Abstract
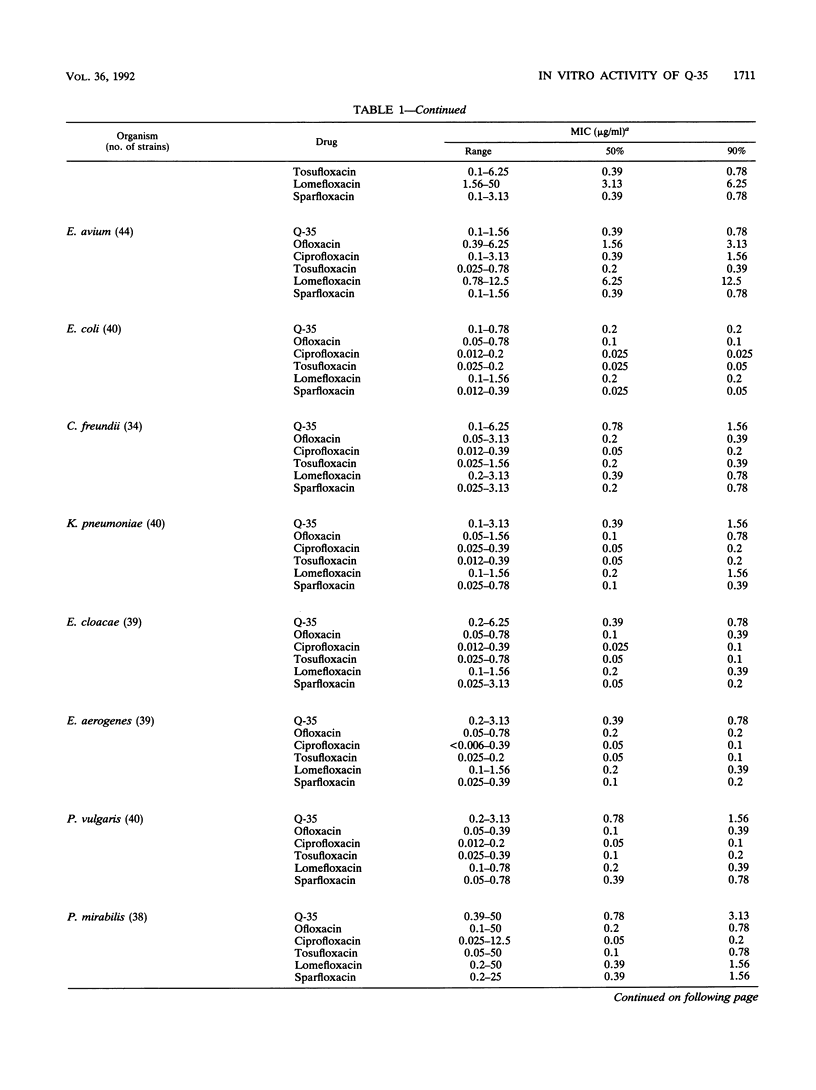
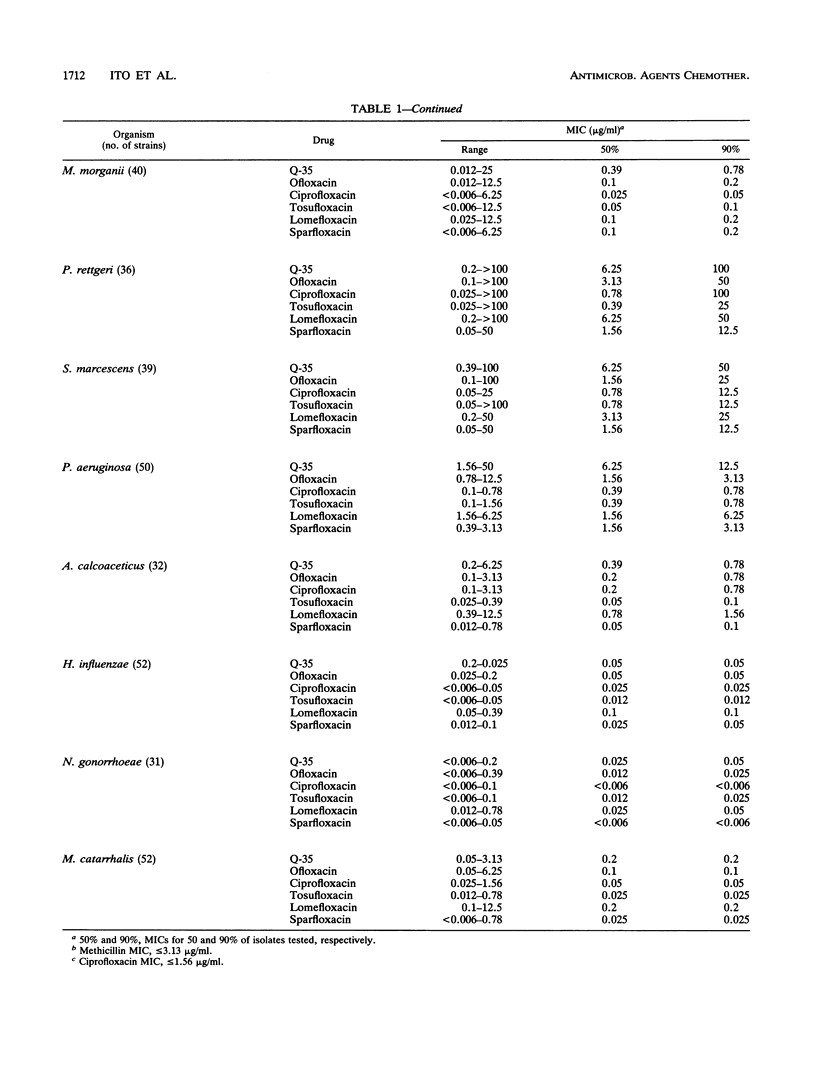
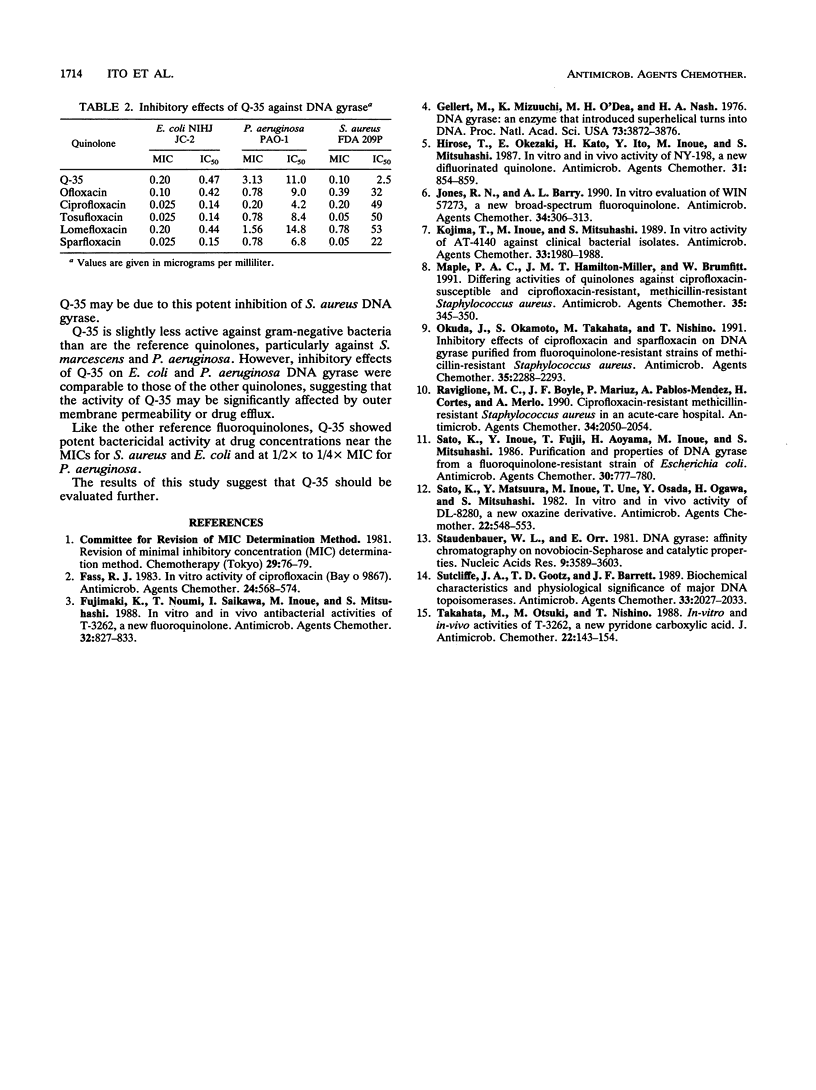
The in vitro activity of Q-35, an 8-methoxy fluoroquinolone, was compared with those of ofloxacin, ciprofloxacin, tosufloxacin, lomefloxacin, and sparfloxacin. The MICs of Q-35 for 90% of strains tested (MIC90s) of Staphylococcus aureus, methicillin-resistant S. aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, and Streptococcus pyogenes were 0.2, 6.25, 0.2, 0.39, and 0.39 micrograms/ml, respectively. The activity of Q-35 was 4- to 16-fold greater than those of ofloxacin, ciprofloxacin and lomefloxacin but equal to those of tosufloxacin and sparfloxacin against these organisms. For 82 ciprofloxacin-resistant staphylococci (MIC90 = 100 micrograms/ml), Q-35 was the most active of the new quinolones tested (MIC90 = 6.25 micrograms/ml). The MIC90s of Q-35 against Escherichia coli, Enterobacter aerogenes, and Pseudomonas aeruginosa were 0.2, 0.78, and 12.5 micrograms/ml, respectively, and Q-35 was 2- to 16-fold less active than the other quinolones tested. Q-35 showed potent bactericidal activity and inhibited the supercoiling activity of DNA gyrase of S. aureus, E. coli, and P. aeruginosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fass R. J. In vitro activity of ciprofloxacin (Bay o 9867). Antimicrob Agents Chemother. 1983 Oct;24(4):568–574. doi: 10.1128/aac.24.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki K., Noumi T., Saikawa I., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activities of T-3262, a new fluoroquinolone. Antimicrob Agents Chemother. 1988 Jun;32(6):827–833. doi: 10.1128/aac.32.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T., Okezaki E., Kato H., Ito Y., Inoue M., Mitsuhashi S. In vitro and in vivo activity of NY-198, a new difluorinated quinolone. Antimicrob Agents Chemother. 1987 Jun;31(6):854–859. doi: 10.1128/aac.31.6.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. In vitro evaluation of WIN 57273, a new broad-spectrum fluoroquinolone. Antimicrob Agents Chemother. 1990 Feb;34(2):306–313. doi: 10.1128/aac.34.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Inoue M., Mitsuhashi S. In vitro activity of AT-4140 against clinical bacterial isolates. Antimicrob Agents Chemother. 1989 Nov;33(11):1980–1988. doi: 10.1128/aac.33.11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. Differing activities of quinolones against ciprofloxacin-susceptible and ciprofloxacin-resistant, methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Feb;35(2):345–350. doi: 10.1128/aac.35.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J., Okamoto S., Takahata M., Nishino T. Inhibitory effects of ciprofloxacin and sparfloxacin on DNA gyrase purified from fluoroquinolone-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Nov;35(11):2288–2293. doi: 10.1128/aac.35.11.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviglione M. C., Boyle J. F., Mariuz P., Pablos-Mendez A., Cortes H., Merlo A. Ciprofloxacin-resistant methicillin-resistant Staphylococcus aureus in an acute-care hospital. Antimicrob Agents Chemother. 1990 Nov;34(11):2050–2054. doi: 10.1128/aac.34.11.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. A., Gootz T. D., Barrett J. F. Biochemical characteristics and physiological significance of major DNA topoisomerases. Antimicrob Agents Chemother. 1989 Dec;33(12):2027–2033. doi: 10.1128/aac.33.12.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata M., Otsuki M., Nishino T. In-vitro and in-vivo activities of T-3262, a new pyridone carboxylic acid. J Antimicrob Chemother. 1988 Aug;22(2):143–154. doi: 10.1093/jac/22.2.143. [DOI] [PubMed] [Google Scholar]



