70:1069–1076. doi: 10.1046/j.1471-4159.1998.70031069.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann NY Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Krijnse-Locker J, Parton RG, Fuller SD, Griffiths G, Dotti CG. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol Biol Cell. 1995;6:1315–1332. doi: 10.1091/mbc.6.10.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksir C, Mellor G, Hart C, Gerhardt GA. Nicotine enhances dopamine clearance in rat nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:151–156. doi: 10.1016/0278-5846(94)00111-t. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem Res. 2004;29:1405–1409. doi: 10.1023/b:nere.0000026404.08779.43. [DOI] [PubMed] [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol. 2002;61:436–445. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Melikian HE, McDonald JK, Gu H, Rudnick G, Moore KR, Blakely RD. Human norepinephrine transporter. Biosynthetic studies using a site-directed polyclonal antibody. J Biol Chem. 1994;269:12290–12297. [PubMed] [Google Scholar]
- Melikian HE, Ramamoorthy S, Tate CG, Blakely RD. Inability to N-glycosylate the human norepinephrine transporter reduces protein stability, surface trafficking, and transport activity but not ligand recognition. Mol Pharmacol. 1996;50:266–276. [PubMed] [Google Scholar]
- Middleton LS, Cass WA, Dwoskin LP. Nicotinic receptor modulation of dopamine transporter function in rat striatum and medial prefrontal cortex. J Pharmacol Exp Ther. 2004;308:367–377. doi: 10.1124/jpet.103.055335. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–498. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Psychopharmacology. 1992;108:460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJ, Wang YT, Niznik HB. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Xu Y, Brenner MB. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994;263:387–90. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Melikian HE, Qian Y, Blakely RD. Biosynthesis, N-glycosylation, and surface trafficking of biogenic amine transporter proteins. Methods Enzymol. 1998;296:347–370. doi: 10.1016/s0076-6879(98)96026-8. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Carr LA, Garner AC. Stimulation of [3H]dopamine release by nicotine in rat nucleus accumbens. J Neurochem. 1987;49:1449–1454. doi: 10.1111/j.1471-4159.1987.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Apparsundaram S, Gerhardt GA. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol Aging. 2003;24:1147–1154. doi: 10.1016/s0197-4580(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Hoffman AF, David DJ, Adams CE, Gerhardt GA. Nicotine-evoked nitric oxide release in the rat hippocampal slice. Neurosci Lett. 1998;255:127–130. doi: 10.1016/s0304-3940(98)00725-3. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Taubenblatt P, Dedieu JC, Gulik-Krzywicki T, Morel N. VAMP (synaptobrevin) is present in the plasma membrane of nerve terminals. J Cell Sci. 1999;112:3559–3567. doi: 10.1242/jcs.112.20.3559. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- Vezina P, Blanc G, Glowinski J, Tassin JP. Nicotine and morphine differentially activate brain dopamine in prefrontocortical and subcortical terminal fields: effects of acute and repeated injections. J Pharmacol Exp Ther. 1992;261:484–490. [PubMed] [Google Scholar]
- Volz TJ, Schenk JO. L-Arginine increases dopamine transporter activity in rat striatum via a nitric oxide synthase-dependent mechanism. Synapse. 2004;54:173–182. doi: 10.1002/syn.20075. [DOI] [PubMed] [Google Scholar]
- Westfall TC, Grant H, Perry H. Release of dopamine and 5-hydroxytryptamine from rat striatal slices following activation of nicotinic cholinergic receptors. Gen Pharmacol. 1983;14:321–325. doi: 10.1016/0306-3623(83)90037-x. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther. 1999;289:266–277. [PubMed] [Google Scholar]
- Zahniser NR, Doolen S. Chronic and acute regulation of Na+/Cl- -dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol Ther. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47:80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Ayers JT, Ghosheh OA, Sumithran SP, Crooks PA, Dwoskin LP. N-n-Alkylnicotinium and N-n-alkylpyridinium analogs inhibit of dopamine transporter function: Selectivity as nicotinic receptor antagonists. Drug Dev Res. 2003;60:270–284. [Google Scholar]


