Abstract
Programmed RNA breakage is an emerging theme underlying cellular responses to stress, virus infection and defense against foreign species. In many cases, site-specific cleavage of the target RNA generates 2′,3′ cyclic phosphate and 5′-OH ends. For the damage to be repaired, both broken ends must be healed before they can be sealed by a ligase. Healing entails hydrolysis of the 2′,3′ cyclic phosphate to form a 3′-OH and phosphorylation of the 5′-OH to form a 5′-PO4. Here, we demonstrate that a polynucleotide kinase-phosphatase enzyme from Clostridium thermocellum (CthPnkp) can catalyze both of the end-healing steps of tRNA splicing in vitro. The route of tRNA repair by CthPnkp can be reprogrammed by a mutation in the 3′ end-healing domain (H189D) that yields a 2′-PO4 product instead of a 2′-OH. Whereas tRNA ends healed by wild-type CthPnkp are readily sealed by T4 RNA ligase 1, the H189D enzyme generates ends that are spliced by yeast tRNA ligase. Our findings suggest that RNA repair enzymes can evolve their specificities to suit a particular pathway.
INTRODUCTION
‘RNA repair’ is a versatile mechanism to rectify programmed breaks in tRNAs and mRNAs incurred during tRNA processing and under conditions of cellular stress. Examples include virus-mediated tRNA repair to thwart a host antiviral response to bacteriophage infection (1), splicing of intron-containing tRNAs (2) and unconventional mRNA splicing during the unfolded protein response (3,4). In each of these cases, the inciting event is a site-specific endonuclease cleavage of the target RNA to generate a 2′,3′ cyclic phosphate end and a 5′-OH end. Both broken ends must be healed before they can be sealed by an RNA ligase. Healing entails hydrolysis of the 2′,3′ cyclic phosphate to form a 3′-OH and phosphorylation of the 5′-OH to form a 5′-PO4.
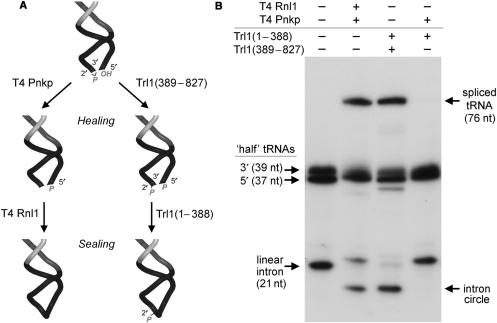
Two pathways of tRNA repair have been delineated, which proceed through distinct 3′ end-healing steps that result in different products of the RNA ligase reaction (1,2). The first pathway is catalyzed by the familiar T4 enzymes polynucleotide kinase-phosphatase (Pnkp) and RNA ligase 1 (Rnl1). Pnkp is a bifunctional enzyme that remodels the ends of the broken tRNA by converting the 2′,3′ cyclic phosphate to a 3′-OH, 2′-OH and by phosphorylating the 5′-OH end to form a 5′-PO4. Rnl1 then joins the 3′-OH and 5′-PO4 RNA ends to form a standard 3′–5′ phosphodiester at the repair junction (Figure 1A) (1). The second pathway is catalyzed by yeast tRNA ligase (Trl1), a multifunctional enzyme composed of separable healing and sealing domains (5–10). The healing domain, Trl1(389–827), consists of a cyclic phosphodiesterase (CPD) module that hydrolyzes the 2′,3′ cyclic phosphate of the proximal tRNA half-molecule to a 3′-OH, 2′-PO4 and a polynucleotide kinase module that converts the tRNA 5′-OH to a 5′-PO4 (Figure 1A). The ligase domain, Trl1(1–388), then joins the healed ends to form a tRNA with a 2′-PO4, 3′–5′ phosphodiester at the splice junction. [The junction 2′-PO4 is ultimately removed by a phosphotransferase, Tpt1, that is specific to the yeast tRNA repair pathway (11)].
Figure 1.
Pathway choice in tRNA splicing is dictated by the outcome of 3′ end healing. (A) Distinctive phage and yeast pathways of tRNA repair. In phage tRNA restriction-repair, the 2′,3′ cyclic phosphate and 5′-OH ends of the broken tRNA are healed by T4 Pnkp, which completely removes the phosphate at the 3′ end and phosphorylates the 5′ terminus. T4 Rnl1 then seals the 3′-OH and 5′-PO4 termini to form a standard 3′–5′ phosphodiester linkage. In yeast tRNA splicing, the ends are healed by Trl1 kinase-CPD domain (389–827), which generate a 3′-OH, 2′-PO4 on the proximal tRNA half and a 5′-PO4 on the distal half, and then sealed by a Trl1 ligase domain (1–388). The yeast pathway leaves a 2′-PO4 at the splice junction. (B) Reaction mixtures (10 µl) containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 5 mM DTT, 25 µM ATP, 25 µM GTP, 140 fmol 32P-labeled cleaved tRNA and 1 pmol of T4 Rnl1, T4 Pnkp, Trl1(1–388) or Trl1(389–827), where indicated by + over the lanes, were incubated for 30 min at 37°C. The reactions were quenched by adding 10 µl of 95% formamide, 50 mM EDTA. The samples were heated at 95°C for 1 min and then analyzed by electrophoresis through a 15-cm 15% polyacrylamide gel containing 7 M urea in 45 mM Tris-borate, 1 mM EDTA. The products were visualized by autoradiography.
Recent studies highlight a plethora of target-specific endoribonuclease toxins in bacteria (12–18) and fungi (19) and their role in defending the organism against non-self species and viruses. This raises the question of whether RNA repair systems exist in bacterial cells as a means of evading programmed RNA breakage. Our identification of candidate RNA healing and sealing enzymes in several bacterial proteomes (20,21) suggests an RNA repair capacity, but the scant knowledge of the genetics and ecology of those bacteria provides no clues to what types of RNA damage might be subject to repair. Here, we demonstrate that an enzyme from Clostridium thermocellum (CthPnkp) can catalyze both of the end-healing steps of tRNA splicing in vitro. The biochemical pathway of tRNA repair by the CthPnkp can be reprogrammed by a mutation in the 3′ end-healing domain that yields a 2′-PO4 product instead of a 2′-OH.
MATERIALS AND METHODS
Recombinant T4 and yeast tRNA repair enzymes
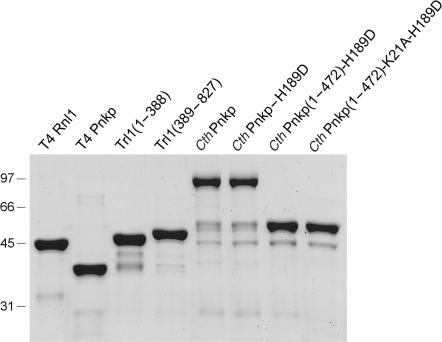
His10-tagged versions of T4 Rnl1, T4 Pnkp and yeast Trl1(1–388) were produced in E. coli and purified from soluble lysates by Ni-agarose chromatography as described previously (8,22,23). Yeast Trl1(389–827) was produced in E. coli as a His-Smt3 fusion and recovered from a soluble extract by Ni-agarose chromatography. The His-Smt3 tag was removed with the Smt3-specific protease Ulp1 and the tag-free Trl1(398–927) protein was recovered in the flow-through fraction during a second round of Ni-agarose chromatography. SDS-PAGE analysis of the enzyme preparations is shown in Figure 2.
Figure 2.
Purification of recombinant RNA repair enzymes. Aliquots (5 µg) of the indicated recombinant proteins were analyzed by SDS-PAGE. The Coomassie blue-stained gel is shown. The positions and sizes (kDa) of marker polypeptides are indicated on the left.
Recombinant CthPnkp
Wild-type CthPnkp and mutated or truncated versions of CthPnkp were produced in E. coli as His10-tagged fusions and purified from soluble bacterial extracts by Ni-agarose chromatography (24,25). The 200-mM imidazole eluates were dialyzed against 150 mM NaCl in buffer A (50 mM Tris-HCl, pH 8.0, 10% glycerol, 1 mM EDTA, 1 mM β-mercaptoethanol) and then applied to 1-ml columns of DEAE-Sephacel that had been equilibrated in buffer A. The columns were washed with 5 ml of buffer A and then eluted stepwise with 200 and 500 mM NaCl in buffer A. The CthPnkp proteins were recovered in the DEAE flow-through, apparently free of contaminating nucleic acids (which were detected in the 0.5 M NaCl eluate). SDS-PAGE analysis of the CthPnkp preparations is shown in Figure 2.
Synthesis of pre-tRNA in vitro
The intron-containing pre-tRNA is a chimera consisting of the mature tRNA sequence of plant pre-tRNATyr plus the intron and anticodon of Methanocaldococcus pre-tRNATrp (26). This pre-tRNA was generated by in vitro transcription of BstN1-cut plasmid pNtY9-T7-M1 by T7 RNA polymerase in the presence of [α32P]ATP. A reaction mixture (100 µl) containing 80 mM HEPES-KOH (pH 7.5), 24 mM MgCl2, 40 mM DTT, 2 mM spermidine, 2.5 mM CTP, UTP and GTP, 0.5 mM [α-32P]ATP, 5 µg template DNA, 120 units RNAsin (Promega), 0.5 units yeast inorganic pyrophosphatase (Sigma) and 300 units T7 RNA polymerase (New England Biolabs) was incubated for 3 h at 37°C. The labeled pre-tRNA was purified by electrophoresis through an 8% polyacrylamide-urea gel. The pre-tRNA was eluted from an excised gel slice in 650 µl of 10 mM Tris-HCl (pH 8.0), 1 mM EDTA. This procedure yielded 380–420 pmol of pre-tRNA.
Cleavage of the pre-tRNA with archaeal-splicing endonuclease
pre-tRNA cleavage reaction mixtures (100 µl) containing 50 mM Tris-HCl (pH 7.5), 100 mM KCl, 10 mM MgCl2, 1 mM DTT, 40 µM spermine, 40 pmol [32P[AMP-labeled pre-tRNA and 1.5 µg Methanocaldococcus jannaschii tRNA-splicing endonuclease (produced in E. coli as a His10 fusion and purified by Ni-agarose chromatography) were incubated for 20 min at 65°C. The RNA products were phenol-extracted, precipitated with ethanol (28–32 pmol of cleaved tRNA recovered), resuspended in 100 µl of 10 mM Tris-HCl (pH 8.0), 1 mM EDTA and stored at −20°C.
RESULTS AND DISCUSSION
Distinct tRNA repair pathways in vitro
The finding that the essential healing and sealing components of the yeast tRNA-splicing system can be replaced in vivo by their bacteriophage analogs (27) attested to the portability of RNA repair systems from widely distant taxa. However, certain restrictions apply. T4 Pnkp can substitute for the yeast kinase-CPD domain only in tandem with T4 Rnl1 and the yeast ligase domain functions only in tandem with yeast kinase-CPD. Because the kinase modules of the phage and yeast healing enzymes are structurally homologous and the reaction products are identical (a 5′-PO4 RNA end), the critical factor appears to be the distinctive 3′-OH, 2′-PO4 end configuration generated by yeast CPD versus the 3′-OH, 2′-OH end produced by T4 Pnkp. In other words, the yeast tRNA ligase appears to require the 2′-PO4 terminus to seal tRNAs in vivo.
To better delineate the specificity of end-healing and end-sealing during tRNA repair/splicing, we exploited an in vitro tRNA-splicing system designed by Englert and Beier (26). The broken tRNA substrate was generated by treating a 32P-labeled intron-containing pre-tRNA with a tRNA-splicing endonuclease, which led to quantitative release of a linear 21-nt intron and the formation of two ‘half-tRNA’ molecules: a 37-nt 5′ fragment and a 39-nt 3′-fragment (Figure 1B). Reaction of the broken RNA with a combination of yeast Trl1(1–388) and Trl1(389–827) resulted in a mature spliced tRNA and the circularization of the intron. Splicing of the tRNA halves and intron circularization were also seen with T4 Rnl1 plus T4 Pnkp. However, the ligase domain of yeast Trl1 was unable to splice the tRNA or circularize the intron when paired with T4 Pnkp (Figure 1B). This in vitro observation echoes precisely the inability of Trl1(1–388) to function together with T4 Pnkp in vivo. This incompatibility reflects a requirement for a 3′-OH, 2′-PO4 terminus in order for the ligase component of Trl1 to seal the broken RNA ends in vitro.
Clostridium Pnkp performs the end-healing steps of tRNA repair
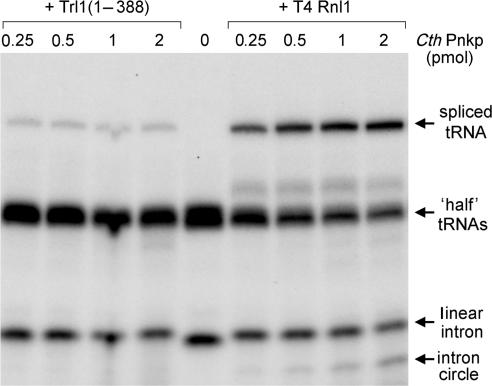
CthPnkp catalyzes the phosphorylation of 5′-OH termini of DNA or RNA polynucleotides and the dephosphorylation of 2′,3′ cyclic phosphate, 2′-phosphate and 3′-phosphate ribonucleotides (21,24,25). These characteristics are consistent with a role in end healing during RNA or DNA repair. CthPnkp is an 870-aa polypeptide composed of three catalytic domains: an N-terminal module that resembles the kinase domain of T4 Pnkp, a central phosphoesterase module and a C-terminal module that resembles the adenylyltransferase domain of polynucleotide ligases. The distinctive feature of CthPnkp vis a vis known repair enzymes is that its 3′ end modification component belongs to the binuclear metallophosphoesterase superfamily (24,25). The salient question is whether CthPnkp can perform a bona fide RNA repair reaction and, if so, to which of the two known repair pathways it adheres. We found that CthPnkp functioned in tRNA splicing in vitro as the sole source of end-healing activity when added in tandem with T4 Rnl1, resulting in the formation of a spliced tRNA product and an intron circle (Figure 3). However, splicing activity was attenuated severely when yeast Trl1(1–388) replaced phage Rnl1 as the source of the RNA sealing activity (Figure 3). We surmise that the predominant outcome of CthPnkp healing of a tRNA 2′,3′ cyclic phosphate end is not the 3′-OH, 2′-PO4 required by yeast tRNA ligase.
Figure 3.
Wild-type CthPnkp elects the phage tRNA repair pathway. Reaction mixtures (20 µl) containing 50 mM Tris-acetate (pH 6.5), 5 mM MgCl2, 0.5 mM MnCl2, 50 µM ATP, 200 fmol radiolabeled cleaved tRNA substrate, either 1 pmol Trl1(1–388) or 1 pmol T4 Rnl1, and CthPnkp as specified were incubated for 30 min at 37°C. The products were resolved by PAGE and visualized by autoradiography.
Mechanism of 3′ end healing by CthPnkp
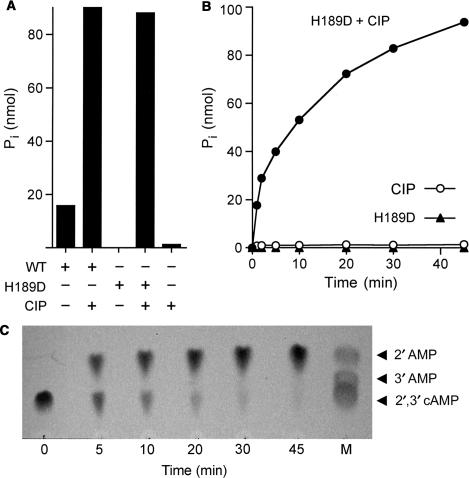
To better address how CthPnkp acts at a 2′,3′ cyclic phosphate end, we compared the release of inorganic phosphate (Pi) from 2′,3′ cAMP by CthPnkp alone versus a duplicate sample in which the products were treated with calf intestinal phosphatase (CIP) prior to assay of Pi release (Figure 4A). Whereas CthPnkp converted 16% of the input 2′,3′ cAMP to Pi, treatment of the reaction product with CIP increased the yield of Pi to 90% of the input 2′,3′ cAMP substrate. (Treatment with CIP alone released <2% of the Pi from 2′,3′ cAMP.) These results indicate that a nucleoside 2′,3′ cyclic phosphate is converted by CthPnkp to a phosphomonoester prior to the release of Pi. The transient nature of the phosphomonoester intermediate would account for why CthPnkp-mediated tRNA repair proceeds down the bacteriophage-type pathway.
Figure 4.
CthPnkp-H189D opens a 2′,3′ cyclic phosphodiester to form a 2′-phosphomonoester. (A) Reaction mixtures (10 µl) containing 50 mM Tris-HCl (pH 7.5), 0.5 mM MnCl2, 10 mM 2′,3′ cAMP (Sigma) and either 4 µg wild-type CthPnkp, 1.4 µg CthPnkp-H189D or 1 unit CIP (New England Biolabs) where indicated by + were incubated for 30 min at 45°C. The reactions were quenched by adding 1 µl of 0.5 M EDTA. Phosphate release was measured colorimetrically using the Malachite green reagent (BIOMOL Research Laboratories). (B) Reaction mixtures containing (per 10 µl) 50 mM Tris-HCl (pH 8.0), 0.5 mM MnCl2, 10 mM 2′,3′ cAMP and either 1.4 µg of CthPnkp-H189D and 1 U of CIP (closed circle), 1.4 µg of CthPnkp-H189D alone (closed triangle) or 1 U of CIP alone (open circle) were incubated at 45°C. Aliquots (10 µl) were withdrawn at the times specified and quenched immediately with EDTA. Phosphate release is plotted as a function of time. (C) A reaction mixture containing (per 10 µl) 50 mM Tris-HCl (pH 8.0), 0.5 mM MnCl2, 10 mM 2′,3′ cAMP and 1.4 µg of CthPnkp-H189D was incubated at 45°C. Samples (10 µl) were withdrawn at the times specified and quenched immediately with EDTA. Aliquots (1 µl) of each sample were applied to a cellulose-F TLC plate (EMD Chemicals). Markers 2′AMP, 3′AMP and 2′,3′ cAMP (5 nmol each) were spotted in lane M. The TLC plate was developed with buffer containing saturated ammonium sulfate:3 M sodium acetate:isopropanol (80:6:2), in which the order of migration away from the origin (Rf) is 2′,3′ cyclic phosphate <3′-phosphate <2′-phosphate (28,29). The nucleotides were visualized by photography under UV light. All species on the TLC plate that were detected by UV are shown in the photograph.
Studies of the catalytic mechanism of the CthPnkp phosphoesterase have shown it to be plastic, insofar as certain mutations in the active site alter the reaction outcome (25). In particular, an H189D change abolished the phosphomonoesterase activity with p-nitrophenylphosphate as a substrate, without affecting the phosphodiesterase activity with bis-p-nitrophenylphosphate (25). Here, we find that CthPnkp-H189D failed to release inorganic phosphate from 2′,3′ cAMP, while converting 88% of the input 2′,3′ cAMP to a monoester that was hydrolyzed by CIP (Figure 4A). The reaction in substrate excess proceeded to near completion with apparent pseudo-first-order kinetics (Figure 4B) at an initial rate of 20 s−1. The transformation of CthPnkp into a CPD-only end-healing enzyme by the H189D mutation allowed us to probe the outcome of the CPD reaction, by performing TLC analysis of the reaction mixture as a function of reaction time in the presence of CthPnkp only (no CIP). This revealed a single-step conversion of 2′,3′ cAMP to a faster moving product that comigrated with 2′ AMP (Figure 4C). We infer that a 2′ phosphomonoester is the relevant intermediate in tRNA end healing by wild-type CthPnkp.
The H189D change alters the pathway of tRNA repair
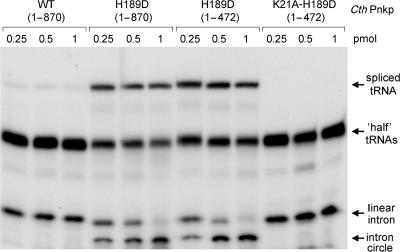
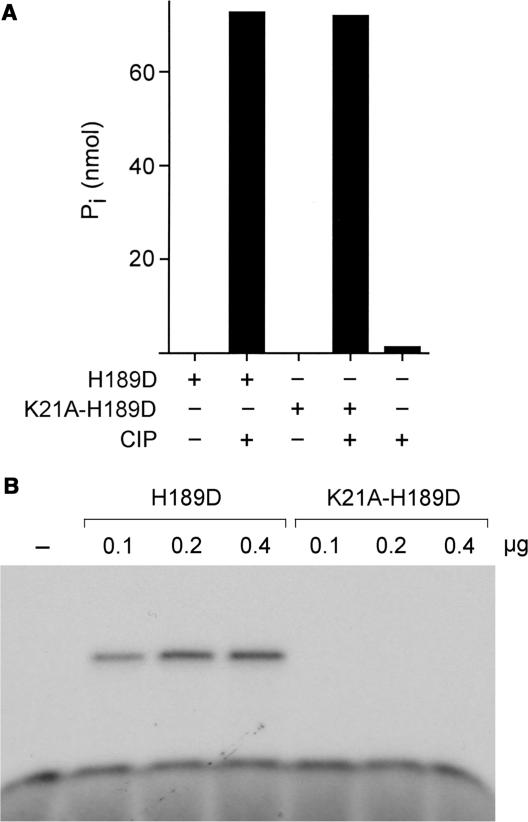
Testing the activity of CthPnkp-H189D in tRNA splicing revealed that its RNA repair specificity had been transformed so that its healing activity was now channeled through the yeast-type repair pathway. H189D exhibited a gain of function in working with the yeast ligase to perform both tRNA splicing and intron circularization (Figure 5). CthPnkp-H189D had no repair activity in the absence of Trl1(1–388) (not shown). Moreover, a truncated version of H189D (aa 1–472) containing just the kinase and phosphoesterase modules retained full activity in tRNA splicing in combination with Trl1(1–388) (Figure 5). Thus, the C-terminal adenylyltransferase domain of CthPnkp was noncontributory to the observed tRNA repair activity. We introduced into the H189D-healing domain a second mutation in the ATP-binding motif of the polynucleotide kinase module. The K21A change abolished 5′ kinase activity, without impacting the cyclic phosphoesterase function (Figure 6). The K21A-H189D double mutant was inert in tRNA repair (Figure 5), proving that the bacterial enzyme is the source of the 5′ end-healing function for tRNA splicing in vitro.
Figure 5.
The H189D change redirects CthPnkp down the yeast tRNA-splicing pathway. Reaction mixtures (20 µl) containing 50 mM Tris-acetate (pH 6.5), 0.5 mM MnCl2, 50 µM ATP, 5 mM MgCl2, 200 fmol of cleaved tRNA substrate, 1 pmol Trl1(1–388) and wild-type CthPnkp, or mutated versions as specified were incubated for 30 min at 37°C. The products were resolved by PAGE and visualized by autoradiography.
Figure 6.
The K21A mutation of CthPnkp abolishes the 5′ kinase activity without affecting the 2′,3′ phosphodiesterase. (A) Phosphodiesterase reaction mixtures (10 µl) containing 50 mM Tris-HCl (pH 7.5), 0.5 mM MnCl2, 10 mM 2′,3′ cAMP and either 0.8 µg CthPnkp(1–472)-H189D, 0.8 µg CthPnkp(1–472)-K21A-H189D or 1 unit CIP (where indicated by +) were incubated for 30 min at 45°C. Phosphate release was measured by using the Malachite green reagent. (B) Polynucleotide kinase reaction mixtures (10 µl) containing 50 mM Tris-acetate (pH 7.0), 5 mM DTT, 100 µM [γ32P]ATP, 10 µM 5′-OH oligodeoxynucleotide 12-mer 5′-CACTATCGGAAT and CthPnkp(1–472)-H189D or CthPnkp(1–472)-K21A-H189D as specified were incubated for 30 min at 45°C. The reactions were quenched with 10 µl of 95% formamide, 50 mM EDTA. The products were analyzed by urea-PAGE and visualized by autoradiography.
CONCLUSIONS
The present study illuminates several aspects of RNA repair: (i) the demonstration of a repair-competent RNA end-healing enzyme encoded by a bacterium (to our knowledge, the first case so documented); (ii) repair pathway choice based on the compatibility of the 3′ end-healing and sealing reaction specificities; and (iii) the ability to reprogram the choice of repair pathway by a single missense mutation in the 3′ end-healing component. These properties suggest that RNA repair enzymes and pathways can quickly evolve (or co-evolve) their specificities to suit a particular biological context, e.g. the CPD reaction chemistry and stringent specificity of yeast Trl1 for a 3′-OH, 2′-PO4 terminus ensures avoidance of sealing RNA ends not generated by programmed cleavage events (27).
Although CthPnkp is clearly tRNA repair-competent in vitro, our initial attempts to replace Trl1(389–827) with CthPnkp-H189D in vivo in yeast were not successful. We suspect that the non-portability of this RNA repair enzyme reflects idiosyncratic aspects of foreign protein expression or localization in budding yeast, rather than any mechanistic deficit, insofar as we were also unable to complement a Saccharomyces cerevisiae trl1Δ strain with the Trl1 ortholog from fission yeast Schizosaccharomyces pombe.
Based on the detection of CthPnkp-like proteins with similar size and domain organization in many other bacterial genera (Bacillus, Helicobacter, Fusobacterium, Herpetosiphon and Maricaulis), we propose that bacterial RNA repair is hiding in plain sight.
ACKNOWLEDGEMENTS
We thank Markus Englert and Hildburg Beier for the generous gift of plasmids containing the hybrid pre-tRNA and archaeal-splicing endonuclease genes. This work was supported by NIH grant GM42498. S.S. is an American Cancer Society Research Professor. Funding to pay the Open Access publication charge was provided by NIH grant GM42498.
Conflict of interest statement. None declared.
REFERENCES
- 1.Amitsur M, Levitz R, Kaufman G. Bacteriophage T4 anticodon nuclease, polynucleotide kinase, and RNA ligase reprocess the host lysine tRNA. EMBO J. 1987;6:2499–2503. doi: 10.1002/j.1460-2075.1987.tb02532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abelson J, Trotta CR, Li H. tRNA splicing. J. Biol. Chem. 1998;273:12685–12688. doi: 10.1074/jbc.273.21.12685. [DOI] [PubMed] [Google Scholar]
- 3.Sidrauski C, Cox JS, Walter P. tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell. 1996;87:405–413. doi: 10.1016/s0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez TN, Sidrauski C, Dörfler S, Walter P. Mechanism of non-spliceosomal mRNA splicing in the unfolded protein response pathway. EMBO J. 1999;18:3119–3132. doi: 10.1093/emboj/18.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greer CL, Peebles CL, Gegenheimer P, Abelson J. Mechanism of action of a yeast RNA ligase in tRNA splicing. Cell. 1983;32:537–546. doi: 10.1016/0092-8674(83)90473-7. [DOI] [PubMed] [Google Scholar]
- 6.Apostol BL, Westaway SK, Abelson J, Greer CL. Deletion analysis of a multifunctional yeast tRNA ligase polypeptide: identification of essential and dispensable functional domains. J. Biol. Chem. 1991;266:7445–7455. [PubMed] [Google Scholar]
- 7.Westaway SK, Belford HG, Apostol BL, Abelson J, Greer CL. Novel activity of a yeast ligase deletion polypeptide: evidence for GTP-dependent tRNA splicing. J. Biol. Chem. 1993;268:2435–2443. [PubMed] [Google Scholar]
- 8.Sawaya R, Schwer B, Shuman S. Genetic and biochemical analysis of the functional domains of yeast tRNA ligase. J. Biol. Chem. 2003;278:43298–43398. doi: 10.1074/jbc.M307839200. [DOI] [PubMed] [Google Scholar]
- 9.Wang LK, Shuman S. Structure-function analysis of yeast tRNA ligase. RNA. 2005;11:966–975. doi: 10.1261/rna.2170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LK, Schwer B, Englert M, Beier H, Shuman S. Structure-function analysis of the kinase-CPD domain of yeast tRNA ligase (Trl1) and requirements for complementation of tRNA splicing by a plant Trl1 homolog. Nucleic Acids Res. 2006;34:517–527. doi: 10.1093/nar/gkj441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinelli SL, Kierzek R, Turner DH, Phizicky EM. Transient ADP-ribosylation of a 2′-phosphate implicated in its removal from ligated tRNA during splicing in yeast. J. Biol. Chem. 1999;274:2637–2644. doi: 10.1074/jbc.274.5.2637. [DOI] [PubMed] [Google Scholar]
- 12.Blanga-Kanfi S, Amitsur M, Azem A, Kaufmann G. PrrC-anticodon nuclease: functional organization of a prototypal bacterial restriction RNase. Nucleic Acids Res. 2006;34:3209–3219. doi: 10.1093/nar/gkl415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa T, Tomita K, Ueda T, Watanabe K, Uozumi T, Masaki H. A cytotoxic ribonuclease targeting specific tRNA anticodons. Science. 1999;283:2097–2100. doi: 10.1126/science.283.5410.2097. [DOI] [PubMed] [Google Scholar]
- 14.Tomita K, Ogawa T, Uozumi T, Watanabe K, Masaki H. A cytotoxic ribonuclease which specifically cleaves four isoaccepting arginine tRNAs at their anticodon loops. Proc. Natl. Acad. Sci. USA. 2000;97:8278–8283. doi: 10.1073/pnas.140213797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graille M, Mora L, Buckingham RH, van Tilbeurgh H, de Zamaroczy M. Structural inhibition of the colicin D tRNase by the tRNA-mimicking immunity protein. EMBO J. 2004;23:1474–1482. doi: 10.1038/sj.emboj.7600162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YL, Elias Y, Huang RH. Structural and mutational studies of the catalytic domain of colicin E5: a tRNA-specific ribonuclease. Biochemistry. 2005;44:10494–10500. doi: 10.1021/bi050749s. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhang J, Hara H, Kato I, Inouye M. Insights into the mRNA cleavage mechanism by MazF, an mRNA interferase. J. Biol. Chem. 2005;280:3143–3150. doi: 10.1074/jbc.M411811200. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Zhang Y, Teh JS, Zhang J, Connell N, Rubin H, Inouye M. Characterization of mRNA interferases from Mycobacterium tuberculosis. J. Biol. Chem. 2006;281:18638–18643. doi: 10.1074/jbc.M512693200. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Huang B, Esberg A, Johanson M, Byström AS. The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. RNA. 2005;11:1648–1654. doi: 10.1261/rna.2172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins A, Shuman S. An RNA ligase from Deinococcus radiodurans. J. Biol. Chem. 2004;279:50654–50661. doi: 10.1074/jbc.M407657200. [DOI] [PubMed] [Google Scholar]
- 21.Martins A, Shuman S. An end-healing enzyme from Clostridium thermocellum with 5′ kinase, 2′,3′ phosphatase, and adenylyltransferase activities. RNA. 2005;11:1271–1280. doi: 10.1261/rna.2690505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang LK, Shuman S. Domain structure and mutational analysis of T4 polynucleotide kinase. J. Biol. Chem. 2001;276:26868–26874. doi: 10.1074/jbc.M103663200. [DOI] [PubMed] [Google Scholar]
- 23.Wang LK, Ho CK, Pei Y, Shuman S. Mutational analysis of bacteriophage T4 RNA ligase 1: different functional groups are required for the nucleotidyl transfer and phosphodiester bond formation steps of the ligation reaction. J. Biol. Chem. 2003;278:29454–29462. doi: 10.1074/jbc.M304320200. [DOI] [PubMed] [Google Scholar]
- 24.Keppetipola N, Shuman S. Mechanism of the phosphatase component of Clostridium thermocellum polynucleotide kinase-phosphatase. RNA. 2006;12:73–82. doi: 10.1261/rna.2196406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keppetipola N, Shuman S. Distinct enzymic functional groups are required for the phosphomonoesterase and phosphodiesterase activities of Clostridium thermocellum polynucleotide kinase-phosphatase. J. Biol. Chem. 2006;281:19251–19259. doi: 10.1074/jbc.M602549200. [DOI] [PubMed] [Google Scholar]
- 26.Englert M, Beier H. Plant tRNA ligases are multifunctional enzymes that have diverged in sequence and substrate specificity from RNA ligases of other phylogenetic origins. Nucleic Acids Res. 2005;33:388–399. doi: 10.1093/nar/gki174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwer B, Sawaya R, Ho CK, Shuman S. Portability and fidelity of RNA-repair systems. Proc. Natl. Acad. Sci. USA. 2004;101:2788–2793. doi: 10.1073/pnas.0305859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genshik P, Billym E, Saniewicz M, Filipowicz W. The human RNA 3′-terminal phosphate is a member of a new family of proteins conserved in eukarya, bacteria, and archaea. EMBO J. 1997;16:2955–2967. doi: 10.1093/emboj/16.10.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yakunin AF, Proudfoot M, Kuznetsova E, Savchenko A, Brown G, Arrowsmith CH, Edwards AM. The HD domain of the Escherichia coli tRNA nucleotidyltransferase has 2′,3′-cyclic phosphodiesterase, 2′-nucleotidase, and phosphatase activities. J. Biol. Chem. 2004;279:36819–36827. doi: 10.1074/jbc.M405120200. [DOI] [PubMed] [Google Scholar]