Abstract
The repair of DNA double-strand breaks (DSB) requires processing of the broken ends to complete the ligation process. Recently, it has been shown that DNA polymerase μ (polμ) and DNA polymerase λ (polλ) are both involved in such processing during non-homologous end joining in vitro. However, no phenotype was observed in animal models defective for either polμ and/or polλ. Such observations could result from a functional redundancy shared by the X family of DNA polymerases. To avoid such redundancy and to clarify the role of polμ in the end joining process, we generated cells over-expressing the wild type as well as an inactive form of polμ (polμD). We observed that cell sensitivity to ionizing radiation (IR) was increased when either polμ or polμD was over-expressed. However, the genetic instability in response to IR increased only in cells expressing polμD. Moreover, analysis of intrachromosomal repair of the I-SceI-induced DNA DSB, did not reveal any effect of either polμ or polμD expression on the efficiency of ligation of both cohesive and partially complementary ends. Finally, the sequences of the repaired ends were specifically affected when polμ or polμD was over-expressed, supporting the hypothesis that polμ could be involved in the repair of a DSB subset when resolution of junctions requires some gap filling.
INTRODUCTION
The DNA polymerase μ (polμ) (1) belongs to the X family of DNA polymerases comprising the long time identified and extensively studied DNA polymerase β and the recently discovered DNA polymerase λ (polλ) (2,3). Polμ and polλ share some homology and both have been described as participating in vitro in the repair of DNA double-strand breaks (DSB) by non-homologous end joining (NHEJ) (4,5). The hallmark of animals or cells defective in one of the components of the NHEJ pathway is an increased sensitivity to ionizing radiation (IR) (6–8). However, cells isolated from animals knocked out for polλ or polμ do not present differences in sensitivity to any DNA damaging agents (9–11). Furthermore, mice defective for both polλ and polμ fail to reveal increased sensitivity to IR (10). These results suggest that an alternative pathway could deal with the DNA breaks normally managed by polλ and polμ, or that these polymerases are not involved in NHEJ in vivo. We showed, however, in a previous work that the expression of an inactive form of polλ sensitized mammalian cells to IR (12). In order to better understand the cellular role of polμ in NHEJ, we generated cells over-expressing either the wild type form of polμ or an inactive form of polμ to determine any interference with NHEJ. We used cells containing an integrated chromosomal substrate allowing the evaluation of the I-SceI-induced DSB repair by NHEJ (13). We observed a sensitization of cells expressing polμD toward IR. The joining efficiency of the different DNA ends was not affected, but the molecular analysis of the repaired junctions from incompletely complementary DNA ends revealed a specific decrease in the proportion of DNA ends undergoing DNA synthesis.
MATERIALS AND METHODS
Cells
The C’10 and A’7H cell lines were cultured in DMEM medium (GIBCO BRL, Eragny, France) as previously described (13). The C’10pI2 and A’7HpI4 clones, the C’10μ2 and A’7Hμ2 clones and the C’10μDB and A’7HμD5 clones were obtained after transfection with the empty pIRESpuro2 vector (Clontech, Heidelberg, Germany) or the pIRESpuro2 vector containing the cDNA coding for the human wild-type DNA polymerase μ (polμ) or the inactive form of DNA polymerase μ (polμD), respectively. Individual clones were obtained after transfection with jetPEI (Qbiogen, Illkirch, France) and puromycin selection (5 μg/ml).
Generation of cells expressing polμ isoforms
Polμ expressing plasmids pIRES-polμ and pIRES-polμD were constructed by PCR amplification from a P17-His vector plasmid carrying the cDNA sequence of the polμ gene (3). The polμ catalytically inactive mutants were constructed from P17-His plasmid, using the Quick Change mutagenesis kit (Stratagene, La Jolla, CA, USA) according to the manufacturer's instructions. Residues D330 and D332 in polμ were replaced by Ala using two primers also containing a new BsmI site used for mutation selection. The resulting constructs were sequenced and error-free vectors were used to transfect cells.
DNA polymerase purification and activity assay
The WT and catalytically mutated forms of polμ on p17 plasmid were sequenced and introduced into BL21(DE3) bacteria before purification. The different polμ proteins were purified by Nickel chelation chromatography according to Novagen's suggestions with the following modifications. Cells were lysed by freezing for 16 h at −70°C and slowly thawing on ice in the presence of 20 mg/ml lysozyme and 1% Triton X100. Proteins were concentrated and stored in buffer containing 50 mM Tris pH 7.5, 100 mM NaCl, 0.1 mM EDTA, 1 mM DTT and 20% Glycerol. Protein purity was estimated as >90% by visual inspection of Coomassie Blue-stained 12% SDS–polyacrylamide gels. Standard primer extension reactions were performed at 37°C for 1 h as previously described (14).
Western blotting
Total cellular protein extracts (50 μg) were separated by 10% SDS-PAGE (PolyAcrylamide Gel Electrophoresis) and transferred to PVDF membrane. Polμ was detected using polyclonal antibody (1/500) (AbCam, Cambridge, UK) followed by incubation with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG, and revealed using the ECL system (Amersham, Freiburg, Germany). Equal loading was determined using monoclonal anti-actin antibody (1/5000) (AC10, Sigma, Lyon, France).
Measurement of NHEJ by FACS after induction of DSBs
Cells (5 × 105) were plated and allowed to attach overnight. Expression of the meganuclease I-SceI in cells was achieved by transient transfection of the expression plasmid pCMV –I-SceI (15) using Jet-PEI (Q-BIOgen, Illkrich, France). Seventy-two hours after transfection, cells were dissociated with PBS/EDTA (50 mM), washed in PBS and fixed in PBS/2% PAF for 20 min at room temperature. Cells were then stained with anti-H2Kd (1/30 mouse isotype, SF1-1.1, Pharmigen), or anti-CD4 (1/30 rat isotype, H129.19, Pharmigen), or anti-CD8 (1/30 rat isotype, 53-6.7, Pharmigen) for 30 min at room temperature in PBS/0.5% BSA. Cells were then incubated with anti-mouse-FITC (1/530 mouse isotype, F-2761, Molecular probe) for 30 min at room temperature. The frequency of H2K events allowed us to estimate the efficiency of I-SceI transfection and activity. Scoring of the NHEJ events affecting CD4 and CD8 was performed by flow cytometry using FACScan (Becton Dickinson, San Diego, CA, USA).
Enrichment of CD4+ -expressing cells by MACS
Cells were dissociated with PBS/EDTA (50 mM) and washed with PBS/0.5% BSA/2 mM EDTA. Cells (1 × 106) were stained with anti-CD4 for 15 min at room temperature, then incubated with beads coated with goat anti-rat IgG (Miltenyi Biotec, Bergisch Gladbach, Germany) in PBS/0.5% BSA/2 mM EDTA for 15 min at room temperature. After washing, the positively stained cells were separated onto miniMACS columns and enriched by ∼30%.
Junction sequence analysis
Genomic DNA was prepared from a population of CD4 enriched cells (Puregene, Gentra Systems, Hameenlinna, Finland), and junctions of deletion events (CD4+) were amplified by PCR with the following primers (CMV1: 5′-TGGCCCGCCTGGCATTATGCCCAG-3′ and CD4int: 5′-GCTGCCCCAGAATCTTCCTCT-3′). PCR products of 800 bp were obtained after 35 cycles of amplification with Taq polymerase (Biolabs, Beverly, MA, USA) (13). The PCR products were cloned into Topo-TA system (Invitrogen, Carlsbad, CA, USA), which allows isolation of individual clones, and sequenced on one strand.
Cytotoxicity studies
Cytotoxicities were determined by clonogenic assay (16). For IR, cells (400 or 800) were plated in 25 cm2 flasks and allowed to attach overnight. Cells were subsequently irradiated in growth medium with a Co-source irradiator (IBL 437C type H, Oris industries SA, Gif sur Yvette, France). For camptothecin (CPT), mitomycin C (MMC) and methyl methanesulfonate (MMS) (Sigma, France) cytotoxicities, cells (400) were plated in 6-well plates and allowed to attach overnight. Cells were treated in growth medium with increasing concentrations of drug for 24 h (for CPT) or 1 h (for MMS and MMC). After 8 days post-treatment incubation, the plates were washed with PBS and colonies were fixed with methanol, stained with crystal violet solution and colonies of more than 50 cells were counted. Survival was expressed as the plating efficiency of treated cells relative to the untreated control cells. The results are the mean ± SD of three independent experiments. Where absent, the error bars are smaller than the symbols.
Karyotype analyses
Cells were irradiated at 2 Gy, and metaphase spreads were prepared as described (17). Chromosomal aberrations were analysed using a Nikon microscope (TE300) (objective × 100). Results are the sum of four separate preparations of metaphases resulting from two independent irradiation experiments.
Statistical analysis
Student's t paired analysis was used to examine differences between two sets of results for the measurement of frequencies of NHEJ events. The genetic instability frequency was assessed for significance by χ2 testing. Trend P-values of less than or equal to 0.05 were considered significant.
RESULTS
Ectopic expression of polμ sensitizes cells to ionizing radiation
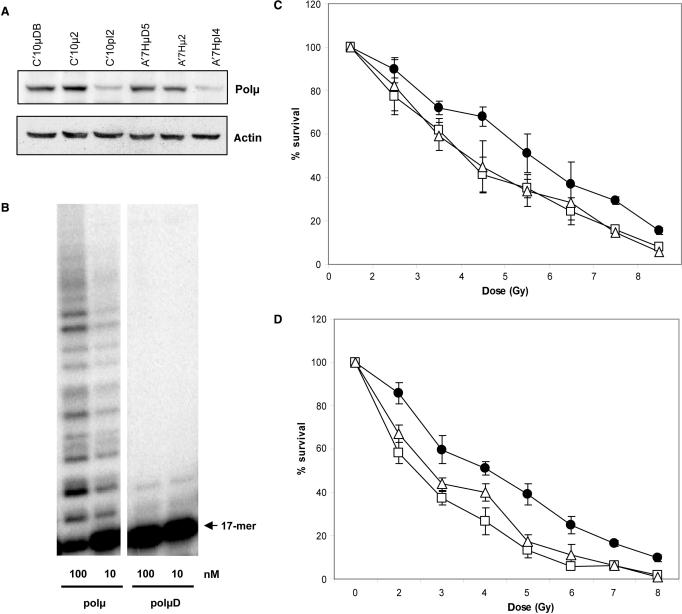
An inactive form of polμ (polμD) was generated by changing the amino acids Asp330 and Asp332 to Ala. Primer extension assay confirmed the absence of DNA polymerase activity of this protein (Figure 1B) (12). We generated stable cell lines over-expressing the different forms of polμ in Chinese Hamster Ovary (CHO) cell lines. We used two CHO cell lines (C’10 and A’7H) that contain an integrated chromosomal substrate designed to allow specific generation of DSB by the rare cutting enzyme I-SceI (13). The different isoforms of polμ were expressed approximately four times more as compared to the corresponding parental cell lines (Figure 1A). Since the antibody we used was not previously characterized, we performed western blotting with purified polμ protein and extracts from cells over-expressing polλ, the homolog of polμ, as positive and negative controls (see Supplementary Data).
Figure 1.
Cellular sensitivity to ionizing radiation. (A) Expression of the different isoforms of polμ in the C’10 and A’7H cell lines, C’10pI2 and A’7HpI4 are parental cells transfected with the empty vector, C’10μ2 and A’7Hμ2 are parental cells transfected with the WT form of polμ, C’10μDB and A’7HμD5 are parental cells transfected with the inactive form of polμ. (B) DNA polymerase assay. Gel activity assay of active and inactive polμ. PolμD represents the inactive protein. The enzyme activity was evaluated with different amounts of purified protein as measured by extension of labelled primer (17-mer) annealed to a 60-mer template. (C) Cell survival after IR in the different C’10 cell lines (D) Cell survival after IR in the different A’7H cell lines. Black dots represent the parental cell line transfected with empty vector, open triangles represent the polμ over-expressing cell lines and the open squares represent the polμD expressing cell lines. Results are the mean ± SD of three independent experiments. When not apparent, the error bars are smaller than the symbols.
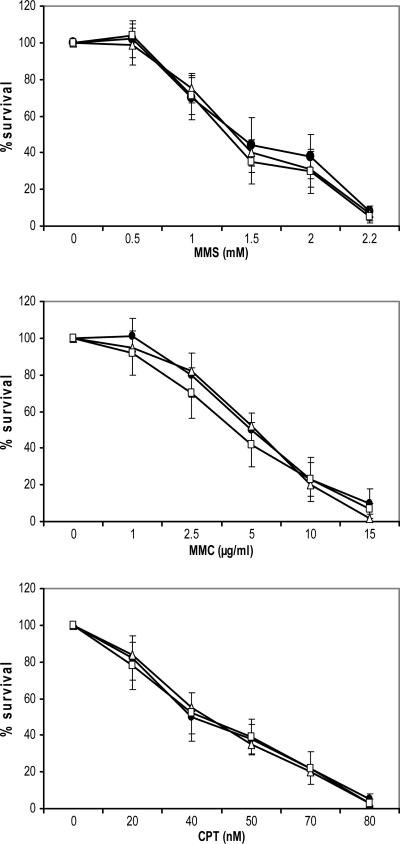
We first examined sensitivity to IR because increased sensitivity to IR is a hallmark of defective DSB repair by non-homologous end joining (NHEJ). Cells expressing polμD showed increased sensitivity to IR compared to control cells (Figure 1C and D). Surprisingly, cells over-expressing the WT form were as sensitive to IR as the polμD expressing cells (Figure 1C and D). We also tested cytotoxicity to additional DNA damaging agents such as MMS, MMC and CPT, and we did not observe any differences in cell toxicity between control cells and cells expressing the different forms of polμ (Figure 2).
Figure 2.
Cellular sensitivity to DNA damaging agents. Cell survival after treatment with methyl methane sulfonate (MMS), mitomycin C (MMC) or camptothecin (CPT) in cells expressing the different forms of polμ. Black dots represent the parental cell line transfected with empty vector, open triangles represent the polμ over expressing cell lines and the open squares represent the polμD expressing cell lines. Results are the mean ± SD of three independent experiments.
The expression of polμD produces genetic instability
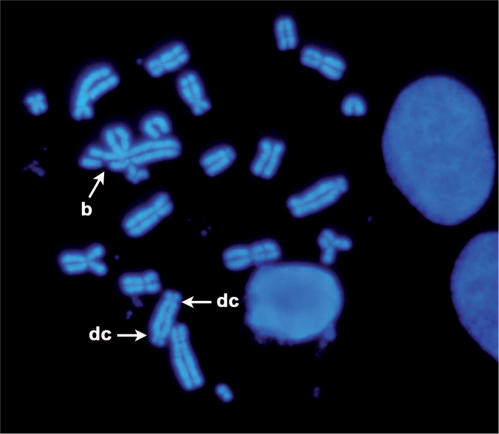
Since NHEJ deficiency leads to chromosomal aberrations, we examined genetic instability by performing karyotype analyses on metaphases from cells exposed to IR. Scoring of chromosomal aberrations included chromosome breaks, triradial, dicentric and end-to-end fusion of chromosomes (Figure 3). Chromosomal analysis showed that the expression of polμD significantly increased (P < 0.05) the genetic instability observed in IR-treated cells as compared to control cells (Table 1). Of note is that over-expression of polμ WT form did not induce a statistically significant increase of genetic instability as compared to control cells (P > 0.05).
Figure 3.
Example of chromosome aberrations observed in polμD expressing cells 24 h after IR exposure (2 Gy), b: break; dc: dicentric.
Table 1.
Evaluation of chromosomal instability in response to IR of cells expressing different forms of polμ
| Control | Polμ | PolμD | |
|---|---|---|---|
| Nb aberration/Nb analysed chrom. | 14/1368 | 17/1367 | 35/1664 |
| Frequency (%) | 1.02 | 1.24 | 2.10 |
The expression of polμD does not alter the efficiency of I-SceI-induced NHEJ
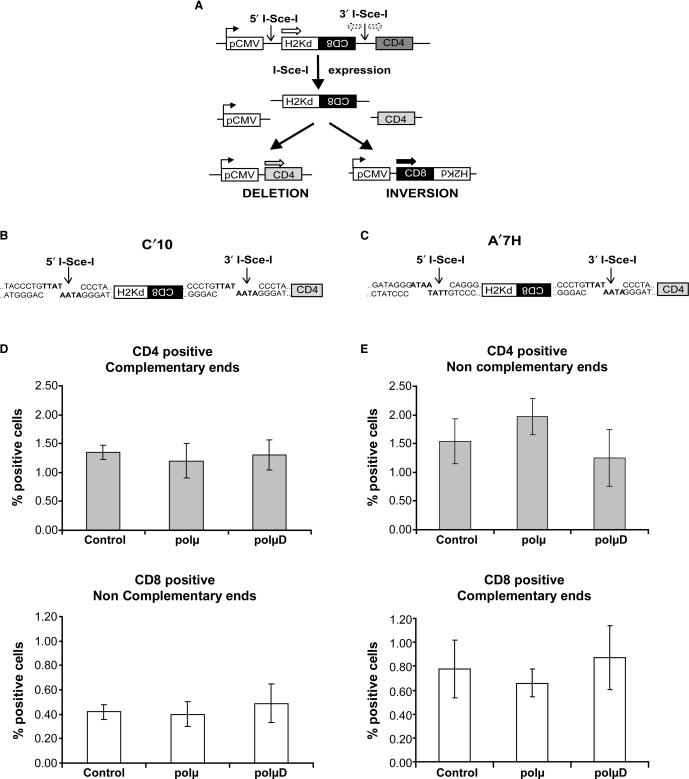
We use two different cell lines to analyse the involvement of polμ on different types of DSB repaired by NHEJ. Both cell lines possess two integrated I-SceI sites. In the absence of I-SceI expression, only the H2-Kd gene is expressed. After transient transfection with a plasmid coding for I-SceI, cleavage at the I-SceI sites leads to excision of the internal fragment H2-Kd/CD8. Subsequent joining of the DNA ends then leads to two different measurable events. Either the pCMV promoter is ligated to the CD4 fragment, leading to deletion of the excised fragment and expression of the CD4 gene (left portion of Figure 4A), or the pCMV promoter is ligated to the excised fragment after inversion, leading to expression of the CD8 gene (right portion of Figure 4A). In the C’10 cell line the two I-SceI sites are in direct orientation, creating complementary ends for deletion events that generate CD4 gene expression, but incompletely complementary ends for inversion events (CD8) (Figure 4B). No significant differences were observed between cells expressing the different isoforms of polμ regarding the frequency of both CD4 (P = 0.2 and P = 0.35 for polμ and polμD expressing cells compared with parental cells, respectively) and CD8 events (P = 0.3 and P = 0.17 for polμ and polμD expressing cells compared with parental cells, respectively) (Figure 4D).
Figure 4.
Intrachromosomal NHEJ in cells expressing the WT and the inactive forms of polμ. (A) Substrate used to measure NHEJ. The only expressed gene is H2-Kd, which is under the control of the pCMV promoter. CD8 is not expressed because of its in inverted orientation. CD4 is not expressed because it is too far from the pCMV promoter. Two I-SceI sites are present in non-coding sequences. After cleavage by I-SceI, the internal H2-Kd/CD8 fragment is excised. Re-joining of the DNA ends can lead to two different measurable events: deletion that leads to expression of the CD4 gene, or inversion that leads to expression of the CD8 gene. (B and C) Representation of the sequences of the I-SceI restriction sites in the C’10 and A’7H cell lines, respectively. In the C’10 cells the two I-SceI sites are in direct orientation, whereas in the A’7H cells the two I-SceI sites are in inverted orientation. (D and E) Evaluation of the frequencies of the deletion (CD4) and inversion (CD8) events in the different cell lines. Results are the mean ± SD of three independent experiments.
In the second cell line (A’7H) the two I-SceI sites are in inverted orientation, creating incompletely complementary ends for deletion events (CD4), but complementary ends for inversion events (CD8) (Figure 4C). As observed for the C’10 cell line, no significant differences were observed with the A’7H cell line regarding the frequency of both CD4 (P = 0.45 and P = 0.1 for polμ and polμD cells compared with parental cells, respectively) and CD8 events (P = 0.16 and P = 0.34 for polμ and polμD cells compared with parental cells, respectively) (Figure 4E).
Evaluation of the quality of the repair of complementary and partially complementary DNA ends
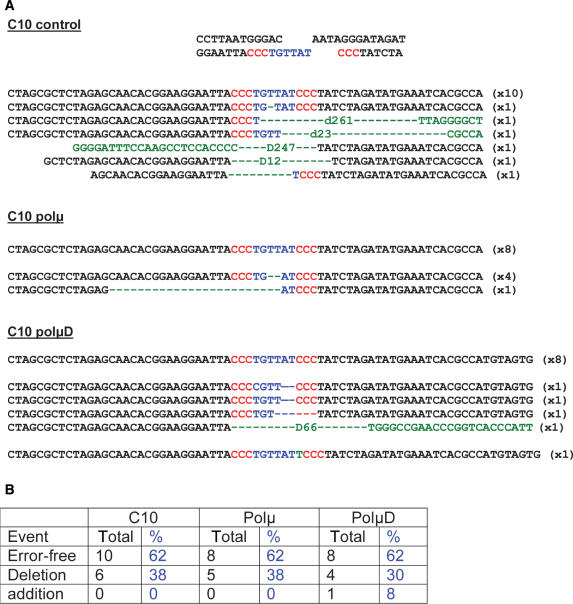
Although no significant differences in the generation of CD4 and CD8 events were observed between cell lines expressing the different isoforms of polμ, we decided to examine the quality of the repaired end junctions. We sequenced the I-SceI break site from CD4 positive cells generated from complementary (C’10 cell line) or partially complementary (A’7H cell line) DNA ends. The analysis of the junction sequences obtained from C’10 cell lines enriched for CD4 gene expression, focused on the repair of complementary ends for which no polymerization event is normally required (Figure 5A). The sequences obtained showed no significant differences in junction fidelity. In all cell lines, we observed the same proportion (62%) of error-free repair (Figure 5B). In three cell lines, a lower proportion (38%) of sequences showed erroneous repair. In most cases, deletions were observed. However, the largest deletions (more than 200 bp) were detected only in the control cell line (Figure 5B). We observed the addition of one nucleotide at the break site only in one sequence from the polμD expressing cells. In summary, we consider that the repair junctions are similar in the cell lines over-expressing either the WT polμ or the polμD isoform, revealing that polμ has no impact on the repair of fully complementary ends.
Figure 5.
Sequencing of the repair junctions. (A) Sequences of the repair junctions obtained in different C’10 cell lines presenting complementary ends. (B) Percentage of the different types of end junctions in the control, polμ and polμD expressing cells.
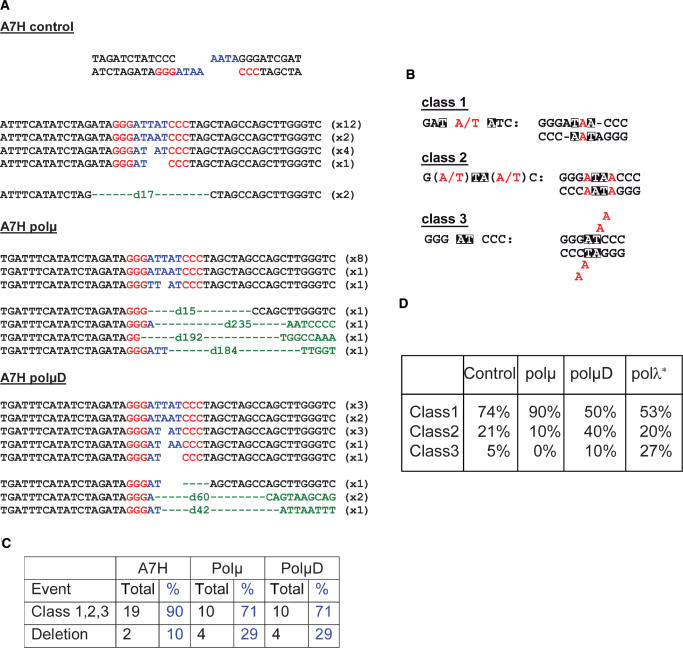
We then sequenced the repair junctions from CD4 enriched A’7H cells for which the junction involved partially complementary ends (Figure 6A). Sequence analysis revealed an increased percentage of deletions (10–29%) in cells over-expressing the WT or the polμD as compared to the control cell line (Figure 6C). These results showed that in contrast to the C’10 cell line, both forms of polμ induced a decrease in the use of the four protruding nucleotides. Precise examination of the processing of the four protruding nucleotides can be done and three classes of events delineated, depending on the partial pairing of the four protruding nucleotides, leading to gaps (class 1), mismatches (class 2) and flap structures (class 3) (Figure 6B). We observed that the over-expression of the WT polμ form increased the percentage of class 1 events (from 74 to 90%), whereas such events were reduced in the polμD cells (from 74 to 50%) as compared to the control cell line (Figure 6D). Concerning the class 2 events, we observed the opposite result: cells expressing the WT polμ displayed a decrease (from 21 to 10%) of such events, which were conversely increased (from 21 to 40%) in polμD cells. Analysis and interpretation of the class 3 events were not possible due to the low number of sequences obtained. This analysis showed symetrical opposite effects of the expression of the two different isoforms of polμ, the WT form of polμ facilitating generation of class 1 events that are the events requiring gap filling by DNA synthesis. On the other hand, the expression of polμD preferentially drove processing of the protruding nucleotides to the class 2 events that do not require DNA synthesis, but rather mismatch pairing.
Figure 6.
Sequencing of the repair junctions. (A) Sequences of the repair junctions obtained in different A’7H cell lines presenting partially complementary ends. (B) Models for the different classes of end joining events involving the four protruding nucleotides of the I-SceI site cleavage, using micro-homologies. Black squares, location of micro-homologies. (C) Percentage of different types of end junctions in control, polμ and polμD expressing cells. (D) Specific analysis of the events involving only the four protruding nucleotides. *Adapted from Capp et al. (12).
DISCUSSION
The use of an inactive form of polμ allowed us to investigate the involvement of polμ in the processing of DNA DSB. As previously observed for polλ (12), expression of an inactive form of polμ sensitized cells to death by IR. We surmize that the absence of phenotype to IR treatment of polλ−/−, polμ−/− and polλ−/− /polμ−/− cells (9–11), could be due to redundancy of other DNA polymerases, or to the adaptation and compensatory effect of another DNA repair pathway that handles DNA damage. Cell death by IR was similar in cells expressing the WT form of polμ and cells expressing the polμD (Figure 1C and D). This could result from an inhibitory effect of polμ, preventing the repair of DNA damage by other DNA polymerases specialized in DSB repair, such as polλ, or by an alternative DNA repair pathway. Indeed, if the presence of a BRCT domain in polμ is responsible for the interaction with the NHEJ factors (4,5), a dominant negative effect could be caused by the over-expression of the BRCT domain of wild-type polμ that could titrate some of the NHEJ factors. The absence of an effect on cytotoxicity to other DNA damaging agents (MMS, MMC and CPT), favours the hypothesis of polμ being a specialized DNA polymerase for processing IR-induced damage. Interestingly, Mahajan et al. observed an induction of polμ expression after exposure to IR, but not after exposure to UV or MMC (18). Although the cells over-expressing either form of polμ showed the same profile of IR sensitivity, only cells expressing the inactive form of polμ presented a significant higher chromosomal instability in response to IR (Table 1). Such a discrepancy between the two cell lines suggests that over-expression of the WT polμ form did not produce the same clastogenic events as polμD expression. However, the two types of events produced were equally deleterious in the two cell lines.
We subsequently analysed the frequency and quality of some NHEJ events following specific induction of DSB by the I-SceI rare cutting enzyme. The scoring of inversion and deletion events after induction of DSB did not show significant differences in cells expressing the WT or the inactive form of polμ. No differences were observed in the frequency of NHEJ events, whether the DNA ends generated in the two different cell lines were complementary or partially complementary. The results obtained with the polμD form are in accordance with the previous results obtained when comparing polλ, polβ and polμ in the same system (12). Altogether, these results show that the joining efficiency of the different DNA ends is not affected, providing evidence that the over-expression of the different forms of polμ is not acting as a dominant negative of the overall NHEJ process due to titration of NHEJ factors. In contrast, we previously observed that the expression of a polλ inactive form (12) decreased the efficiency of partially complementary end processing. This inhibitory effect could result from a higher affinity of polλ for this type of DNA ends as compared with polμ. In this case the inactive polλ could be stuck on the DNA ends, preventing its processing by another DNA polymerase such as polμ. On the contrary, we propose that the polμD has a lower affinity for this type of DNA ends as compared with polλ and could then be displaced by the endogenous polλ. The sequencing of the repair junctions confirms that in the presence of complementary ends, the expression of both WT and inactive forms of polμ has no effect on the processing of the DNA ends (Figure 5). This suggests that no DNA polymerase is recruited at the break site. In contrast, when the DNA ends are partially complementary, the over-expression of both forms of polμ increases the percentage of deletions (from 10 to 29%) (Figure 6C). Therefore, both forms of polμ decrease the efficiency of processing of the four protruding nucleotides, leading to an increase in deletion frequency that could explain the higher sensitivity to IR of both cell lines.
A close examination of the events resulting from processing of the four protruding nucleotides at the break site, revealed significant changes in the proportion of the three classes of events accounting for such processes. Events involving gap filling (class 1) were increased in cells over-expressing the polμ WT form, whereas they were decreased in cells expressing polμD. One hypothesis is that excess of WT polμ protects the four protruding nucleotides and improves gap filling, because in this case the DNA polymerization step is no longer the limitating step. On the other hand, presence of the polμD inhibits only partially the process of gap filling, because the endogenous polλ could still perform some gap filling due to a stronger affinity for the DNA ends as compared with polμ. As demonstrated by the structural data obtained by Moon et al. (19), the 8-kDa domain of polμ binds the 5′-phosphate of the downstream strand in gapped DNA. This interaction is certainly lower than that of polλ due to fewer interactions (19). Thus, cells could compensate the effects of polμD expression by increasing the proportion of class 2 events, which do not require gap filling and DNA synthesis. In the case of over-expression of the WT polμ form the proportion of class 2 events is decreased, perhaps because there is more gap filling due to the presence of more available DNA polymerase. Comparison with previous results obtained from over-expression of the WT polλ, show a different processing of the four protruding nucleotides of the DNA break. Over-expression of polλ decreases the proportion of class 1 events, has no effect on class 2 events, but increases class 3 events (Figure 6) (12). This could result from the ability of polλ to produce deletions at high rate when filling short gaps and to generate -2 base frameshifts (20). Altogether, our results show that polμ participates in the cellular response to IR, but with an impact different from that of polλ as regards the processing of DNA breaks. In our system, the lesser impact of the inactive polμ form as compared to the polλ inactive form, could result from a lower affinity of polμ to the template (19). While one of the defining catalytic properties of polμ is the repair of substrates lacking a template strand, our system does not allow the examination of these kinds of events. The study of substrate specificity for each DNA polymerase on DSB should elucidate whether both polλ and polμ cooperate to repair DSB, or if they are mutually exclusive.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
We thank Dr Said Aoufouchi for providing the DNA polymerase μ cDNA. J.-P.C. is a fellowship recipient from the French MENRT. We thank Dr Caroline Monod for her critical reading of the manuscript. This work was supported by ACI Cancéropôle 2004–2007 (grant to CC), Ligue Nationale contre le Cancer ‘équipe labellisée’ (grant to JSH). Funding to pay the Open Access publication charges for this article was provided by ACI Cancéropôle 2004–2007.
Conflict of interest statement. None declared.
REFERENCES
- 1.Dominguez O, Ruiz JF, Lain de Lera T, Garcia-Diaz M, Gonzalez MA, Kirchhoff T, Martinez AC, Bernad A, Blanco L. DNA polymerase mu (Pol mu), homologous to TdT, could act as a DNA mutator in eukaryotic cells. EMBO J. 2000;19:1731–1742. doi: 10.1093/emboj/19.7.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Diaz M, Dominguez O, Lopez-Fernandez LA, de Lera LT, Saniger ML, Ruiz JF, Parraga M, Garcia-Ortiz MJ, Kirchhoff T, et al. DNA polymerase lambda (Pol lambda), a novel eukaryotic DNA polymerase with a potential role in meiosis. J. Mol. Biol. 2000;301:851–867. doi: 10.1006/jmbi.2000.4005. [DOI] [PubMed] [Google Scholar]
- 3.Aoufouchi S, Flatter E, Dahan A, Faili A, Bertocci B, Storck S, Delbos F, Cocea L, Gupta N, et al. Two novel human and mouse DNA polymerases of the polX family. Nucleic Acids Res. 2000;28:3684–3693. doi: 10.1093/nar/28.18.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Lu H, Tippin B, Goodman MF, Shimazaki N, Koiwai O, Hsieh CL, Schwarz K, Lieber MR. A biochemically defined system for mammalian nonhomologous DNA end joining. Mol. Cell. 2004;16:701–713. doi: 10.1016/j.molcel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Nick McElhinny SA, Havener JM, Garcia-Diaz M, Juarez R, Bebenek K, Kee BL, Blanco L, Kunkel TA, Ramsden DA. A gradient of template dependence defines distinct biological roles for family X polymerases in nonhomologous end joining. Mol. Cell. 2005;19:357–366. doi: 10.1016/j.molcel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Otevrel T, Gao Y, Cheng HL, Seed B, Stamato TD, Taccioli GE, Alt FW. The XRCC4 gene encodes a novel protein involved in DNA double-strand break repair and V(D)J recombination. Cell. 1995;83:1079–1089. doi: 10.1016/0092-8674(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 7.Riballo E, Critchlow SE, Teo SH, Doherty AJ, Priestley A, Broughton B, Kysela B, Beamish H, Plowman N, et al. Identification of a defect in DNA ligase IV in a radiosensitive leukaemia patient. Curr. Biol. 1999;9:699–702. doi: 10.1016/s0960-9822(99)80311-x. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Jin S, Gao Y, Weaver DT, Alt FW. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc. Natl Acad. Sci. USA. 1997;94:8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi Y, Watanabe M, Okada Y, Sawa H, Takai H, Nakanishi M, Kawase Y, Suzuki H, Nagashima K, et al. Hydrocephalus, situs inversus, chronic sinusitis, and male infertility in DNA polymerase lambda-deficient mice: possible implication for the pathogenesis of immotile cilia syndrome. Mol. Cell. Biol. 2002;22:2769–2776. doi: 10.1128/MCB.22.8.2769-2776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertocci B, De Smet A, Weill JC, Reynaud CA. Nonoverlapping functions of DNA polymerases mu, lambda, and terminal deoxynucleotidyltransferase during immunoglobulin V(D)J recombination in vivo. Immunity. 2006;25:31–41. doi: 10.1016/j.immuni.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Bertocci B, De Smet A, Berek C, Weill JC, Reynaud CA. Immunoglobulin kappa light chain gene rearrangement is impaired in mice deficient for DNA polymerase mu. Immunity. 2003;19:203–211. doi: 10.1016/s1074-7613(03)00203-6. [DOI] [PubMed] [Google Scholar]
- 12.Capp JP, Boudsocq F, Bertrand P, Laroche-Clary A, Pourquier P, Lopez BS, Cazaux C, Hoffmann JS, Canitrot Y. The DNA polymerase lambda is required for the repair of non-compatible DNA double strand breaks by NHEJ in mammalian cells. Nucleic Acids Res. 2006;34:2998–3007. doi: 10.1093/nar/gkl380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guirouilh-Barbat J, Huck S, Bertrand P, Pirzio L, Desmaze C, Sabatier L, Lopez BS. Impact of the KU80 pathway on NHEJ-induced genome rearrangements in mammalian cells. Mol. Cell. 2004;14:611–623. doi: 10.1016/j.molcel.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Boudsocq F, Iwai S, Hanaoka F, Woodgate R. Sulfolobus solfataricus P2 DNA polymerase IV (Dpo4): an archaeal DinB-like DNA polymerase with lesion-bypass properties akin to eukaryotic poleta. Nucleic Acids Res. 2001;29:4607–4616. doi: 10.1093/nar/29.22.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang F, Han M, Romanienko P, Jasin M. Homology-directed repair is a major double-strand-break repair pathway in mammalian cells. Proc. Natl Acad. Sci. USA. 1998;95:5172–5177. doi: 10.1073/pnas.95.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frechet M, Canitrot Y, Bieth A, Dogliotti E, Cazaux C, Hoffmann JS. Deregulated DNA polymerase beta strengthens ionizing radiation-induced nucleotidic and chromosomal instabilities. Oncogene. 2002;21:2320–2327. doi: 10.1038/sj.onc.1205295. [DOI] [PubMed] [Google Scholar]
- 17.Bergoglio V, Pillaire MJ, Lacroix-Triki M, Raynaud-Messina B, Canitrot Y, Bieth A, Gares M, Wright M, Delsol G, et al. Deregulated DNA polymerase beta induces chromosome instability and tumorigenesis. Cancer Res. 2002;62:3511–3514. [PubMed] [Google Scholar]
- 18.Mahajan KN, Nick McElhinny SA, Mitchell BS, Ramsden DA. Association of DNA polymerase mu (pol mu) with Ku and ligase IV: role for pol mu in end-joining double-strand break repair. Mol. Cell Biol. 2002;22:5194–5202. doi: 10.1128/MCB.22.14.5194-5202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon AF, Garcia-Diaz M, Bebenek K, Davis BJ, Zhong X, Ramsden DA, Kunkel TA, Pedersen LC. Structural insight into the substrate specificity of DNA polymerase μ. Nat. Struct. Mol. Biol. 2007;14:45–53. doi: 10.1038/nsmb1180. [DOI] [PubMed] [Google Scholar]
- 20.Bebenek K, Garcia-Diaz M, Blanco L, Kunkel TA. The frameshift infidelity of human DNA polymerase lambda. Implications for function. J. Biol. Chem. 2003;278:34685–34690. doi: 10.1074/jbc.M305705200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.