Abstract
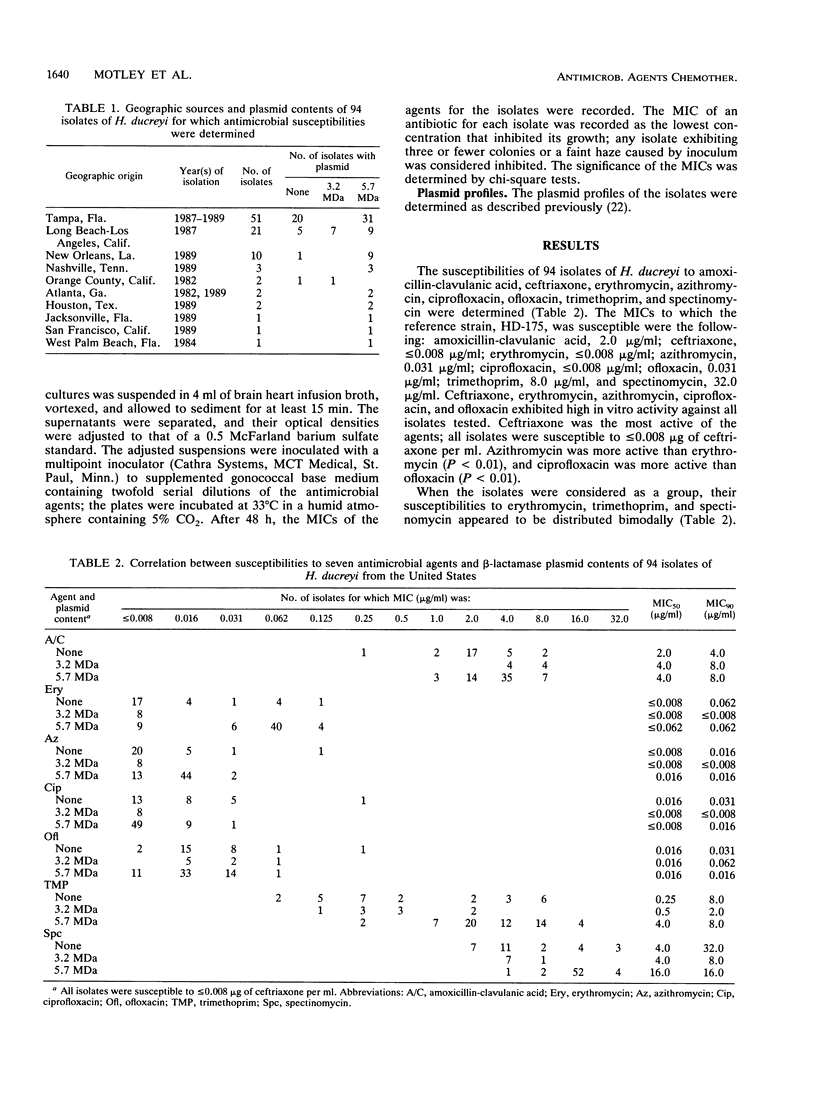
We determined the susceptibilities of 94 strains of Haemophilus ducreyi isolated in various municipalities in the United States between 1982 and 1989 to the following antimicrobial agents: amoxicillin-clavulanic acid, ceftriaxone, erythromycin, azithromycin, ciprofloxacin, ofloxacin, trimethoprim, and spectinomycin. Ceftriaxone (MIC, less than or equal to 0.008 micrograms/ml), azithromycin (MIC, less than or equal to 0.125 micrograms/ml), erythromycin (MIC, less than or equal to 0.125 micrograms/ml), ciprofloxacin (MIC, less than or equal to 0.25 micrograms/ml), and ofloxacin (MIC, less than or equal to 0.25 micrograms/ml) were highly active against all isolates. Amoxicillin-clavulanic acid (MICs, 0.25 to 8.0 micrograms/ml), trimethoprim (MICs, 0.06 to 16.0 micrograms/ml), and spectinomycin (MICs, 2.0 to greater than or equal to 32.0 micrograms/ml) were less active against these isolates. Isolates possessing the 5.7-MDa beta-lactamase plasmid were less susceptible to erythromycin, trimethoprim, and spectinomycin than were isolates possessing the 3.2-MDa beta-lactamase plasmid. The susceptibilities of plasmidless isolates to erythromycin, trimethoprim, and spectinomycin were distributed bimodally; the median MIC for the more susceptible plasmidless isolates corresponded to that for isolates with the 3.2-MDa plasmid, and the median MIC for the less susceptible plasmidless isolates corresponded to that for isolates with the 5.7-MDa plasmid. Thus, plasmid profiles may be valuable markers for geographical variations in antimicrobial susceptibilities of H. ducreyi strains that may indicate the relative efficacy of regimens for the treatment of chancroid. Of the regimens recommended by the U.S. Public Health Service for the treatment of chancroid, our results support the use of erythromycin, ceftriaxone, and ciprofloxacin, and perhaps ofloxacin, but suggest that amoxicillin-clavulanic acid and sulfamethoxazole-trimethoprim should be used with caution.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeck D., Johnson A. P., Dangor Y., Ballard R. C. Antibiotic susceptibilities and plasmid profiles of Haemophilus ducreyi isolates from southern Africa. J Antimicrob Chemother. 1988 Oct;22(4):437–444. doi: 10.1093/jac/22.4.437. [DOI] [PubMed] [Google Scholar]
- Anderson B., Albritton W. L., Biddle J., Johnson S. R. Common beta-lactamase-specifying plasmid in Haemophilus ducreyi and Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1984 Feb;25(2):296–297. doi: 10.1128/aac.25.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgeri Y. R., Ballard R. C., Duncan M. O., Mauff A. C., Koornhof H. J. Antimicrobial susceptibility of 103 strains of Haemophilus ducreyi isolated in Johannesburg. Antimicrob Agents Chemother. 1982 Oct;22(4):686–688. doi: 10.1128/aac.22.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J., Meier M., Ehrman N., Maclean I., Slaney L., Albritton W. L. Molecular epidemiology of beta-lactamase-specifying plasmids of Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jun;21(6):857–863. doi: 10.1128/aac.21.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangor Y., Miller S. D., Exposto F. da L., Koornhof H. J. Antimicrobial susceptibilities of southern African isolates of Haemophilus ducreyi. Antimicrob Agents Chemother. 1988 Sep;32(9):1458–1460. doi: 10.1128/aac.32.9.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon J. A., Tostowaryk W., Pauzé M. Effects of different media and methods of inoculum preparation on results of antimicrobial susceptibility testing of Neisseria gonorrhoeae by agar dilution. Antimicrob Agents Chemother. 1987 Nov;31(11):1744–1749. doi: 10.1128/aac.31.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon J. A., Yeung K. H. Beta-lactamase plasmids and chromosomally mediated antibiotic resistance in pathogenic Neisseria species. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S125–S133. doi: 10.1128/cmr.2.suppl.s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylewski J., Nsanze H., D'Costa L., Slaney L., Ronald A. Trimethoprim sulphamoxole in the treatment of chancroid. Comparison of two single dose treatment regimens with a five day regimen. J Antimicrob Chemother. 1985 Jul;16(1):103–109. doi: 10.1093/jac/16.1.103. [DOI] [PubMed] [Google Scholar]
- Fast M. V., Nsanze H., D'Costa L. J., Karasira P., Maclean I. W., Piot P., Albritton W. L., Ronald A. R. Antimicrobial therapy of chancroid: an evaluation of five treatment regimens correlated with in vitro sensitivity. Sex Transm Dis. 1983 Jan-Mar;10(1):1–6. [PubMed] [Google Scholar]
- Girouard Y. C., Maclean I. W., Ronald A. R., Albritton W. L. Synergistic antibacterial activity of clavulanic acid and amoxicillin against beta-lactamase-producing strains of Haemophilus ducreyi. Antimicrob Agents Chemother. 1981 Jul;20(1):144–145. doi: 10.1128/aac.20.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978 Apr;13(4):608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Slutchuk M., Scatliff J., Sherman E., Wilt J. C., Ronald A. R. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev Infect Dis. 1980 Nov-Dec;2(6):867–879. doi: 10.1093/clinids/2.6.867. [DOI] [PubMed] [Google Scholar]
- Handsfield H. H., Totten P. A., Fennel C. L., Falkow S., Holmes K. K. Molecular epidemiology of Haemophilus ducreyi infections. Ann Intern Med. 1981 Sep;95(3):315–318. doi: 10.7326/0003-4819-95-3-315. [DOI] [PubMed] [Google Scholar]
- Jones B. M., Kinghorn G. R., Duerden B. I. In vitro activity of azithromycin and erythromycin against organisms associated with bacterial vaginosis and chancroid. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):551–553. doi: 10.1007/BF01962614. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Gavan T. L., Thornsberry C., Fuchs P. C., Gerlach E. H., Knapp J. S., Murray P., Washington J. A., 2nd Standardization of disk diffusion and agar dilution susceptibility tests for Neisseria gonorrhoeae: interpretive criteria and quality control guidelines for ceftriaxone, penicillin, spectinomycin, and tetracycline. J Clin Microbiol. 1989 Dec;27(12):2758–2766. doi: 10.1128/jcm.27.12.2758-2766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Le Saux N. M., Slaney L. A., Plummer F. A., Ronald A. R., Brunham R. C. In vitro activity of ceftriaxone, cefetamet (Ro 15-8074), ceftetrame (Ro 19-5247; T-2588), and fleroxacin (Ro 23-6240; AM-833) versus Neisseria gonorrhoeae and Haemophilus ducreyi. Antimicrob Agents Chemother. 1987 Jul;31(7):1153–1154. doi: 10.1128/aac.31.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol P. J., Ronald A. R. The plasmids of Haemophilus ducreyi. J Antimicrob Chemother. 1984 Dec;14(6):561–564. [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989 Apr;2(2):137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson-Le Pors M. J., Casin I., Ortenberg M., Perol Y. In-vitro susceptibility of thirty strains of Haemophilus ducreyi to several antibiotics including six cephalosporins. J Antimicrob Chemother. 1983 Mar;11(3):271–280. doi: 10.1093/jac/11.3.271. [DOI] [PubMed] [Google Scholar]
- Sarafian S. K., Knapp J. S. Molecular epidemiology, based on plasmid profiles, of Haemophilus ducreyi infections in the United States. Results of surveillance, 1981-1990. Sex Transm Dis. 1992 Jan-Feb;19(1):35–38. doi: 10.1097/00007435-199201000-00007. [DOI] [PubMed] [Google Scholar]
- Schmid G. P., Sanders L. L., Jr, Blount J. H., Alexander E. R. Chancroid in the United States. Reestablishment of an old disease. JAMA. 1987 Dec 11;258(22):3265–3268. [PubMed] [Google Scholar]
- Schmid G. P. The treatment of chancroid. JAMA. 1986 Apr 4;255(13):1757–1762. [PubMed] [Google Scholar]
- Schmid G. P. Treatment of chancroid, 1989. Rev Infect Dis. 1990 Jul-Aug;12 (Suppl 6):S580–S589. doi: 10.1093/clinids/12.supplement_6.s580. [DOI] [PubMed] [Google Scholar]
- Sturm A. W. Comparison of antimicrobial susceptibility patterns of fifty-seven strains of Haemophilus ducreyi isolated in Amsterdam from 1978 to 1985. J Antimicrob Chemother. 1987 Feb;19(2):187–191. doi: 10.1093/jac/19.2.187. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Echeverria P., Hanchalay S., Pitarangsi C., Slootmans L., Piot P. Antimicrobial susceptibility and characterization of outer membrane proteins of Haemophilus ducreyi isolated in Thailand. J Clin Microbiol. 1985 Mar;21(3):442–444. doi: 10.1128/jcm.21.3.442-444.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Pitarangsi C., Echeverria P., Panikabutra K., Suvongse C. Comparative study of ceftriaxone and trimethoprim-sulfamethoxazole for the treatment of chancroid in Thailand. J Infect Dis. 1985 Nov;152(5):1002–1006. doi: 10.1093/infdis/152.5.1002. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Handsfield H. H., Peters D., Holmes K. K., Falkow S. Characterization of ampicillin resistance plasmids from Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Apr;21(4):622–627. doi: 10.1128/aac.21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R. A., Mabey D. C., Bello C. S., Felmingham D. The comparative in-vitro activity of twelve 4-quinolone antimicrobials against Haemophilus ducreyi. J Antimicrob Chemother. 1985 Aug;16(2):165–168. doi: 10.1093/jac/16.2.165. [DOI] [PubMed] [Google Scholar]
- Zenilman J. M., Whittington W. L., Frazier D., Rice R. J., Knapp J. S. Penicillinase-producing Neisseria gonorrhoeae in Dade County, Florida: phenotypic characterization of isolates from 1983, 1984, and 1986. Sex Transm Dis. 1988 Jul-Sep;15(3):158–163. doi: 10.1097/00007435-198807000-00010. [DOI] [PubMed] [Google Scholar]



