Abstract
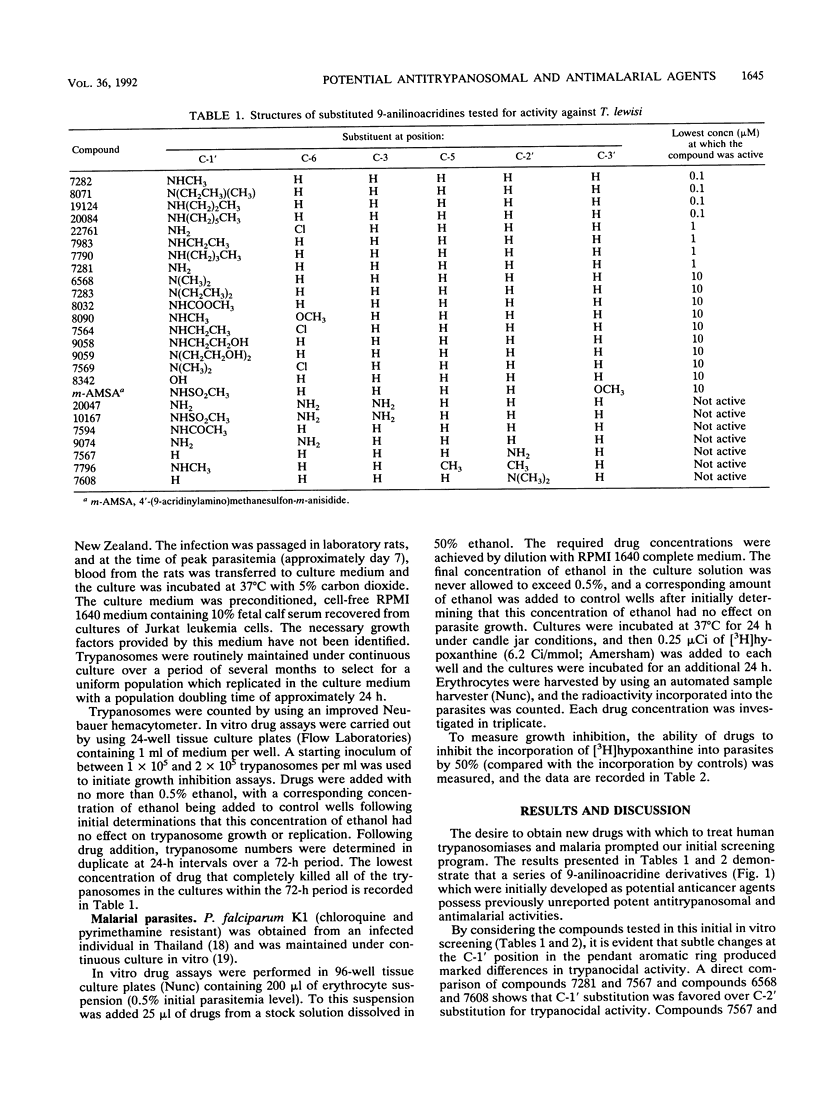
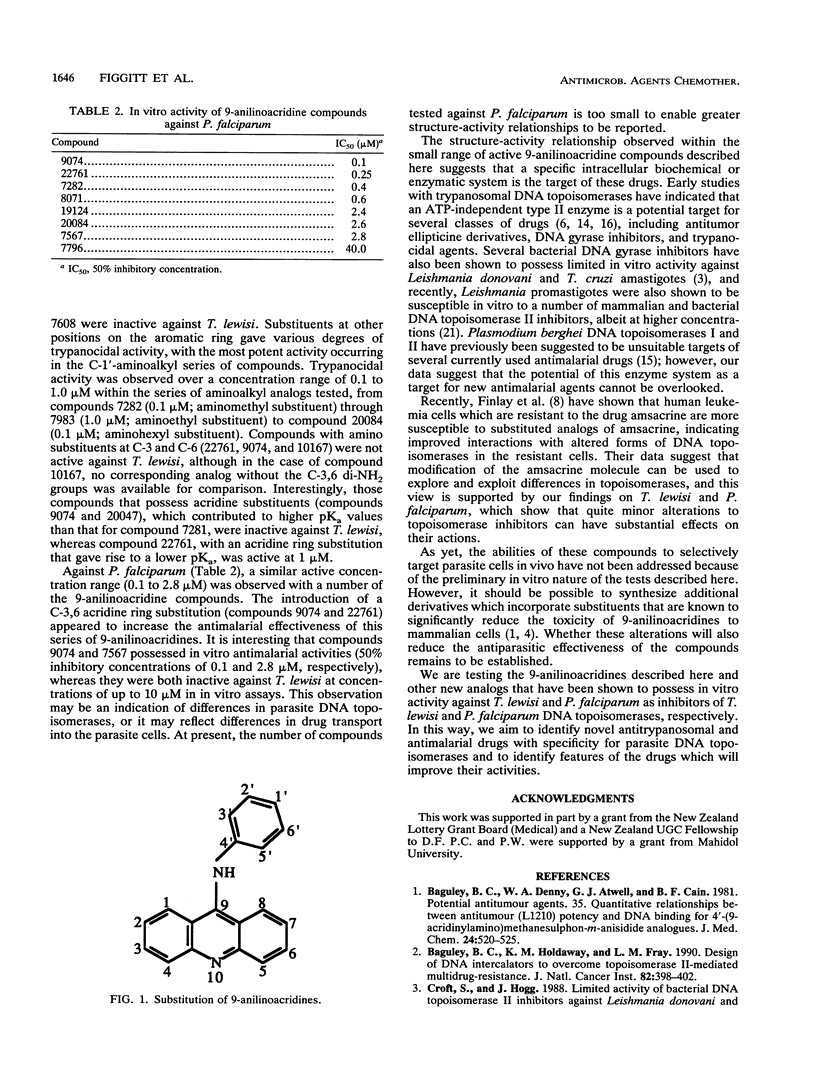
The requirement for rational drug design in the search for new agents that are active against parasitic protozoa prompted our in vitro studies with a group of 9-anilinoacridines. In vitro growth assays with Trypanosoma lewisi identified a series of C-1' alkylaminoacridines which possess previously unreported potent growth-inhibitory activities against T. lewisi at a concentration range of 0.1 to 1 microM. In contrast, several 9-anilinoacridines that possess acridine ring NH2 substituents at C-3 and C-6 were inactive against T. lewisi, but they possessed strong activity against Plasmodium falciparum at a concentration range of 0.1 to 2.8 microM. In mammalian cells, amsacrine [4'-(9-acridinylamino)methanesulfon-m-anisidide] inhibits DNA topoisomerase II; however, amsacrine was only weakly active against T. lewisi. Such differences in the patterns of susceptibility of mammalian cells, T. lewisi, and P. falciparum to these 9-anilinoacridines may reflect enzyme differences between different parasites and mammalian cells that can be exploited by further improvements in drug design.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Denny W. A., Atwell G. J., Cain B. F. Potential antitumor agents. 35. Quantitative relationships between antitumor (L1210) potency and DNA binding for 4'-(9-acridinylamino)methanesulfon-m-anisidide analogues. J Med Chem. 1981 May;24(5):520–525. doi: 10.1021/jm00137a009. [DOI] [PubMed] [Google Scholar]
- Baguley B. C., Holdaway K. M., Fray L. M. Design of DNA intercalators to overcome topoisomerase II-mediated multidrug resistance. J Natl Cancer Inst. 1990 Mar 7;82(5):398–402. doi: 10.1093/jnci/82.5.398. [DOI] [PubMed] [Google Scholar]
- Croft S. L., Hogg J. Limited activity of bacterial DNA topoisomerase II inhibitors against Leishmania donovani and Trypanosoma cruzi amastigotes in vitro. Trans R Soc Trop Med Hyg. 1988;82(6):856–856. doi: 10.1016/0035-9203(88)90017-x. [DOI] [PubMed] [Google Scholar]
- Douc-Rasy S., Kayser A., Riou J. F., Riou G. ATP-independent type II topoisomerase from trypanosomes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7152–7156. doi: 10.1073/pnas.83.19.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douc-Rasy S., Riou J. F., Ahomadegbe J. C., Riou G. ATP-independent DNA topoisomerase II as potential drug target in trypanosomes. Biol Cell. 1988;64(2):145–156. doi: 10.1016/0248-4900(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H. Future prospects for the chemotherapy of human trypanosomiasis. 1. Novel approaches to the chemotherapy of trypanosomiasis. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):613–617. doi: 10.1016/0035-9203(90)90124-w. [DOI] [PubMed] [Google Scholar]
- Finlay G. J., Baguley B. C., Snow K., Judd W. Multiple patterns of resistance of human leukemia cell sublines to amsacrine analogues. J Natl Cancer Inst. 1990 Apr 18;82(8):662–667. doi: 10.1093/jnci/82.8.662. [DOI] [PubMed] [Google Scholar]
- Fu S., Xiao S. H. Pyronaridine: A new antimalarial drug. Parasitol Today. 1991 Nov;7(11):310–313. doi: 10.1016/0169-4758(91)90267-r. [DOI] [PubMed] [Google Scholar]
- Pepin J., Milord F., Guern C., Schechter P. J. Difluoromethylornithine for arseno-resistant Trypanosoma brucei gambiense sleeping sickness. Lancet. 1987 Dec 19;2(8573):1431–1433. doi: 10.1016/s0140-6736(87)91131-7. [DOI] [PubMed] [Google Scholar]
- Peters W. The prevention of antimalarial drug resistance. Pharmacol Ther. 1990;47(3):499–508. doi: 10.1016/0163-7258(90)90067-c. [DOI] [PubMed] [Google Scholar]
- Rewcastle G. W., Baguley B. C., Atwell G. J., Denny W. A. Potential antitumor agents. 52. Carbamate analogues of amsacrine with in vivo activity against multidrug-resistant P388 leukemia. J Med Chem. 1987 Sep;30(9):1576–1581. doi: 10.1021/jm00392a009. [DOI] [PubMed] [Google Scholar]
- Riou G., Douc-Rasy S., Kayser A. Inhibitors of trypanosome topoisomerases. Biochem Soc Trans. 1986 Apr;14(2):496–499. doi: 10.1042/bst0140496. [DOI] [PubMed] [Google Scholar]
- Riou J. F., Gabillot M., Philippe M., Schrevel J., Riou G. Purification and characterization of Plasmodium berghei DNA topoisomerases I and II: drug action, inhibition of decatenation and relaxation, and stimulation of DNA cleavage. Biochemistry. 1986 Apr 8;25(7):1471–1479. doi: 10.1021/bi00355a001. [DOI] [PubMed] [Google Scholar]
- Shapiro T. A., Englund P. T. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc Natl Acad Sci U S A. 1990 Feb;87(3):950–954. doi: 10.1073/pnas.87.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaithong S., Beale G. H., Chutmongkonkul M. Susceptibility of Plasmodium falciparum to five drugs: an in vitro study of isolates mainly from Thailand. Trans R Soc Trop Med Hyg. 1983;77(2):228–231. doi: 10.1016/0035-9203(83)90080-9. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Werbovetz K. A., Lehnert E. K., Macdonald T. L., Pearson R. D. Cytotoxicity of acridine compounds for Leishmania promastigotes in vitro. Antimicrob Agents Chemother. 1992 Feb;36(2):495–497. doi: 10.1128/aac.36.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]


