Abstract
Nerve growth factor (NGF) is able to restore spatial learning and reverse forebrain cholinergic neuron atrophy when administered intracerebrally to behaviorally impaired aged rats. In the present study, behaviorally unimpaired, middle-aged rats (14–16 months old) received transplants of ex vivo transduced, clonal NGF-secreting immortalized neural progenitor cells, bilaterally in the nucleus basalis and septum. During the subsequent 9 months the aged control animals developed the expected impairment in spatial learning in the water maze task, whereas the animals with NGF-secreting grafts maintained a performance level not different from the 12-month-old control rats. The marked age-induced atrophy (−25%) of the cholinergic neurons in medial septum and nucleus basalis, seen in the aged control rats, was not present in the NGF-treated aged animals. 3H-labeled thymidine autoradiography showed that the transduced cells survived well and had become integrated into the host tissue surrounding the injection sites, and reverse transcription–PCR analysis revealed expression of the NGF transgene, at both 4 and 9 months postgrafting, in the grafted tissue. The results show that long-term supply of NGF from ex vivo transduced immortalized neural progenitor cells locally within the nucleus basalis and septum can prevent the subsequent development of age-dependent neuronal atrophy and behavioral impairments when the animals reach advanced age.
Keywords: gene therapy, Alzheimer’s disease, memory, p75NTR
Studies in rodents have shown that the basal forebrain cholinergic system undergoes progressive degenerative changes with advancing age, and that the magnitude of these changes generally is correlated with the severity of the behavioral impairments that the aged animals exhibit in various learning and memory tasks (1–3). Indeed, several lines of evidence indicate that normal cortical and hippocampal function depends on the modulatory afferent control exerted by the two principal basal forebrain cholinergic projection systems originating in the septal–diagonal band area and the nucleus basalis (1–4).
Injections or infusions of exogenous nerve growth factor (NGF) have been successfully used to reverse the age-dependent atrophic changes in the cholinergic forebrain neurons, as well as the performance of the aged animals in spatial memory tasks (5–10). NGF is a neurotrophic factor with potent neuroprotective effects on TrkA/p75NTR receptor bearing cholinergic neurons in the central nervous system (CNS), both in vitro and in vivo (11–13). Although there is little evidence for a specific deficit of NGF in the brains of cognitively impaired animals (14, 15), the results obtained with acute administration of NGF in aged animals suggest that increased supply or availability of NGF to the forebrain cholinergic neurons during aging may be able to stimulate their function and reverse their atrophic state.
The usefulness of intracerebroventricular or systemic administration of NGF may be limited by deleterious side effects such as hyperalgesia, hypophagia, and weight loss (16, 17). For this reason, long-term administration of NGF may require low-level, localized intraparenchymal delivery directly to the area containing the receptive neurons. Work during the last few years has shown that ex vivo gene transfer techniques can provide an interesting alternative for long-term delivery of therapeutically active proteins, which may circumvent the drawbacks associated with chronic intracerebral infusions. In aged rats, intracerebral transplantation of either fibroblasts (18) or immortalized neural progenitor cells (19, 20) engineered to secrete NGF has thus successfully been used to reverse cholinergic neuron atrophy and improve the aged rats’ spatial learning performance in the Morris water maze task. These results show that cellular implants with an estimated secretion rate of 10–100 ng NGF/day, placed in either nucleus basalis or septum, are as efficient as intraventricular infusions of 1–6 μg NGF/day, indicating that local cell-based delivery is a highly efficient route of administration of neurotrophic factors. Polymer capsules containing NGF-secreting fibroblasts have yielded similar results (21), but in this case the capsules were implanted intraventricularly.
So far, all studies on intracerebral NGF delivery during aging have been concerned with amelioration of already established deficits in aged animals, and the maximum duration of intracerebral NGF delivery has been 4 weeks in the studies using injections or infusions (see table in ref. 21), and 10 weeks in the studies using ex vivo gene transfer (20). The present study was designed to investigate to what extent long-term local NGF delivery by transplants of NGF-secreting immortalized neural progenitor cells in nucleus basalis and septum, implanted in middle-aged rats at an early presymptomatic stage, can prevent the development of cholinergic neuron atrophy and behavioral impairments over the subsequent 9 months, when the animals have reached advanced age.
METHODS
Neural Stem Cells for ex Vivo Gene Transfer of NGF.
The production and characterization of the control- or NGF-secreting clonal cell lines used in the present work have been described in detail elsewhere (22); the parental neural stem cell line was the E16 rat hippocampus-derived HiB5 cell line (23), made to express NGF by retroviral transduction, by using a construct without internal promoters or marker/selection genes. Cells were cultured in DMEM (GIBCO) supplemented with 10% fetal bovine serum, 2 mM glutamine, and 10,000 units/ml streptomycin/penicillin. Before grafting, the cells were labeled in culture for 72 h at 10 μCi/ml with 3H-labeled thymidine (Amersham); for grafting, a single cell suspension at 150,000 cells/μl was prepared by trypsinization from near to confluent monolayers.
Animal Groups and Surgery.
Eighty-three middle-aged (14- to 16-month-old) female Sprague–Dawley rats were tested in the Morris water maze for spatial navigation performance during 1 week, as previously described (19, 25), following a four-trials-per-day schedule (pregraft test in Fig. 1A). Forty-seven nonimpaired animals [defined as those showing a swim distance to find the hidden platform within the mean ± 2 standard deviations of the same score in a group of young (3-month-old) animals (n = 10)] were allocated to the following groups: intact (n = 10, no transplantation surgery), control-graft (n = 12, receiving grafts of unmodified HiB5 cells), or NGF-graft (n = 25, receiving a transplant of the NGF-secreting clone of HiB5 named E8 in previous studies; ref. 22). Ten of the behaviorally impaired animals were used for expression studies (see below), and the rest were allocated to a different study.
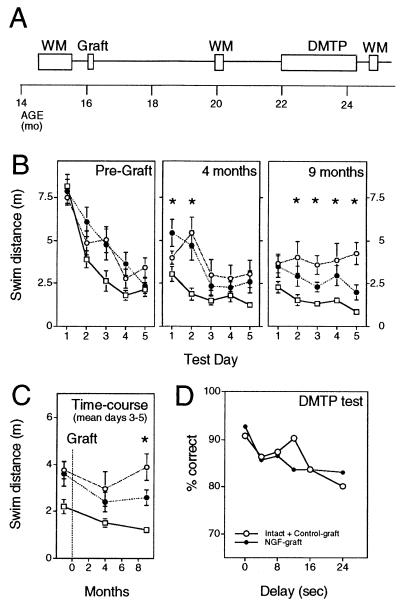
Figure 1.
Experimental design and behavioral testing. (A) Schematic illustration of the experimental schedule, from the pregrafting behavioral test in the Morris water maze (WM) to the last test, performed at 9 months postgrafting. During each WM test, individual animals were trained for 5 days, four trials per day; the boxes represent the actual time required to test all the animals participating in the experiment. (B) Performance in the WM tests (distance swum to find the hidden platform, mean ± SEM), before grafting, and 4 and 9 months postgrafting. Open squares, adult rats; open circles, int+con-graft aged group; closed circles, NGF-grafted aged animals. For the 9-month postgrafting test, a two-way ANOVA reported significant differences among groups (F2,27 = 11.69, P = 0.0003). The asterisks in the diagram denote a significant difference among groups for individual testing days (P < 0.05, one-way ANOVA). For the 9-month test, int+con was different from adult at all days but the first one and different from the NGF- group at days 3 and 5; the NGF group was not different from adult animals at days 1 and 2, and 4 and 5. (C) Temporal changes in task performance (average of data from days 3–5) from mid- to advanced age. The int+con-graft group did not improve performance with consecutive tests, whereas both NGF-graft and adult groups did. A two-way ANOVA reported significant differences both among groups and between tests (F2,27 = 14.96, P = 0.0001, and F2,27 = 3.65, P = 0.033, respectively). One-way ANOVA indicated that all three groups were different from each other at the 9-month test (P < 0.05, posthoc Fisher PLSD). A one-tailed paired t test, comparing pregraft vs. 9-month test values yielded significant differences for NGF-graft and adult groups, but not for the int+con-graft group (int+con-graft, P = 0.4231; NGF-graft, P = 0.0159; adult, P = 0.0099). The intact and control-graft groups did not differ in their performance in any of the tests (P > 0.05 in all cases). For C, the actual values for the 9-month test were: intact = 3.8 ± 1.03 m, control-graft = 3.95 ± 0.76 m. (D) Performance in the DMTP test in the operant test apparatus; experimental groups did not differ either in the performance with no delays (one-way ANOVA at 0 sec delay, F1,16 = 0.09, P = 0.77), nor at increasing delays (two-factor ANOVA: groups F1,16 = 0.0053, P = 0.94; repeated measures, F5,16 = 1.8619, P = 0.1121). Overlapping SEM bars are omitted for clarity.
All animals receiving grafts of neural stem cells were bilaterally implanted at both the medial septum (MS) and the nucleus basalis magnocellularis (NBM), receiving a total of 1.2 million cells (8 deposits × 150,000 cells per deposit). Details of the transplantation procedure can be found elsewhere (19, 20).
Behavioral Testing.
The animals were studied for their performance in a working memory/learning task in the water maze test, as described before (19, 25). Two postgrafting rounds of testing in the water maze were undertaken at 4 and 9 months postsurgery, each consisting of 5 testing days with four trials per day. The hidden platform remained in the same quadrant of the pool for the duration of the experiment, and starting locations were randomized. Spatial probe trials (removed platform) conducted at the end of day 5 in each test were not informative enough in the present experiment because of the low training load (two to three crossings over the 10-cm diameter platform location).
Fourteen of the 47 animals died during testing, and at the time of perfusion noticeable spontaneously occurring pituitary tumors were found in 11 animals; they were, therefore, not considered in the final analysis. The final group composition was eight rats in the combined Intact+ control-graft groups (three intact and five control-cells grafted), and nine in the NGF-graft group. Intact and control animals were combined in a single group because they did not differ in either performance in any of the behavioral tests nor in the histological analysis. Ten adult animals (3 months old) were taken in parallel (12 months old at the end of the experiment).
Testing in the operant test apparatus for the standard delayed-matching-to-position (DMTP) task was performed as described (24); 6 intact, 6 control-graft, and 14 NGF-grafted animals randomly sampled from the whole groups were tested; learning of the task took 10 weeks for the aged animals (at an age of 20–23 months); no difference was observed between the groups either during the learning period or in the level of performance at the end of the test.
Histology.
After completion of the experiment the animals were intracardially perfused with ice-cold buffered paraformaldehyde and their brains were sectioned for histological assessment of number and size of the forebrain cholinergic neurons after immunostaining for the p75NTR neurotrophin receptor by using a monoclonal antibody generated from the 192-IgG hybridoma line (courtesy of E. Johnson, Washington University, St. Louis, MO). Sections were analyzed by using the cast (computer-assisted stereological toolbox)-grid software (Olympus, Denmark) operating an Olympus microscope equipped with a motorized stage. Both MS and NBM (defined as in refs. 19, 20, and 22) were bilaterally examined, and the results of the morphometric analyses are given as total cell counts for both sides combined.
Reverse Transcriptase (RT)-PCR Detection of Transgene Expression.
Long-term in vivo expression of the NGF-coding retroviral vector and significant production of NGF bioactive protein by the grafted cells (assessed both as protein and bioactivity) have previously been demonstrated as long as 10 weeks postgrafting (19, 20, 22). To answer the question of whether or not the retroviral vector in the NGF-secreting neural stem cells could be expressed for the duration of the experiment at the target regions in the grafted brain, an independent group of 10 animals was bilaterally implanted into the NBM, as above, with control or NGF-secreting cells (left or the right hemisphere, respectively; septal grafts were not used because of the easiness of contamination of the control-grafted hemisphere with NGF-cells at this location); one animal died during the 9-month postoperative period, three rats were sacrificed at 4 months, and the remaining six were sacrificed at 9 months postgrafting. Dissected frozen tissue containing the NBM region was processed for RT-PCR of the retroviral mRNA (19, 20, 22); two micrograms of total RNA was reverse-transcribed to cDNA, and one-fourth of the RT products were subjected to amplification by PCR by using a primer set that specifically amplifies the retroviral transcript coding for mNGF in the NGF-stem cells (see ref. 22 for further methodological details and sequences). PCR products were separated by agarose electrophoresis, transferred to nylon membranes, and hybridized with a specific probe, POLYAS36, directed against the cloning site in the retroviral plasmid (22). Standards were generated by mixing known number of cells taken from fresh, dividing cultures with an equal weight of rat brain tissue (from intact animals) equivalent to the dissected pieces from grafted animals and run in parallel with the experimental samples.
RESULTS
NGF Prevents the Development of Cognitive Impairments with Aging.
Middle-aged Sprague–Dawley rats were tested in the Morris water maze to select those showing no signs of impairments (within 2 SD of the young control group). Consistent with previous reports (25), we found 36 animals that were already impaired (43% of the population) and 47 nonimpaired animals (57%). After grafting, the animals were subsequently tested for their performance in the same task 4 and 9 months later (see scheme in Fig. 1A), the last test being performed at the age of 23–25 months.
The control animals not receiving any extra neurotrophic support (int+con group) developed the expected deficits in the water maze task; by the end of the 9-month period, the distance swum to find the hidden platform was 4.26 ± 0.65 m, which was significantly longer when compared with the adult animals (0.84 ± 0.13 m) [P < 0.05, one-way ANOVA, post-hoc Fisher probable least-squares difference (PLSD)] (Fig. 1B). In contrast, the aged animals receiving NGF-producing cell implants maintained their performance at a level not significantly different from adult animals (swim distance = 1.97 ± 0.44 m, P > 0.05) and significantly different from the int+con-graft group (P < 0.05). Combined analysis of the three consecutive tests (average of data from days 3 to 5, Fig. 1C) indicated that both the adult and NGF-graft groups improved their performance as a consequence of increased training, resulting in a reduced swim distance (and escape latency) to find the hidden platform at the end of the experiment. In contrast, the int+con graft rats did not improve their performance in spite of receiving the same training load (one-tailed, paired t test comparing the average performance of days 3–5 from the pregraft test to the 9-month test: adult, P < 0.01; aged int+con-graft, P = 0.4231; aged NGF-graft, P = 0.0159).
Swim speeds did not differ among experimental groups in any of the tests (day 5, int+con vs. NGF; pretest, P = 0.1433; 4-month test, P = 0.6397; 9-month test, P = 0.3278), which indicates that the differences in performance in the water maze task were not due to motor deficits, and that the improvements observed in the NGF-grafted rats are not due to NGF effects on this parameter. Motor coordination and attention were studied in more detail in an operant test apparatus by using the delayed-matching-to-position (DMPT) task, where both groups of aged rats reached more than 90% performance after a training period of 10 weeks (percentage of correct choices int+con-graft = 90.8 ± 5, NGF-graft = 92.7 ± 3.7, one-way ANOVA at 0 sec delay, F1,16 = 0.09, P = 0.77) (Fig. 1D); analysis of nose-poke frequency indicated the same degree of attention to the task in both groups of animals (1.59 ± 0.17 and 1.77 ± 0.15 pokes per sec, int+con-graft and NGF-graft group, respectively). Body weight at the end of the experiment did not differ between groups (int+con graft = 395 ± 19 g; NGF-graft = 386 ± 19 g), and mortality rate was equal in the two groups (see Methods).
Long-Term Survival of the Grafted Immortalized Progenitors and Persistent Transgene Expression.
Nine months postgrafting, after completion of the behavioral tests, 3H-labeled thymidine autoradiography revealed viable grafts in all animals transplanted with either control or NGF cells (Fig. 2). As observed in previous studies, the grafted stem cells had integrated into the host brain within a radius of approximately 1–1.5 mm around the implantation site, both in the NBM region (Fig. 2A) and in the MS (Fig. 2B), thus covering those regions where the NGF-sensitive basal forebrain cholinergic neurons are located. The grafted cells were found cytoarchitecturally well integrated in the host tissue, some of them in close apposition to the neuronal cell bodies (Fig. 2C).
Figure 2.
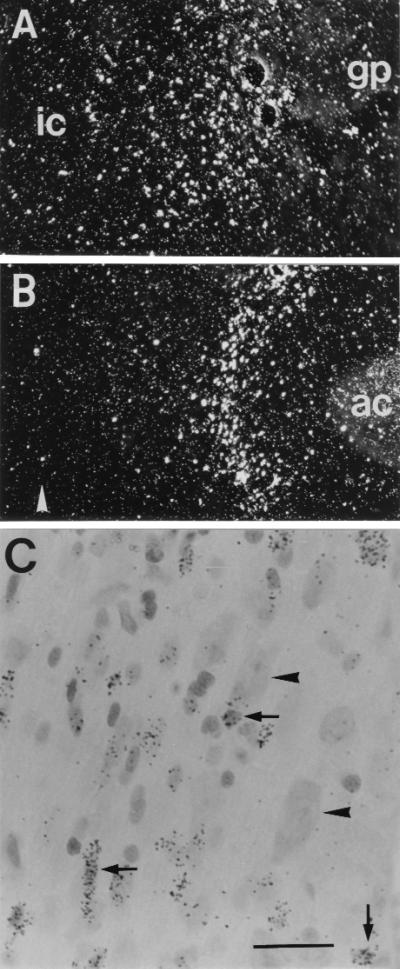
Survival and integration of transplanted neural stem cells. Dark-field images of autoradiograms of [3H]thymidine-labeled NGF-secreting cells grafted into the NBM or MS region (A and B). The cells had migrated out from the original implantation site and become integrated in the surrounding brain parenchyma (ic, internal capsule; gp, globus pallidus; ac, anterior commissure; arrowhead in B denotes the midline). (C) A bright field view of a Nissl-stained section at the MS target location, showing grafted cells (covered with silver grains, arrows) intermingled with host neurons (arrowheads). [Bar = 100 μm (A and B) or 15 μm (C).]
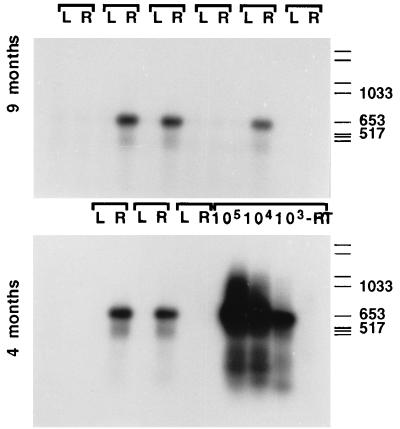
RT-PCR assessment of expression of the retroviral transcript coding for NGF in tissue samples from the grafted NBM region (Fig. 3) revealed expression of the NGF transgene in two of three rats at 4 months and in three of six rats at 9 months postgrafting. This extends our previous observations, where continued in vivo expression of NGF was shown at both the RNA and protein level at 10 weeks after transplantation in the NGF-HiB5 cells (19, 20).
Figure 3.
RT-PCR amplification of the retroviral NGF transcript demonstrates long-term in vivo expression in samples from grafted animals. These animals received control- or NGF-secreting neural stem cells in the left (L) or right (R) hemispheres, respectively, and were sacrificed at 4 or 9 months postgrafting. Amplification of the retroviral transcript was performed on RNA extracted from frozen, dissected tissue blocks. The standards were prepared in parallel, by using known amounts of cells (103 to 105) taken from dividing cultures and mixed with rat brain tissue before RNA isolation. Lane labeled as −RT corresponds to the standard containing the highest amount of NGF cells, subjected to mock reverse transcription (in the absence of reverse transcriptase) and then run in parallel to the other samples for the PCR amplification. Molecular weight standards are Boehringer Type VI.
NGF Prevents the Age-Induced Cholinergic Neuron Atrophy.
Histological examination of the target neurons detected by immunohistochemistry for the low-affinity neurotrophin receptor (p75NTR) provided data in support of the behavioral differences among int+con-grafted and NGF-grafted animals. The present strain of animals does not undergo any significant reduction in the number of basal forebrain cholinergic neurons with aging, although the animals display a marked cholinergic neuronal atrophy, as assessed with stereological techniques (19, 20). Although the int+con-graft aged animals showed a reduction of cell size of about 25% in both MS and NBM compared with the 12-month-old adult animals (see Table 1), the NGF-cell-grafted aged animals did not show any sign of neuronal atrophy (6–8% increase above values seen in the adult animals, nonsignificant). In addition, an extensive p75NTR immunoreactive network of processes that did not differ from that normally present in adult animals was present in MS and NBM in the NGF-cell-grafted aged animals but not in the int+con-graft aged rats (Fig. 4).
Table 1.
Morphometric analysis of p75NTR-immunoreactive neurons
| Group | Medial septum
|
Nucleus basalis
|
||
|---|---|---|---|---|
| Cell counts | Cell volume, μm3 | Cell counts | Cell volume, μm3 | |
| Adult (12 months old) | 5,269 ± 355 | 7,381 ± 256 | 6,126 ± 271 | 11,332 ± 604 |
| Int+con-graft (aged) | 5,084 ± 292 | 5,554 ± 463*† | 6,418 ± 318 | 8,640 ± 293‡ |
| NGF-graft (aged) | 4,924 ± 314 | 7,847 ± 662 | 6,082 ± 473 | 12,193 ± 433 |
Intact and control-graft groups did not differ in any of the measured parameters (P > 0.05). Medial septum: cell counts, intact = 5,451 ± 684, control-graft = 4,810 ± 488 cells; volume, intact = 5,524 ± 1,117 μm3, control-graft = 5,576 ± 356 μm3. Nucleus basalis: cell counts, intact = 6,361 ± 671 cells, control-graft = 6,462 ± 359 cells; volume, intact = 8,271 ± 582 μm3, control-graft = 8,918 ± 269 μm3.
Different from adult, P < 0.05, one-way ANOVA post-hoc Fisher PLSD.
Different from NGF-graft, P < 0.05, one-way ANOVA post-hoc Scheffe F-test.
Different from adult and NGF-graft, P < 0.05, one-way ANOVA post-hoc Scheffe F-test.
Figure 4.
NGF prevents the age-induced atrophy of forebrain cholinergic neurons. The photomicrographs illustrate p75NTR immunostaining at the level of the NBM (Upper) and MS (Lower) in sections from intact adult or aged animals grafted with control- or NGF-secreting neural stem cells. Note the increased cell body size and intensity of immunostaining of both the neuronal soma and the surrounding network of processes in the NGF-grafted animal. As illustrated in Fig. 2, the grafted cells (not visible here) were cytoarchitecturally integrated in the target region and did not cause any abnormality in the anatomical patterns of the region receiving the graft. (Bar = 200 μm.)
DISCUSSION
The present results demonstrate that continuous local supply of transgenic NGF from implanted neural progenitors into middle-aged cognitively unimpaired rats can prevent the appearance of age-induced decline in cognitive function as assessed in the spatial navigation task. In previous studies NGF has been administered over a 4- to 10-week period to already impaired aged animals by using either implants of NGF-secreting cells (18–21, 26) or intraventricular infusions of the neurotrophic factor (5–8, 10). These studies had shown that neurotrophic stimulation of either of the two principal basal forebrain cholinergic cell groups (MS and NBM) can reverse cholinergic neuron atrophy and significantly improve the performance of cognitively impaired aged rats in the water maze task. Here we report that similar local, low-level delivery of NGF, initiated at a presymptomatic stage, can prevent the appearance of both structural and behavioral changes during the subsequent 9 months. Local NGF administration by NGF-secreting neural progenitors did not seem to induce any adverse behavioral side-effects: supply of transgenic NGF to nonimpaired middle-aged rats did not improve the rat’s performance above that seen in the nongrafted controls, nor did the NGF cells cause any behavioral impairments in either the water maze or the DMPT task, which is in contrast to what has previously been reported after implantation of NGF-secreting fibroblasts or direct intracerebral NGF infusions in young subjects (8, 18).
The immortalized neural progenitor cell line used here, HiB5, exhibits highly suitable properties for intracerebral transplantation. In particular, the ability of HiB5 cells to differentiate into glia-like cells and stably integrate into the host brain makes them very well suited for ex vivo gene transfer to the CNS. Previous results show that the grafted HiB5 cells may divide two to three times during the first 5 days after implantation; during this time they migrate out from the site of implantation to become fully integrated into the surrounding host tissue within an area with a radius of about 1–1.5 mm. The cell number and distribution attained within the first 2 weeks after grafting remain stable over the subsequent months (22, 28). The in vivo expression of the NGF transgene in the NGF-transduced HiB5 cells used here has been examined in some detail. Previous results indicate that NGF-mRNA expression in the grafted cells is reduced by about one order of magnitude within the first 1–2 weeks after grafting but the level of expression then is maintained at a seemingly stable level for at least 10 weeks (19, 22). Consistent with the PCR data, NGF bioassay and NGF ELISA determinations have demonstrated significant bioactive NGF protein levels in the grafted region at both 4 and 10 weeks after transplantation (19, 20). The present results demonstrate that the retroviral vector is still expressed in vivo 9 months after transplantation. Comparisons of the present results with previous analyses performed at shorter survival times do not suggest any substantial change in expression between 2 and 9 months postgrafting, although the negative PCR data obtained in some of the present animals (1/3 at 4 months, 3/6 at 9 months) may be taken to indicate that the NGF expression may become more variable at the longest survival times.
The NGF secretion rate of the present NGF-HiB5 transplants can be estimated at about 150 ng/day on each side (see refs. 19, 20, and 22 for further discussion). By bioassay and NGF-ELISA determinations, the grafted cells have been shown to provide a sustained increase in the tissue NGF content in the grafted NBM area, up to the level normally seen in hippocampus or cortex as assessed at 4 and 10 weeks postgrafting (19, 20). Even though the NGF production of the grafted cells may decline in some animals at longer survival times, we propose that the local supply of low levels of the neurotrophic factor from cells located in the immediate vicinity of the cholinergic target neurons, as obtained here, is sufficient to exert a long-lasting neuroprotective effect during aging.
The results presented here thus demonstrate the usefulness of the ex vivo gene transfer approach for long-term intracerebral delivery of neurotrophic factors, as compared with other alternative methods of administration. Systemically administered neurotrophic factors are not able to cross the blood-brain barrier (BBB), and their diffusion through the brain parenchyma is limited (29), which creates steep concentration gradients when delivered from a point source (30). Indeed, NGF itself has a short half-life (45 min) when delivered to the brain interstitium (31), and its in vivo effects disappear after a few days following administration (32). An alternative to intracerebral delivery is the use of conjugates of trophic factors to antibodies (anti-transferrin receptor-NGF) (9, 33) that would make them transported across the BBB by virtue of specific receptors; however, this strategy results in a nontargeted delivery of the trophic factor to all parts of the nervous system (33). Although behavioral effects have been obtained in aged animals by using this approach (9), complications may arise because most neurons in the peripheral nervous system are endowed with (often multiple) sets of neurotrophin receptors that regulate their survival, growth, or function (12, 34). Additionally, outside the nervous system there are other systems that may be responsive to systemically administered neurotrophic factors, as is the case for memory B lymphocytes and NGF (35).
Age-related changes in the forebrain cholinergic system provide a well characterized and highly useful experimental model of progressive neurodegeneration, associated with severe cognitive decline analogous to dementia in man. This study makes use of this model to explore the efficacy of long-term, low-level targeted intracerebral delivery of a neurotrophic factor as a potential therapeutic strategy to prevent the appearance of both morphological changes and behavioral decline associated with advanced age. The results suggest that targeted gene transfer to vulnerable or affected brain regions can provide interesting new possibilities to achieve long-term delivery of therapeutic proteins within the CNS. Cholinergic neuron atrophy and degeneration is prominent in patients with Alzheimer disease (AD), and although neurodegeneration is widespread in AD, cholinergic neuron dysfunction is believed to play a role in the development of cognitive symptoms, particularly in the early stages of the disease (1). Animal experiments indicate that the basal forebrain cholinergic system is an important modulator of cortical and hippocampal functions and that the subcortical cholinergic afferents play a normal physiological role, e.g., in regulation of attentional processes and short-term spatial or working memory (1, 4). Consistent with this view, it has been shown that selective damage to the basal forebrain cholinergic system (obtained by local injections of the 192IgG-saporin immunotoxin) induces pronounced and long-lasting impairments in the rats’ performance in spatial learning and short-term memory tasks; however, these effects are seen only after extensive lesions, which remove >80–85% of the cholinergic neurons in both NBM and the septal–diagonal band area (24, 36, 37). Less extensive lesions, or lesions restricted to either of the two projection systems, have only marginal effects in these tasks (38–40). Because the forebrain cholinergic system in aged rats is largely spared, though in an atrophic state, it seems likely that the age-dependent behavioral impairments may reflect dysfunctions in both cholinergic and noncholinergic systems. The behavioral improvements obtained after intracerebral administration of NGF, therefore, could be due to effects mediated not only by its known neurotrophic actions on the forebrain cholinergic neurons, but also by effects on other systems in the brain (cf. ref. 41).
Although the cellular and behavioral effects of NGF reported here may readily be explained by a direct action of the neurotrophin at the p75NTR and TrkA receptors, both present in forebrain cholinergic neurons, other indirect beneficial actions of NGF should be considered as well. In the brain of both aged individuals and AD patients, inflammation is being recognized as an important component of a pathophysiological mechanism, contributing to both normal aging and the progression of the disease (42, 43). Interestingly, NGF has been shown to modulate excitotoxic processes in vivo, leading to a substantial rescue of striatal projection neurons that lack any NGF receptor, through a mechanism that could be related to the control of microglial and glial responses to injury, which are central to the inflammatory process (44, 45). Other possible NGF actions, such as preservation of mitochondrial function against oxidative stress, may be applicable as well (46). It is conceivable, therefore, that a combined trophic and antiinflammatory mechanism may be important for the neuroprotective effects seen in the present experimental paradigm.
In conclusion, the present results provide evidence in support of the hypothesis that sustained local supply of a neurotrophic factor at the cell body level can retard or diminish the normal age-induced progressive degenerative changes in the forebrain cholinergic system to a level where no cellular or behavioral changes can be detected at advanced age. The results show, moreover, that genetically modified neural progenitor cells, by virtue of their integrative properties and stability in the adult or aged brain, are highly useful vehicles for continuous local administration of trophic factors to defined target areas within the CNS. Expression of the high-affinity NGF receptor trkA gene is decreased in both AD patients and aged rats, resulting in an impaired retrograde NGF transport in the basal forebrain cholinergic system (47, 48). The ex vivo gene transfer of NGF locally, at the cell body level, therefore may be particularly well suited in the context of age-related neuronal atrophy, because it will circumvent those disease-related pathological processes that affect uptake and retrograde transport of the trophic factor from remote target areas. This approach also may have implications for the design of therapeutic strategies for other progressive neurodegenerative conditions, particularly those that are characterized by protracted deterioration of defined subsets of neurons within the CNS.
Acknowledgments
The authors wish to thank the excellent animal care provided by Sten Nilsson and the expert technical help of Birgit Haraldsson, AnnaKarin Olden, Gertrude Stridsberg, Kerstin Fogelstrom, Ulla Jarl, Alicja Flasch, and Cristina Ciornei. Help with image analyses, data handling, and computer editing of the manuscript by Soledad Conte is gratefully acknowledged. This work was supported by grants from the Swedish Medical Research Council (19X-11632 to A.M.-S. and 04X-3874 to A.B.), the Human Frontier Science Program, the Faculty of Medicine of the University of Lund, and the Segerfalk, Åke Wiberg, and Kock foundations.
ABBREVIATIONS
- RT
reverse transcription
- NGF
nerve growth factor
- CNS
central nervous system
References
- 1.Dunnett S B, Fibiger H C. Prog Brain Res. 1993;98:413–420. doi: 10.1016/s0079-6123(08)62425-5. [DOI] [PubMed] [Google Scholar]
- 2.Finch C E. Trends Neurosci. 1993;16:104–110. doi: 10.1016/0166-2236(93)90134-8. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher M, Colombo P. Curr Opin Neurobiol. 1995;5:161–168. doi: 10.1016/0959-4388(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 4.Everitt B J, Robbins T W. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 5.Fischer W, Wictorin K, Björklund A, Williams L R, Varon S, Gage F H. Nature (London) 1987;329:65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- 6.Fischer W, Björklund A, Chen K, Gage F H. J Neurosci. 1991;11:1889–1906. doi: 10.1523/JNEUROSCI.11-07-01889.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer W, Sirevaag A, Wiegand S J, Lindsay R M, Björklund A. Proc Natl Acad Sci USA. 1994;91:8607–8611. doi: 10.1073/pnas.91.18.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowska A L, Koliatsos V E, Breckler S J, Price D L, Olton D S. J Neurosci. 1994;14:4815–4824. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bäckman C, Rose G M, Hoffer B J, Henry M A, Bartus R T, Friden P, Granholm A C. J Neurosci. 1996;16:5437–5442. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frick K M, Price D L, Koliatsos V E, Markowska A L. J Neurosci. 1997;17:2543–2550. doi: 10.1523/JNEUROSCI.17-07-02543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsay R M, Wiegand S J, Altar C A, Distefano P S. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 12.Lewin G R, Barde Y-A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 13.Rylett R J, Williams L R. Trends Neurosci. 1994;11:486–490. doi: 10.1016/0166-2236(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 14.Hellweg R, Fischer W, Hock W, Gage F H, Björklund A, Thoenen H. Brain Res. 1990;537:123–130. doi: 10.1016/0006-8993(90)90348-f. [DOI] [PubMed] [Google Scholar]
- 15.Crutcher K A, Weingartner K. Neurobiol Aging. 1991;12:449–454. doi: 10.1016/0197-4580(91)90072-r. [DOI] [PubMed] [Google Scholar]
- 16.Williams L R. Exp Neurol. 1991;113:31–37. doi: 10.1016/0014-4886(91)90143-z. [DOI] [PubMed] [Google Scholar]
- 17.Lewin G R, Mendell L M. Trends Neurosci. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- 18.Chen K S, Gage F H. J Neurosci. 1995;15:2819–2825. doi: 10.1523/JNEUROSCI.15-04-02819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Serrano A, Fischer W, Björklund A. Neuron. 1995;15:473–484. doi: 10.1016/0896-6273(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Serrano A, Fischer W, Söderstrom S, Ebendal T, Björklund A. Proc Natl Acad Sci USA. 1996;93:6355–6360. doi: 10.1073/pnas.93.13.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindner M D, Kearns C E, Winn S R, Frydel B, Emerich D F. Cell Transplant. 1996;5:205–223. doi: 10.1177/096368979600500210. [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Serrano A, Lundberg C, Horellou P, Fischer W, Bentlage C, Campbell K, McKay R D G, Mallet J, Björklund A. J Neurosci. 1995;15:5668–5680. doi: 10.1523/JNEUROSCI.15-08-05668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renfranz P J, Cunningham M G, McKay R D G. Cell. 1991;66:713–729. doi: 10.1016/0092-8674(91)90116-g. [DOI] [PubMed] [Google Scholar]
- 24.Leanza G, Muir J, Nilsson O G, Wiley R G, Dunnet S B, Björklund A. Eur J Neurosci. 1996;8:1535–1544. doi: 10.1111/j.1460-9568.1996.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Fischer W, Chen K S, Gage F H, Björklund A. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- 26.Winn S R, Lindner M D, Lee A, Haggett G, Francis J M, Emerich D F. Exp Neurol. 1996;140:126–138. doi: 10.1006/exnr.1996.0123. [DOI] [PubMed] [Google Scholar]
- 27.Pelleymounter M A, Cullen M J, Baker M B, Gollub M, Wellman C. Mol Chem Neuropathol. 1996;29:211–226. doi: 10.1007/BF02815003. [DOI] [PubMed] [Google Scholar]
- 28.Lundberg C, Martínez-Serrano A, Cattaneo E, McKay R D G, Björklund A. Exp Neurol. 1997;145:342–360. doi: 10.1006/exnr.1997.6503. [DOI] [PubMed] [Google Scholar]
- 29.Yan Q, Matheson C, Sun J, Radeke M J, Feinstein S C, Miller J A. Exp Neurol. 1994;127:23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]
- 30.Krewson C E, Klarman M L, Saltzman W M. Brain Res. 1995;680:196–206. doi: 10.1016/0006-8993(95)00261-n. [DOI] [PubMed] [Google Scholar]
- 31.Krewson C E, Saltzman W M. Brain Res. 1996;727:169–181. doi: 10.1016/0006-8993(96)00378-2. [DOI] [PubMed] [Google Scholar]
- 32.Knusel B N, Kaplan D R, Hefti F. Exp Neurol. 1996;139:121–130. doi: 10.1006/exnr.1996.0087. [DOI] [PubMed] [Google Scholar]
- 33.Kordower J H, Charles V, Bayer R, Bartus R T, Putney S, Walus L R, Friden P M. Proc Natl Acad Sci USA. 1994;91:9077–9080. doi: 10.1073/pnas.91.19.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon S B, Priestley J V. Curr Opin Neurobiol. 1995;5:616–624. doi: 10.1016/0959-4388(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 35.Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 36.Waite J J, Chen A D, Wardlow M L, Wiley R G, Lappi A D, Tal L J. Neuroscience. 1995;65:463–476. doi: 10.1016/0306-4522(94)00479-o. [DOI] [PubMed] [Google Scholar]
- 37.Leanza G, Nilsson O G, Wiley R G, Björklund A. Eur J Neurosci. 1995;7:329–343. doi: 10.1111/j.1460-9568.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 38.Torres E M, Perry T A, Blokland A, Wilkinson L S, Wiley R G, Lappi D A, Dunnett S B. Neuroscience. 1994;63:95–122. doi: 10.1016/0306-4522(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 39.Baxter M G, Bucci D J, Sobel T J, Williams M J, Gorman L K, Gallager M. Neuroreport. 1996;7:1417–1420. doi: 10.1097/00001756-199605310-00019. [DOI] [PubMed] [Google Scholar]
- 40.McMahan R W, Sobel T J, Baxter M G. Hippocampus. 1997;7:130–136. doi: 10.1002/(SICI)1098-1063(1997)7:2<130::AID-HIPO2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 41.Mervis R F, Pope D, Lewis R, Dvorak R M, Williams L R. Ann N Y Acad Sci. 1991;640:95–101. doi: 10.1111/j.1749-6632.1991.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 42.Patterson P H. Curr Opin Neurobiol. 1995;5:642–646. doi: 10.1016/0959-4388(95)80070-0. [DOI] [PubMed] [Google Scholar]
- 43.Perry V H, Bell M D, Brown H C, Matyszak M K. Curr Opin Neurobiol. 1995;5:636–641. doi: 10.1016/0959-4388(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Serrano A, Björklund A. J Neurosci. 1996;16:4604–4616. doi: 10.1523/JNEUROSCI.16-15-04604.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levi-Montalcini R, Skaper S D, Dal Toso R, Petrelli L, Leon A. Trends Neurosci. 1996;19:514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- 46.Galpern W R, Matthews R T, Beal M F, Isacson O. Neuroreport. 1996;7:2639–2642. doi: 10.1097/00001756-199611040-00046. [DOI] [PubMed] [Google Scholar]
- 47.Cooper J D, Lindholm D, Sofroniew M V. Neuroscience. 1994;62:625–629. doi: 10.1016/0306-4522(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 48.Mufson E J, Li J M, Sobreviela T, Kordower J H. Neuroreport. 1996;8:25–29. doi: 10.1097/00001756-199612200-00006. [DOI] [PubMed] [Google Scholar]