Abstract
DHEA, together with DHEAS, is the most abundant steroid in the blood of young adult humans. Levels in humans decline with age and during certain types of illness or stress. We have found that DHEA(S) can prevent or reduce the neurotoxic actions in the hippocampus of the glutamate agonists N-methyl-d-aspartic acid (NMDA) both in vitro and in vivo or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainic acid in vitro. Pre-treatment with DHEA (10–100 nM for 6–8 h) protected primary hippocampal cultures from embryonic day 18 (E18) embryos against NMDA-induced toxicity (0.1, 1, 10, and 50 mM). DHEA added either with NMDA (1 mM) or 1 h later had lesser, but still significant, protective actions. DHEAS also reduced NMDA-induced toxicity (1 mM), although the lowest effective dose of DHEAS (100 nM) was higher than that of DHEA (10 nM). DHEA (100 nM) protected cultured neurons against the neurotoxic actions of either AMPA (25 μM) or kainic acid (1 mM) as well. In vivo, s.c. pellets of DHEA, which resulted in plasma levels that resembled those in young adult humans, protected hippocampal CA1/2 neurons against unilateral infusions of 5 or 10 nmol of NMDA. Because the release of glutamate has been implicated in the neural damage after cerebral ischemia and other neural insults, these results suggest that decreased DHEA levels may contribute significantly to the increased vulnerability of the aging or stressed human brain to such damage.
Levels of DHEA, together with DHEAS, peak at ≈20 years of age in humans and then decline ineluctably to reach values of 20–30% at ≈70–80 years of age (1). DHEA(S) levels also are reduced by intercurrent stressful events such as an episode of major depressive disorder (2, 3) or systemic disease (4, 5). Recent evidence shows that DHEA and DHEAS have direct actions on the brain, acting as allosteric modulators of γ-aminobutyric acid type A receptors (6), interacting with voltage-gated Ca2+ channels in CA1 hippocampal neurons (7), reducing aggression, and improving memory in mice (8, 9). The functional and clinical significance of age-related or stress-induced declines in DHEA or DHEAS for neural function is not understood. Both age and stress are associated with neuronal vulnerability to degeneration (10). We have found that DHEA or DHEAS can prevent or reduce the neurotoxic actions of the glutamate agonist N-methyl-d-aspartic acid (NMDA) in the hippocampus both in vitro and in vivo, as well as that of two other glutamate receptor agonists, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainic acid in vitro. Because the release of glutamate has been implicated in the neural damage after cerebral ischemia and other neural insults (11–13), these results suggest that decreased DHEA(S) levels may contribute significantly to the increased vulnerability of the aging or stressed human brain to such damage.
EXPERIMENTAL PROCEDURES
Preparation of Hippocampal Cultures.
Hippocampi were dissected out from E18 Sprague–Dawley rat fetuses and transferred into DMEM (GIBCO/BRL). The tissue was triturated gently by using a glass pipette with a narrow fire-polished tip. After a cell count with a trypan blue vital stain to assess the success of the dissection and triturating procedures, the cell suspensions were cultured at 105 cells per well on 13-mm glass coverslips coated with poly-l-lysine (1 mg/ml; Sigma) and merosin (10 μl/ml; Chemicon). Both poly-l-lysine and merosin (used just before the experiment) were used as adhesive agents, the former giving a charge to the glass coverslip and the latter acting as a biological adhesion molecule. They were used together to enhance adhesion of cells onto the coverslips for the purposes of this type of culture technique. The wells were then flooded with culture medium, a total volume of 500 μl of DMEM supplemented with B27 (1:50) growth medium (GIBCO/BRL), streptomycin (25 μg/ml)/penicillin G sodium (10,000 μg/ml)/amphotericin B (0.85%) (Sigma), and 5% fetal calf serum (GIBCO/BRL). The coverslips were inverted within 24 h to maximize neuronal survival (14, 15). On day 5 (plating day is day 0), the cultures in the medium were changed, and the concentration of fetal calf serum was reduced to 1%. On day 10, the medium was replaced with DMEM supplemented with N-2 growth medium (GIBCO/BRL) but without FCS. We used N-2 (insulin 500 μg/liter/human transferrin 10,000 μg/liter/progesterone 0.63 mg/liter/putrescine 1611 μg/liter/selenite 0.52 mg/liter) because, unlike B-27 [biotin/l-carnitine/corticosterone/ethanolamine/D(+) galactose/reduced glutathione/linoleic acid/linolenic acid/progesterone/putrescine/retinyl acetate/selenium/T3/vitamin E/vitamin E acetate/bovine albumin/catalase/insulin/superoxide dismutase/transferrin], it is limited in the range of anti-oxidants and hormones that it contains.
Toxicity Studies.
To investigate the effect of DHEA(S) on NMDA-induced excitotoxicity in primary hippocampal cultures, mature cultures (older than 10 days in vitro) were incubated with DHEA (100 nM; Sigma) under standard culture conditions for 6–8 h. After that, the cultures were exposed to NMDA for 1 h. After exposure to the toxin, they were then washed with the same medium (without NMDA) and incubated for an additional survival period of 16 h at the end of which they were fixed in either 10% formalin or 4% paraformaldehyde. They were then washed in PBS (pH 7.4) and stored at 4°C until staining.
Immunocytochemal Staining for Glial Fibrillary Acidic Protein (GFAP) and β-Tubulin III.
The cultures were double-stained immunocytochemically by using an mAb to β-tubulin(III) (aβT-III) (Sigma) and a polyclonal antibody to GFAP (aGFAP) (Dako). GFAP is a widely accepted marker of activated astrocytes. It should be noted that all astrocytes in culture are activated. βT-III is a neuron-specific cytoskeletal protein.
The cultures were incubated with 5% normal goat serum in PBS and 0.2% Triton X-100 (TX-PBS) for 1 h at room temperature. They were then incubated with the primary antibodies [GFAP (1:1000) and βT-III(1:500)] in PBS and 0.2% Triton X-100 overnight at 4°C. After that, they were washed three times with PBS and then incubated for 1 h with anti-mouse biotin and anti-rabbit fluorescein secondary antibodies (1:500 in PBS) (Boehringer Mannheim) at room temperature. They were then washed again three times with PBS and incubated for another hour at room temperature with streptavidin sulforhodamine secondary antibody (1:500 in PBS) (Boehringer Mannheim) and Hoechst stain (1:5000) (no. 33342, Sigma) as a nuclear counterstain to enable total cell count. They finally were washed and mounted (inverted) onto clean glass microscope slides with PBS/glycerol mountant (1:1). To prevent evaporation, the edges of the coverslips were sealed to the glass slide with clear nail varnish. After the varnish dried, the slides were cleaned, and stained cells were counted on a fluorescent microscope.
Cell Counts and Data Analysis.
Stained cells were counted in six random 625-μm2 fields across the coverslips by using a minimum of four coverslips per treatment. The identity of coverslips was not known during this procedure. All experiments were repeated across cultures from four different rats for DHEA and three for DHEAS. Multivariate comparisons were made by using ANOVAs and intergroup comparisons by Scheffe tests. Two group comparisons were analyzed with post hoc t tests.
Experiment 1.
To investigate the effect of DHEA on NMDA-induced neurotoxicity, the cultures were treated either with DHEA (100 nM) or vehicle 6–8 h before exposure to incremental doses of NMDA (0.1, 1, 10, and 50 mM). In subsequent in vitro experiments, a dose of 1 mM NMDA was chosen. Culture medium samples were collected at the end of the experiment (50 mM NMDA only), and lactate dehydrogenase was measured spectrofluorometrically (LDL kit; Sigma diagnostics; ref. 16).
Experiment 2.
The above procedures were repeated except that incremental amounts of either DHEA (0, 10, 20, 50, and 100 nM) or DHEAS (0, 10, 50, and 100 nM) were added before 1 mM NMDA. The culture medium from some of the latter experiments was assayed for DHEA by radioimmunoassay to test for possible conversion of DHEAS to DHEA.
Experiment 3.
The significance of the time of application of the hormone was investigated by adding either DHEA or DHEAS (100 nM) to the cultures 6 h before administration of 1 mM NMDA, simultaneously with the NMDA, or 1 h later (after the NMDA was washed off).
In Vivo Infusion of NMDA and Implantation of DHEA.
Intact, male Lister hooded rats (weight 325–375 g) were implanted s.c. with either paraffin or 100% DHEA pellets (weight 120–150 mg) under halothane/N2O anesthesia. Five days later, NMDA (Sigma) (or artificial cerebrospinal fluid) infusions were made into the dorsal hippocampus directly through cannulae introduced in a stereotaxic frame under anesthesia (halothane/N2O) at the required depth (co-ordinates: 2.5 mm below the dura; AP, −4.5, L, +3.3, from bregma, incisor bar −3.3). Two doses of NMDA were used (5 or 10 nmol) and infused in a volume of 1 μl over 5 min. The cannulae were left in place for 5 min. Rats were observed for ≈1 h postinfusion and returned to their cages for 3 days before they were anesthetized and perfused transcardially with an isotonic vascular rinse followed by 10% formalin/1% acetic acid. In addition, five rats were infused with either CSF or NMDA to which had been added 1 μg/ml rabbit IgG, which was then visualized immunocytochemically (results not shown), in all cases confirming that the infusion procedure was reliable.
In the second experiment, the above procedure was repeated with the following exceptions: Rats received paraffin or 50% or 100% DHEA pellets [120–150 mg fused DHEA (100% or diluted with 50% cholesterol)]. At the same time, an intracerebral guide cannula was implanted stereotaxically in the neocortex just above the CA1 region of the dorsal hippocampus (co-ordinates: A, −4.5, L, +3.3, from bregma, V, −1.5 from dura, incisor bar, −3.3). Five days later, they were infused without anesthesia through the guide cannula by inserting an infusion cannula that protruded 1 mm beyond the guide. NMDA (5 nmol) dissolved in 1 μl of artificial CSF was delivered by an infusion pump (1 μl/5 min). The cannulae were left in place for 5 min. Controls received CSF alone. The survival time and analytic procedure were the same as in the first experiment.
Measurement of Lesion Size and DHEA Levels.
Coronal serial frozen sections (40 μm) were cut through the hippocampus and stained with cresyl violet. The series (one in five) was examined, and the section with the largest cross-sectional lesion (i.e., loss of neurons in layers C1/2) was chosen. Degeneration of the pyramidal neurons of CA1 was measured on this section by using an image analysis system (nih image, written by Wayne Rasband). A line was drawn along the lesion, and its length was measured. The identity of the rats was obscured during this procedure. Plasma DHEA levels were measured by radioimmunoassay on blood samples taken at time of death (intra-assay variation 5.2%). Serial measures in other experiments showed that the pellets used sustained relatively constant DHEA levels in the blood throughout durations similar to this one.
RESULTS
Neuroprotective Effects of DHEA(S) on NMDA-Induced Toxicity in Vitro.
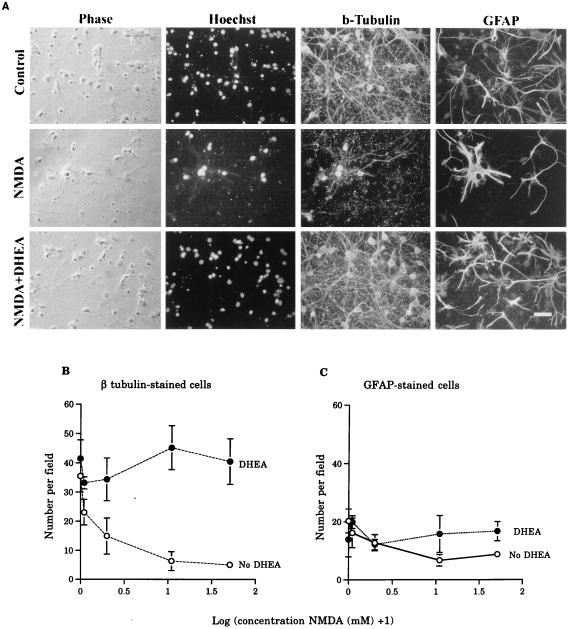
Hippocampal neurons are particularly sensitive to the toxic effects of glutamate analogues (10). We first looked for possible neuroprotective effects of DHEA on glutamate-mediated toxicity on both neurons and glia in vitro. Cells harvested from the hippocampi of E18 embryos were cultured for 10 days and grown in the presence or absence of DHEA (100 nM) for 6–8 h. Individual wells then were treated with incremental doses of NMDA (0.1, 1, 10, and 50 mM for 1 h), fixed, and stained 16 h later histochemically with the Hoechst dye (no. 33342), a nuclear stain (for total cell counts), and immunocytochemically for βT-III (to display putative neurons) and GFAP (for astrocytes). Control cultures contained many large βT-III-positive cells with neuronal morphology and a smaller population of GFAP-positive astrocytes (Fig. 1A). No cells were double-stained for βT-III and GFAP. There was a marked decrease in total cell number after exposure to NMDA, which was prevented by DHEA pretreatment (Fig. 1A). This result was mainly due to the effect of NMDA on neurons. NMDA alone resulted in a significant loss of βT-III-positive neurons, and this loss was prevented by the addition of DHEA to the cultures (Fig. 1B). This protective role was substantial: For example, the number of βT-III-stained neurons remaining after exposure to 1 mM NMDA was 17.4% of control levels in the absence of DHEA and 132% with DHEA added. In contrast, there was no significant effect of NMDA or DHEA on the number of GFAP-positive astrocytes (Fig. 1C). DHEA by itself did not significantly increase the total number of cells in culture compared with controls. Assays of lactic acid dehydrogenase, released into the medium by dead cells confirmed these results. Mean lactic acid dehydrogenase activity in the media of controls was 0.15 ± 0.01 units/liter; this activity was increased by 50 mM NMDA to 0.68 ± 0.27 (P < 0.05 Duncan’s test) but restored to 0.11 ± 0.03 in the presence of DHEA (F = 10.3, P < 0.01; P > 0.05 vs. controls; P < 0.05 vs. NMDA alone; n = 4 wells from one experiment).
Figure 1.
The effect of DHEA on the survival of cells in hippocampal primary cultures exposed to NMDA. (A) Photomicrographs showing the protective effects of DHEA on NMDA-induced toxicity. (Bar = 10 μm.) NMDA clearly reduced the number of phase-bright, Hoechst, and βT-III-stained cells, although a number of astrocytes remains. In the presence of DHEA, a large number of βT-III-positive cells survived exposure to NMDA. (B) Graph showing that, in the absence of DHEA, there was a decline in the number of βT-III-positive cells (log-transformed data ANOVA: main effect NMDA F(4,5) = 4.70, P < 0.007); this was prevented by the application of 100 nM DHEA (main effect DHEA F(1,5) = 30.34, P < 0.0001). There was a significant interaction between these factors (F = 2.95, P < 0.04). Values are mean ± SEM. Each variable is the mean of 4–6 wells. (C) Graph showing that there were no significant effects of either NMDA (F = 2.17, P > 0.05) or DHEA (F = 3.69, P > 0.05) on the survival of GFAP-stained cells in culture.
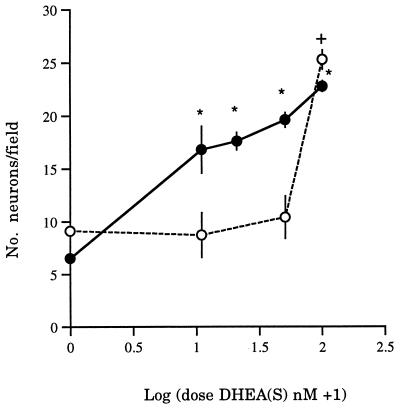
We then investigated the dose–response features of the neuroprotective effect of DHEA(S). Cultures were exposed to incremental amounts of DHEA (0, 10, 20, 50, and 100 nM). The lowest dose (10 nM) exerted significant protection against the toxic actions of 1 mM NMDA (four experiments; F = 51.9, P < 0.001; P < 0.05 Scheffe test) (Fig. 2). Three parallel experiments used similar doses of DHEAS. These experiments showed that, although DHEAS also protected neurons against NMDA, this steroid was effective only at a dose of 100 nM (F = 8.6, P < 0.01) (Fig. 2).
Figure 2.
The effects of incremental doses of either DHEA (•) or DHEAS (○) on the numbers of neurons in cultures exposed to 1 mM NMDA. The means (± SEM) of 3–4 experiments are shown. ∗, DHEA; †, DHEAS; P < 0.05 compared with baseline (no steroid).
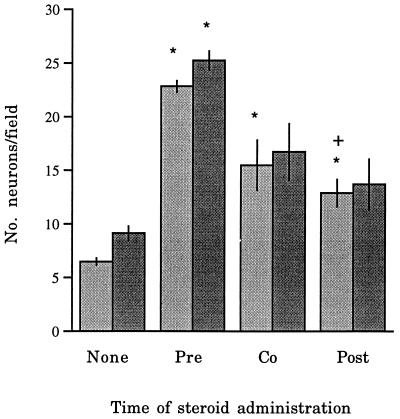
Because it was not clear whether steroid pretreatment was essential for neuroprotection, in the next experiment we added DHEA (100 nM) 6 h before, with, or 1 h after 1 mM NMDA. This showed that co-administration of DHEA also protected neurons, although the effect was less marked than in pretreatment (F = 28.9, P < 0.001; Scheffe test P < 0.05). Postadministration of DHEA also increased neuronal survival compared with cultures without steroid but not to the same extent as pre-treatment (Scheffe test P < 0.05) (Fig. 3). On the other hand, DHEAS was significantly protective only when it was added before NMDA exposure (F = 10.2, P < 0.005) (Fig. 3). There was little conversion of DHEAS to DHEA; radioimmunoassay showed that levels of DHEA in cultures treated with DHEAS were 0.5 ± 0.04 nM (n = 3) compared with 0.51 ± 0.06 nM (n = 3) in untreated controls. DHEA levels at the end of the experiment in DHEA-treated cultures (100 nM) were 32.3 ± 1.3 nM. Sensitivity of the assay was 0.44 nM.
Figure 3.
Graph showing the effect of changing the time of application of either DHEA or DHEAS relative to that of NMDA (1 mM) on neuronal survival. DHEA (░⃞) or DHEAS (▪) was added either 6 h before NMDA (Pre), co-administered with NMDA (Co), or applied 60 minutes afterward (Post). ∗, P < 0.05 (Scheffe test) compared with controls (no steroid added); †, univariate comparisons between pretreatment and other steroid treatments.
Neuroprotective Effects of DHEA on AMPA or Kainate-Induced Toxicity in Vitro.
We then explored the ability of DHEA to protect neurons in vitro against two other glutamate receptor agonists acting on non-NMDA receptors. DHEA (100 nM) added 6 h before either AMPA (25 μM) or kainic acid (1 mM) also protected cultures partially against the toxic actions of either of these agonists (Table 1). In the absence of DHEA, AMPA reduced neuronal cell counts to 17.5% of control values, but these values were restored to 55.8% in the presence of DHEA. Similarly, cell counts were 26.4% of control values after kainic acid but increased to 63.2% after added DHEA.
Table 1.
Effect of DHEA (100 nM) on neurotoxicity induced by either AMPA or kainic acid
| Neurons per field, mean ± SEM | |
|---|---|
| Control (no toxin + vehicle) | 26.9 ± 1.1 |
| AMPA 25 μm + vehicle | 4.7 ± 0.4 |
| AMPA + DHEA 100 nM | 15.0 ± 0.8* |
| Kainic acid 1 mM + vehicle | 7.1 ± 0.5 |
| Kainic acid + DHEA 100 nM | 17.0 ± 0.5* |
The effects of adding DHEA (100 nM) 6 h before either AMPA (25 μM) or kainic acid (1 mM) for 60 min on the mean number of neurons per field (four experiments for each treatment; 4-6 wells per experiment).
P < 0.001 (t test; toxin + vehicle vs. toxin + DHEA).
Neuroprotective Effects of DHEA on NMDA-Induced Toxicity in Vivo.
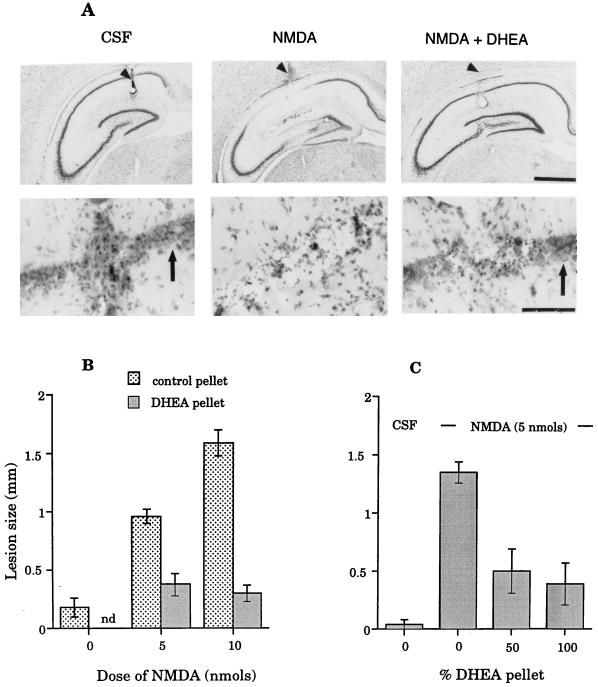
To test the ability of DHEA to protect hippocampal neurons against NMDA-induced neurotoxicity in vivo, rats were implanted s.c. either with a pellet of DHEA (100%) or paraffin (placebo control). After 5 days, rats were reanesthetized, and either 5 or 10 nmol of NMDA was infused unilaterally into the hippocampus. These doses were chosen on the basis of pilot experiments, which showed them to induce moderate but reproducible loss of hippocampal neurons. Brains were examined histologically after 3 days, and, as expected, both doses of NMDA in control (paraffin-implanted) rats resulted in localized degeneration of the hippocampal pyramidal cells in CA1 and CA2 and the areas lying immediately around the tip of the infusion cannula (Fig. 4A). The large neurons of CA1/2 either were completely absent or were severely malformed and clearly in the process of degeneration, resulting in either thinning or absence of this layer. Rats preimplanted with DHEA s.c. had significantly reduced hippocampal lesions after NMDA infusions (Fig. 4 A and B). Overall, the size of the lesions in DHEA-treated rats (measured as the medio-lateral loss of hippocampal pyramidal neurons; details given below) was reduced by 60.4% or 81.1% of their respective controls (rats given either 5 or 10 nmol NMDA but no DHEA). Radioimmunoassays showed that the plasma of control rats contained little or no DHEA (0.47 ± 0.06 ng/ml, n = 4) compared with those with 100% DHEA pellets (16.5 ± 1.8 ng/ml, n = 4). The latter are similar to those in young human adults (1).
Figure 4.
The effects of DHEA on the lesions induced by NMDA infused into the hippocampus of rats. (A) Photomicrographs of sections through the hippocampus of a sham-operated (CSF infusion) animal, a rat infused with 10 nmol of NMDA and no DHEA, and one implanted with a 100% DHEA pellet and then infused with NMDA [Upper, low power (Bar = 1000 μm); Lower, high power (Bar = 100 μm)]. Sections pass through the center of the lesions at the site of the infusion cannula. Arrowheads show the cannula track, and arrows show healthy pyramidal neurons. NMDA in the absence of DHEA caused a clear, reproducible lesion that was prevented by DHEA pretreatment. (B) Graphs showing the effects of DHEA on two doses of NMDA-induced toxicity in rats after infusions in the hippocampus under halothane anesthesia. CSF infusion results in small, barely detectable cell loss; NMDA without DHEA pretreatment results in a dose-dependent lesion that was prevented by s.c. DHEA implants. ANOVAS were carried out on log-transformed data. Main effects: NMDA F(2,40) = 33.31, P < 0.001; DHEA F(2,40) = 47.63, P < 0.01. Values shown are mean ± SEM. (nd, not done). There were no significant differences between the CSF controls and groups receiving NMDA (either 5 or 10 nmol) and DHEA (F = 1.35, P value not significant) (6–11 rats per group). (C) Graphs showing the effects of s.c. DHEA (either 50 or 100% pellets) on the mean lesion size after NMDA (5 nmol) infusions through preimplanted guide cannula. NMDA in the absence of DHEA induced degeneration of the hippocampal pyramidal neurons, as expected (NMDA main effect F(1,24) = 26.97, P < 0.001). This degeneration was greatly reduced in rats receiving either 50% or 100% DHEA s.c. (main effect DHEA F(2,24) = 11.11, P < 0.001) vs. no DHEA. There was no significant difference between the effects of the two doses of DHEA (post hoc t test; df = 11, t = 0.38, P > 0.05), and lesion size in these two groups was not significantly larger than lesions induced by infusions of CSF into control animals (Scheffe test, P > 0.05) (5–7 rats per group).
To exclude possible effects of anesthesia on the neurotoxicity of NMDA or its interaction with DHEA, the above experiment was repeated on unanesthetized animals. Rats were preimplanted with paraffin or 50% or 100% DHEA pellets s.c. and a guide cannula introduced into the brain to lie just above the hippocampus. Five days later, 5 nmol of NMDA was infused into the hippocampus through these guide cannulae in awake animals. The results confirmed those of the previous experiment. DHEA again greatly reduced the neurotoxic effects of this dose of NMDA (Fig. 4C) and was equally effective at both 50% and 100% doses (Scheffe test, P > 0.05). Lesion size was reduced by 63.0% or 71.1% of control values by implanting either 50% or 100% DHEA pellets.
DISCUSSION
The experiments reported here show that the neurosteroid DHEA has powerful ameliorating effects on excitatory amino acid-induced neurotoxicity. This conclusion is strengthened by this effect being demonstrated both in vitro and in vivo for NMDA. In the latter experiments, the pellets of DHEA used in these rats created constant plasma levels lying in the range of those in young human adults (1) (rats normally have very little circulating DHEA). Under these very different conditions, DHEA had a major attenuating effect on the neurotoxic action of NMDA. We used a standard concentration of NMDA in vitro (1 mM) that was slightly higher than that of some previous studies because the experiments were done on inverted cultures (15). Parallel experiments showed that, although DHEAS also protected neurons in vitro against NMDA, it seemed less potent because significant protection only was observed at ≈10 times the lowest effective dose of DHEA (10 nM) used in these experiments. It is therefore interesting to note that, although levels of DHEAS in the blood of adult humans are more than 100 times those of DHEA, in the CSF, the ratio is only about three times (17). This suggests that DHEA may play a predominant role in the combined neuroprotective effects of these steroids in the brain. However, radioimmunoassays showed that the neuroprotective action of DHEAS is not due to conversion to DHEA, so DHEAS may contribute directly to the overall protective actions in species, such as humans, that have appreciable amounts of both steroids in their CSF (17). The protective actions of DHEA(S) stand in contrast to those of glucocortoids, which induce hippocampal pyramidal neuron degeneration (10).
The mechanisms by which DHEA(S) have these neuroprotective effects are not yet evident. Although protection of the large pyramidal neurons of the hippocampus was a prominent feature, other cell types (for example, glia) also may have been affected or might be acting as mediators of this effect. At the molecular level, two attractive hypotheses are either that DHEA(S) reduces the entry of Ca2+ into cells (or otherwise alters free Ca2+ intracellular homeostasis) after NMDA exposure—a process that is known to be a critical step in excitotoxic cell death (18) or that it acts as a glucocorticoid antagonist (19). The first interpretation does not exclude the second because glucocorticoids have been shown to accentuate Ca2+ entry into neurons via voltage-gated channels (20). It is also possible that endogenous corticoids potentiate the action of glutamatergic toxins such as NMDA (21); DHEA may be having its effect by counteracting these steroids, although this is less likely to apply to our in vitro results. DHEA also can have either estrogenic or androgenic effects (22), and this may have altered the process of cell proliferation and differentiation in vitro. So far, attempts to identify a cytoplasmic steroid-like receptor for DHEA have not been convincing. Alternatively, the neuroprotective effects of DHEA may be related to its known action as a γ-aminobutyric acid type A antagonist (6) although whether γ-aminobutyric acid contributes to NMDA-induced toxicity under the conditions used in these experiments remains doubtful. There seems no indication that DHEA is a glutamate (NMDA) receptor-blocking agent; indeed, there is electrophysiological evidence suggesting that acute treatment with DHEA can accentuate the actions of glutamate (23) unlike the longer term actions reported here. Whether DHEA might alter the number or affinity of NMDA-type glutamate receptors awaits further study. However, our finding that DHEA also protects cultured neurons against either AMPA or kainic acid raises questions about the identity of the glutamate receptor involved in these effects. These results suggest that DHEA(S) has a generally protective action against glutamate neurotoxicity. Furthermore, we have preliminary data showing that DHEA also counteracts glucocorticoid-induced neurotoxicity.
We also found that DHEA was able to protect cultured neurons against NMDA when the steroid was added either at the same time as NMDA or even 1 h later. This suggests that DHEA interfered with some process downstream of the initial action of NMDA, although what this might be remains to be determined. DHEAS was less effective when added either with NMDA or subsequent to it, although this conclusion is provisional. Cell counts after addition of DHEAS seemed to show increased survival, but because the control values in this experiment were somewhat higher than for DHEA, differences were not significant (Fig. 3).
The principal significance of the findings reported here is that situations in which DHEA or DHEAS, major steroids in the blood of humans, are lowered either by age, stress, or illnesses such as major depression (2, 3) may increase the vulnerability of the brain to neurotoxic processes involving the release of glutamate, including ischemia and other neurodegenerative conditions (24). These findings point the way toward new preventive treatments for those either at risk for age-related neurodegenerative disorders or additional therapeutic approaches for those suffering acute brain damage.
Acknowledgments
We thank our colleagues J. W. Fawcett and S. B. Dunnett for help with these experiments, J. Bashford for photography, and E. Torres and H. M. Shiers for technical guidance. V.G.K. was supported partly by a postgraduate studentship from the Cambridge Commonwealth Trust; N.H.K. was a third year undergraduate, and C.N.S. holds a research fellowship from the Wellcome Trust.
ABBREVIATIONS
- DHEA
dehydroepiandrosterone
- DHEAS
DHEA sulfate
- NMDA
N-methyl-d-aspartic acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- GFAP
glial fibrillary acidic protein
- βT-III
β-tubulin(III)
- CSF
cerebrospinal fluid
References
- 1.Orentreich N, Brind J L, Vogelman J H, Andres R, Baldwin H. J Clin Endocrin Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 2.Goodyer I M, Herbert J, Altham P M E, Pearson J, Secher S M, Shiers H M. Psychol Med. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- 3.Herbert J, Goodyer I M, Altham P M E, Pearson J, Secher S M, Shiers H M. Psychol Med. 1996;26:257–263. doi: 10.1017/s0033291700034656. [DOI] [PubMed] [Google Scholar]
- 4.Hedman M, Nilsson E, Torre B. Clin Exp Rheumatol. 1992;10:25–30. [PubMed] [Google Scholar]
- 5.Spratt D I, Longcope C, Cox P M, Bigos S T, Wilbur-Welling C. J Clin Endocrin Metab. 1993;72:1542–1547. doi: 10.1210/jcem.76.6.8501162. [DOI] [PubMed] [Google Scholar]
- 6.Majewska M D, Demigoren S, Spivak C E, London E D. Brain Res. 1990;526:143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- 7.French-Mullen J M H, Spence K T. Eur J Pharmacol. 1991;202:269–272. doi: 10.1016/0014-2999(91)90303-8. [DOI] [PubMed] [Google Scholar]
- 8.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlegel M L, Spetz J F, Robel P, Haug M. Physiol Behav. 1985;34:867–870. doi: 10.1016/0031-9384(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 10.Sapolsky R M. Stress, the Aging Brain, and the Mechanisms of Neuron Death. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- 11.Simon R P, Swan J H, Griffiths T, Meldrum B S. Science. 1984;226:850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- 12.Benveniste H, Drejer J, Schousboe A, Diemer N H. J Neurochem. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 13.Choi D W. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 14.Brewer G J, Cotman C W. Brain Res. 1989;494:65–74. doi: 10.1016/0006-8993(89)90144-3. [DOI] [PubMed] [Google Scholar]
- 15.Svendsen C N, Kew J N C, Staley K, Sofroniew M V. J Neurosci. 1994;14:75–87. doi: 10.1523/JNEUROSCI.14-01-00075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh J Y, Choi D W. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 17.Guazzo E P, Kirkpatrick P J, Goodyer I M, Shiers H M, Herbert J. J Clin Endocrin Metab. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- 18.Garthwaite G, Garthwaite J. Neurosci Lett. 1986;66:193–198. doi: 10.1016/0304-3940(86)90189-8. [DOI] [PubMed] [Google Scholar]
- 19.May M, Holmes E, Rogers W, Poth M. Life Sci. 1990;46:1627–1631. doi: 10.1016/0024-3205(90)90394-7. [DOI] [PubMed] [Google Scholar]
- 20.Kerr D, Campbell L, Thibault O, Landfield P. Proc Natl Acad Sci USA. 1992;89:8527–8531. doi: 10.1073/pnas.89.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armanini M P, Hutchins C, Stein B A, Sapolsky R M. Brain Res. 1990;532:7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- 22.Ebeling P, Kiovisto V A. Lancet. 1994;343:1479–1481. doi: 10.1016/s0140-6736(94)92587-9. [DOI] [PubMed] [Google Scholar]
- 23.Bergeron R, de Montigny C, Debonnel G. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton S A, Rosenberg P A. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]