Abstract
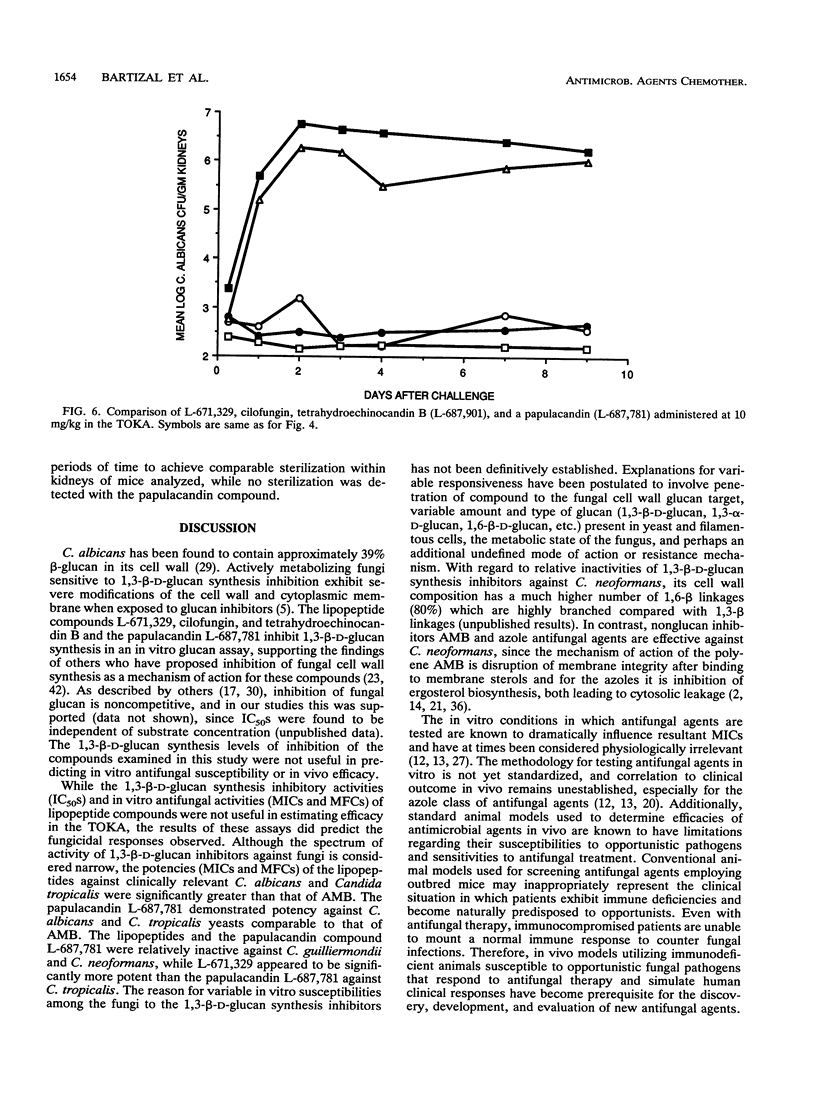
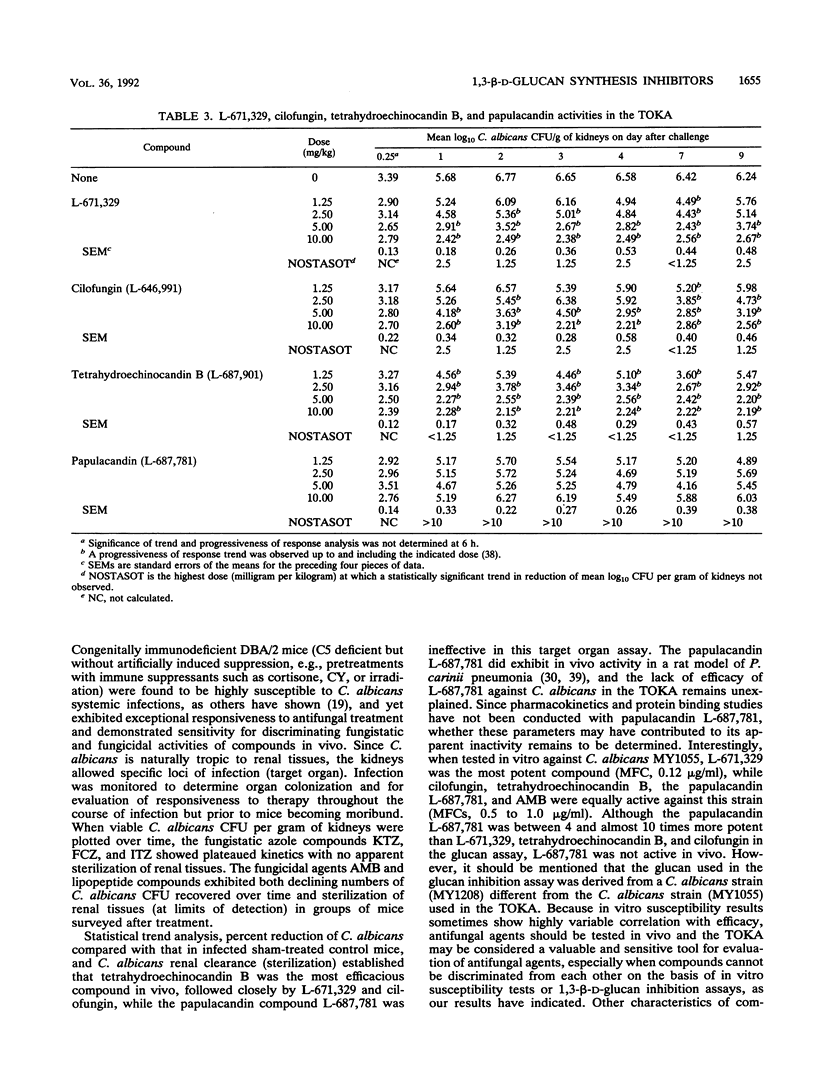
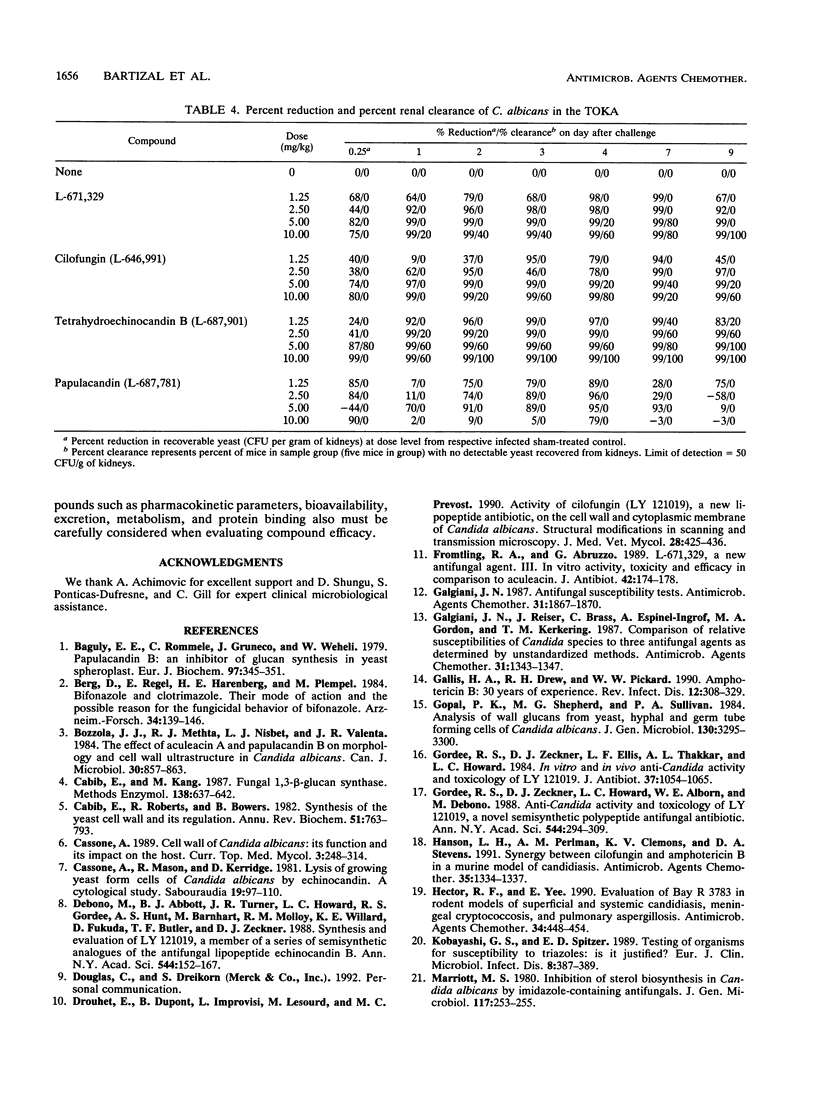
The in vivo anti-Candida activities of 1,3-beta-D-glucan synthesis inhibitors L-671,329, L-646,991 (cilofungin), L-687,901 (tetrahydroechinocandin B), and L-687,781 (a papulacandin analog) were evaluated by utilizing a murine model of disseminated candidiasis that has enhanced susceptibility to Candida albicans but increased sensitivity for discriminating antifungal efficacy. DBA/2 mice were challenged intravenously with 1 x 10(4) to 5 x 10(4) CFU of C. albicans MY1055 per mouse. Compounds were administered intraperitoneally at concentrations ranging from 1.25 to 10 mg/kg of body weight twice daily for 4 days. At 6 h and 1, 2, 3, 4, 7, and 9 days after challenge, five mice per group were sacrificed and their kidneys were homogenized and plated for enumeration of Candida organisms (CFU per gram). Progressiveness of response trends and no-statistical-significance-of-trend doses were derived to rank compound efficacy. 1,3-beta-D-Glucan synthesis 50% inhibitory concentrations were determined by using a C. albicans (MY1208) membrane glucan assay. Candida and Cryptococcus neoformans MICs and minimal fungicidal concentrations were determined by broth microdilution. L-671,329, L-646,991, L-687,901, and L-687,781 showed similar 1,3-beta-D-glucan activities, with 50% inhibitory concentrations of 0.64, 1.30, 0.85, and 0.16 micrograms/ml, respectively. Data from in vitro antifungal susceptibility studies showed that L-671,329, L-646,991, and L-687,901 had similar MICs ranging from 0.5 to 1.0 micrograms/ml, while L-687,781 showed slightly higher MICs of 1.0 to 2.0 micrograms/ml for C. albicans MY1055. Lipopeptide compounds were ineffective against C. neoformans strains.Results from in vivo experiments comparing significant trend and progressiveness in response analyses indicated that L-671,329 and L-646,991 were equipotent but slightly less active than L-687-901, while L-687,781 was ineffective at 10 mg/kg. Fungicidal activities of L-671,329, L-646,991, and L-687,901 were observed in vivo, with significant reduction in Candida CFU per gram of kidneys compared with those in sham-treated mice at doses of > or = 2.5 mg/kg evident as early as 1 day after challenge.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Römmele G., Gruner J., Wehrli W. Papulacandin B: an inhibitor of glucan synthesis in yeast spheroplasts. Eur J Biochem. 1979 Jul;97(2):345–351. doi: 10.1111/j.1432-1033.1979.tb13120.x. [DOI] [PubMed] [Google Scholar]
- Berg D., Regel E., Harenberg H. E., Plempel M. Bifonazole and clotrimazole. Their mode of action and the possible reason for the fungicidal behaviour of bifonazole. Arzneimittelforschung. 1984;34(2):139–146. [PubMed] [Google Scholar]
- Bozzola J. J., Mehta R. J., Nisbet L. J., Valenta J. R. The effect of aculeacin A and papulacandin B on morphology and cell wall ultrastructure in Candida albicans. Can J Microbiol. 1984 Jun;30(6):857–863. doi: 10.1139/m84-133. [DOI] [PubMed] [Google Scholar]
- Cabib E., Kang M. S. Fungal 1,3-beta-glucan synthase. Methods Enzymol. 1987;138:637–642. doi: 10.1016/0076-6879(87)38057-7. [DOI] [PubMed] [Google Scholar]
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- Cassone A., Mason R. E., Kerridge D. Lysis of growing yeast-form cells of Candida albicans by echinocandin: a cytological study. Sabouraudia. 1981 Jun;19(2):97–110. [PubMed] [Google Scholar]
- Debono M., Abbott B. J., Turner J. R., Howard L. C., Gordee R. S., Hunt A. S., Barnhart M., Molloy R. M., Willard K. E., Fukuda D. Synthesis and evaluation of LY121019, a member of a series of semisynthetic analogues of the antifungal lipopeptide echinocandin B. Ann N Y Acad Sci. 1988;544:152–167. doi: 10.1111/j.1749-6632.1988.tb40398.x. [DOI] [PubMed] [Google Scholar]
- Drouhet E., Dupont B., Improvisi L., Lesourd M., Prevost M. C. Activity of cilofungin (LY 121019), a new lipopeptide antibiotic, on the cell wall and cytoplasmic membrane of Candida albicans. Structural modifications in scanning and transmission electron microscopy. J Med Vet Mycol. 1990;28(6):425–436. doi: 10.1080/02681219080000541. [DOI] [PubMed] [Google Scholar]
- Fromtling R. A., Abruzzo G. K. L-671,329, a new antifungal agent. III. In vitro activity, toxicity and efficacy in comparison to aculeacin. J Antibiot (Tokyo) 1989 Feb;42(2):174–178. doi: 10.7164/antibiotics.42.174. [DOI] [PubMed] [Google Scholar]
- Galgiani J. N. Antifungal susceptibility tests. Antimicrob Agents Chemother. 1987 Dec;31(12):1867–1870. doi: 10.1128/aac.31.12.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N., Reiser J., Brass C., Espinel-Ingroff A., Gordon M. A., Kerkering T. M. Comparison of relative susceptibilities of Candida species to three antifungal agents as determined by unstandardized methods. Antimicrob Agents Chemother. 1987 Sep;31(9):1343–1347. doi: 10.1128/aac.31.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis H. A., Drew R. H., Pickard W. W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990 Mar-Apr;12(2):308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- Gopal P. K., Shepherd M. G., Sullivan P. A. Analysis of wall glucans from yeast, hyphal and germ-tube forming cells of Candida albicans. J Gen Microbiol. 1984 Dec;130(12):3295–3301. doi: 10.1099/00221287-130-12-3295. [DOI] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Ellis L. F., Thakkar A. L., Howard L. C. In vitro and in vivo anti-Candida activity and toxicology of LY121019. J Antibiot (Tokyo) 1984 Sep;37(9):1054–1065. doi: 10.7164/antibiotics.37.1054. [DOI] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Howard L. C., Alborn W. E., Jr, Debono M. Anti-Candida activity and toxicology of LY121019, a novel semisynthetic polypeptide antifungal antibiotic. Ann N Y Acad Sci. 1988;544:294–309. doi: 10.1111/j.1749-6632.1988.tb40415.x. [DOI] [PubMed] [Google Scholar]
- Hanson L. H., Perlman A. M., Clemons K. V., Stevens D. A. Synergy between cilofungin and amphotericin B in a murine model of candidiasis. Antimicrob Agents Chemother. 1991 Jul;35(7):1334–1337. doi: 10.1128/aac.35.7.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector R. F., Yee E. Evaluation of Bay R 3783 in rodent models of superficial and systemic candidiasis, meningeal cryptococcosis, and pulmonary aspergillosis. Antimicrob Agents Chemother. 1990 Mar;34(3):448–454. doi: 10.1128/aac.34.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi G. S., Spitzer E. D. Testing of organisms for susceptibility to triazoles: is it justified? Eur J Clin Microbiol Infect Dis. 1989 May;8(5):387–389. doi: 10.1007/BF01964051. [DOI] [PubMed] [Google Scholar]
- Marriott M. S. Inhibition of sterol biosynthesis in Candida albicans by imidazole-containing antifungals. J Gen Microbiol. 1980 Mar;117(1):253–255. doi: 10.1099/00221287-117-1-253. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuda S., Tegoshi T. Yeast glucan in the cyst wall of Pneumocystis carinii. J Protozool. 1989 Jan-Feb;36(1):21S–22S. doi: 10.1111/j.1550-7408.1989.tb02674.x. [DOI] [PubMed] [Google Scholar]
- Miyata M., Kitamura J., Miyata H. Lysis of growing fissin-yeast cells induced by aculeacin A, a new antifungal antibiotic. Arch Microbiol. 1980 Aug;127(1):11–16. doi: 10.1007/BF00414349. [DOI] [PubMed] [Google Scholar]
- Murgui A., Elorza M. V., Sentandreu R. Tunicamycin and papulacandin B inhibit incorporation of specific mannoproteins into the wall of Candida albicans regenerating protoplasts. Biochim Biophys Acta. 1986 Dec 10;884(3):550–558. doi: 10.1016/0304-4165(86)90207-2. [DOI] [PubMed] [Google Scholar]
- Orlean P. A. (1,3)-beta-D-Glucan synthase from budding and filamentous cultures of the dimorphic fungus Candida albicans. Eur J Biochem. 1982 Oct;127(2):397–403. doi: 10.1111/j.1432-1033.1982.tb06885.x. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Gerarden T., Yu M., Wenzel R. P. Influence of in vitro susceptibility testing conditions on the anti-candidal activity of LY121019. Diagn Microbiol Infect Dis. 1988 Sep;11(1):1–9. doi: 10.1016/0732-8893(88)90067-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Sentandreu R. Fungal cell wall synthesis and assembly. Curr Top Med Mycol. 1989;3:168–217. doi: 10.1007/978-1-4612-3624-5_8. [DOI] [PubMed] [Google Scholar]
- Sawistowska-Schröder E. T., Kerridge D., Perry H. Echinocandin inhibition of 1,3-beta-D-glucan synthase from Candida albicans. FEBS Lett. 1984 Jul 23;173(1):134–138. doi: 10.1016/0014-5793(84)81032-7. [DOI] [PubMed] [Google Scholar]
- Schmatz D. M., Romancheck M. A., Pittarelli L. A., Schwartz R. E., Fromtling R. A., Nollstadt K. H., Vanmiddlesworth F. L., Wilson K. E., Turner M. J. Treatment of Pneumocystis carinii pneumonia with 1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5950–5954. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. E., Giacobbe R. A., Bland J. A., Monaghan R. L. L-671,329, a new antifungal agent. I. Fermentation and isolation. J Antibiot (Tokyo) 1989 Feb;42(2):163–167. doi: 10.7164/antibiotics.42.163. [DOI] [PubMed] [Google Scholar]
- Shematek E. M., Braatz J. A., Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of beta-(1 leads to 3)glucan synthetase. J Biol Chem. 1980 Feb 10;255(3):888–894. [PubMed] [Google Scholar]
- Shematek E. M., Cabib E. Biosynthesis of the yeast cell wall. II. Regulation of beta-(1 leads to 3)glucan synthetase by ATP and GTP. J Biol Chem. 1980 Feb 10;255(3):895–902. [PubMed] [Google Scholar]
- Shepherd M. G. Cell envelope of Candida albicans. Crit Rev Microbiol. 1987;15(1):7–25. doi: 10.3109/10408418709104445. [DOI] [PubMed] [Google Scholar]
- Taft C. S., Stark T., Selitrennikoff C. P. Cilofungin (LY121019) inhibits Candida albicans (1-3)-beta-D-glucan synthase activity. Antimicrob Agents Chemother. 1988 Dec;32(12):1901–1903. doi: 10.1128/aac.32.12.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. H. Suggested mechanisms for the antimycotic activity of the polyene antibiotics and the N-substituted imidazoles. J Antimicrob Chemother. 1986 Mar;17(3):269–279. doi: 10.1093/jac/17.3.269. [DOI] [PubMed] [Google Scholar]
- Traxler P., Tosch W., Zak O. Papulacandins--synthesis and biological activity of papulacandin B derivatives. J Antibiot (Tokyo) 1987 Aug;40(8):1146–1164. doi: 10.7164/antibiotics.40.1146. [DOI] [PubMed] [Google Scholar]
- Tukey J. W., Ciminera J. L., Heyse J. F. Testing the statistical certainty of a response to increasing doses of a drug. Biometrics. 1985 Mar;41(1):295–301. [PubMed] [Google Scholar]
- VanMiddlesworth F., Omstead M. N., Schmatz D., Bartizal K., Fromtling R., Bills G., Nollstadt K., Honeycutt S., Zweerink M., Garrity G. L-687,781, a new member of the papulacandin family of beta-1,3-D-glucan synthesis inhibitors. I. Fermentation, isolation, and biological activity. J Antibiot (Tokyo) 1991 Jan;44(1):45–51. doi: 10.7164/antibiotics.44.45. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., McEntee C., Dixon D. M. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J Clin Microbiol. 1987 May;25(5):931–932. doi: 10.1128/jcm.25.5.931-932.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann C. F., Liesch J. M., Schwartz R. E. L-671,329, a new antifungal agent. II. Structure determination. J Antibiot (Tokyo) 1989 Feb;42(2):168–173. doi: 10.7164/antibiotics.42.168. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Hiratani T., Baba M., Osumi M. Effect of aculeacin A, a wall-active antibiotic, on synthesis of the yeast cell wall. Microbiol Immunol. 1985;29(7):609–623. doi: 10.1111/j.1348-0421.1985.tb00865.x. [DOI] [PubMed] [Google Scholar]



