Abstract
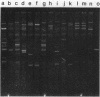
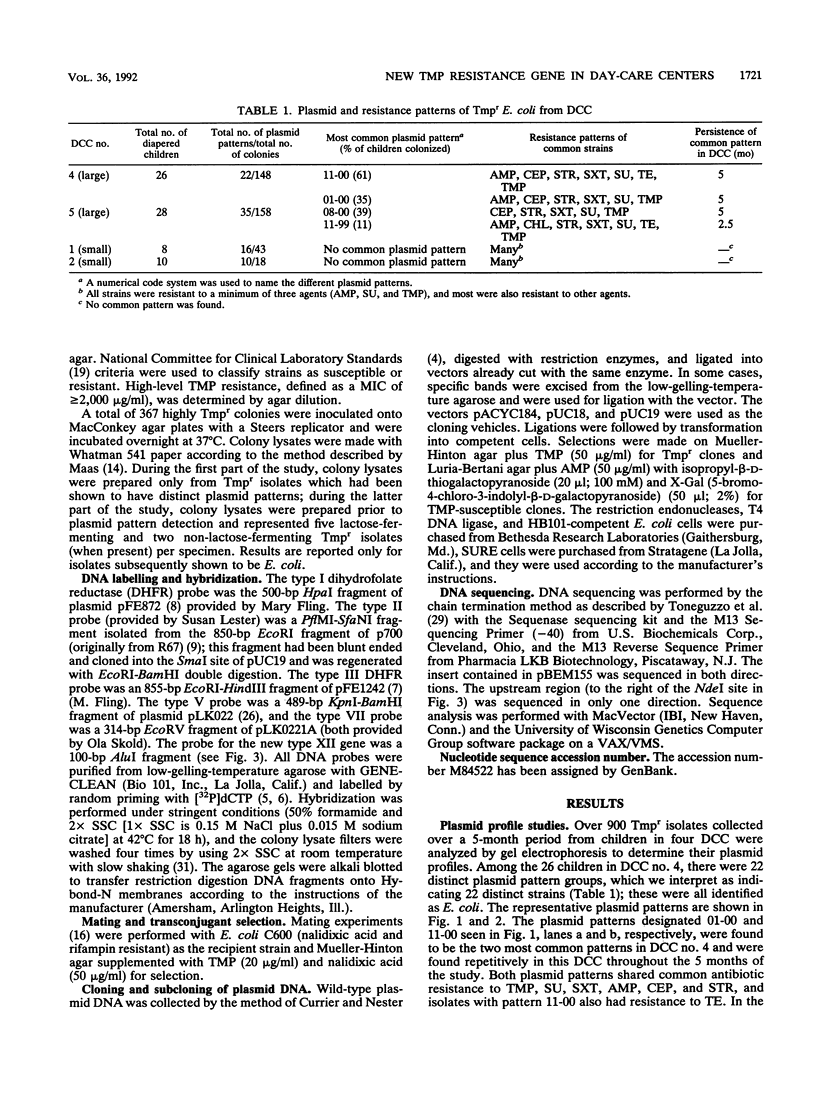
In our ongoing studies of trimethoprim resistance (Tmpr) in day-care centers (DCC), we have shown a high rate of fecal colonization with Tmpr Escherichia coli and, using total plasmid content analysis, have shown that this is due to a diversity of strains. In the present study, we analyzed 367 highly Tmpr (MIC, greater than or equal to 2,000 micrograms/ml) isolates of E. coli from 72 children over a 5-month period and found at least 83 distinct plasmid patterns, indicating that at least 83 strains were involved. Several strains were particularly common in a given DCC, including one found in 61% of children with Tmpr E. coli; these common strains usually persisted within a DCC for several months. Colony lysates were hybridized with gene probes for dihydrofolate reductases (DHFR) types I, II, III, V, and VII; 21% hybridized under stringent conditions, and all of these were with type I (17%) or type V (4%) probes. Tmpr was cloned from a probe-negative Tmpr transconjugant, and an intragenic probe was prepared from this clone. Approximately 21% of the Tmpr E. coli strains (76 isolates) in the DCC were found to have this new gene, 74 of which were in one DCC. The DNA sequence of this gene was determined, and the predicted amino acid sequence was shown to have between 32% and 39% identity with the amino acid sequences for types I, III, V, VI, and VII and the partial sequence of type IV and approximately 26% identity with types IX and X DHFR. This confirms the uniqueness of this gene, which has tentatively been named dhfrxii, and its translation product, DHFR type XII.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Towner K. J., Carter G. I., Thomson C. J., Young H. K. The type VII dihydrofolate reductase: a novel plasmid-encoded trimethoprim-resistant enzyme from gram-negative bacteria isolated in Britain. J Antimicrob Chemother. 1989 Aug;24(2):111–119. doi: 10.1093/jac/24.2.111. [DOI] [PubMed] [Google Scholar]
- Barg N. L., Hutson F. S., Wheeler L. A., Thomson C. J., Amyes S. G., Wharton M., Schaffner W. Novel dihydrofolate reductases isolated from epidemic strains of trimethoprim/sulfamethoxazole-resistant Shigella sonnei. J Infect Dis. 1990 Aug;162(2):466–473. doi: 10.1093/infdis/162.2.466. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fling M. E., Kope J., Richards C. Characterization of plasmid pAZ1 and the type III dihydrofolate reductase gene. Plasmid. 1988 Jan;19(1):30–38. doi: 10.1016/0147-619x(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Fling M. E., Richards C. The nucleotide sequence of the trimethoprim-resistant dihydrofolate reductase gene harbored by Tn7. Nucleic Acids Res. 1983 Aug 11;11(15):5147–5158. doi: 10.1093/nar/11.15.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling M. E., Walton L., Elwell L. P. Monitoring of plasmid-encoded, trimethoprim-resistant dihydrofolate reductase genes: detection of a new resistant enzyme. Antimicrob Agents Chemother. 1982 Nov;22(5):882–888. doi: 10.1128/aac.22.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein F. W., Papadopoulou B., Acar J. F. The changing pattern of trimethoprim resistance in Paris, with a review of worldwide experience. Rev Infect Dis. 1986 Sep-Oct;8(5):725–737. doi: 10.1093/clinids/8.5.725. [DOI] [PubMed] [Google Scholar]
- Huovinen P. Trimethoprim resistance. Antimicrob Agents Chemother. 1987 Oct;31(10):1451–1456. doi: 10.1128/aac.31.10.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson C., Sköld O. Appearance of a new trimethoprim resistance gene, dhfrIX, in Escherichia coli from swine. Antimicrob Agents Chemother. 1991 Sep;35(9):1891–1899. doi: 10.1128/aac.35.9.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Alvarado T., Kim K. H., Vorachit M., Jayanetra P., Levine M. M., Prenzel I., Fling M., Elwell L., McCracken G. H. Increasing resistance to trimethoprim-sulfamethoxazole among isolates of Escherichia coli in developing countries. J Infect Dis. 1985 Dec;152(6):1107–1113. doi: 10.1093/infdis/152.6.1107. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Moellering R. C., Jr Aminoglycoside-modifying enzymes among clinical isolates of Acinetobacter calcoaceticus subsp. anitratus (Herellea vaginicola): explanation for high-level aminoglycoside resistance. Antimicrob Agents Chemother. 1979 Feb;15(2):190–199. doi: 10.1128/aac.15.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Rensimer E. R., DuPont H. L. Emergence of high-level trimethoprim resistance in fecal Escherichia coli during oral administration of trimethoprim or trimethoprim--sulfamethoxazole. N Engl J Med. 1982 Jan 21;306(3):130–135. doi: 10.1056/NEJM198201213060302. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Rensimer E. R. Transfer of trimethoprim resistance from fecal Escherichia coli isolated during a prophylaxis study in Mexico. J Infect Dis. 1983 Apr;147(4):724–728. doi: 10.1093/infdis/147.4.724. [DOI] [PubMed] [Google Scholar]
- Parsons Y., Hall R. M., Stokes H. W. A new trimethoprim resistance gene, dhfrX, in the In7 integron of plasmid pDGO100. Antimicrob Agents Chemother. 1991 Nov;35(11):2436–2439. doi: 10.1128/aac.35.11.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reves R. R., Fong M., Pickering L. K., Bartlett A., 3rd, Alvarez M., Murray B. E. Risk factors for fecal colonization with trimethoprim-resistant and multiresistant Escherichia coli among children in day-care centers in Houston, Texas. Antimicrob Agents Chemother. 1990 Jul;34(7):1429–1434. doi: 10.1128/aac.34.7.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reves R. R., Murray B. E., Pickering L. K., Prado D., Maddock M., Bartlett A. V., 3rd Children with trimethoprim- and ampicillin-resistant fecal Escherichia coli in day care centers. J Infect Dis. 1987 Nov;156(5):758–762. doi: 10.1093/infdis/156.5.758. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström L., Rådström P., Swedberg G., Sköld O. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol Gen Genet. 1988 Aug;213(2-3):191–201. doi: 10.1007/BF00339581. [DOI] [PubMed] [Google Scholar]
- Sundström L., Vinayagamoorthy T., Sköld O. Novel type of plasmid-borne resistance to trimethoprim. Antimicrob Agents Chemother. 1987 Jan;31(1):60–66. doi: 10.1128/aac.31.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson C. J., Barg N., Amyes S. G. N-terminal amino acid sequence of the novel type IIIb trimethoprim-resistant plasmid-encoded dihydrofolate reductase from Shigella sonnei. J Gen Microbiol. 1990 Apr;136(4):673–677. doi: 10.1099/00221287-136-4-673. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Glynn S., Levi E., Mjolsness S., Hayday A. Use of a chemically modified T7 DNA polymerase for manual and automated sequencing of supercoiled DNA. Biotechniques. 1988 May;6(5):460–469. [PubMed] [Google Scholar]
- Trimethoprim resistance; epidemiology and molecular aspects. J Med Microbiol. 1990 Jan;31(1):1–19. doi: 10.1099/00222615-31-1-1. [DOI] [PubMed] [Google Scholar]
- Volz K. W., Matthews D. A., Alden R. A., Freer S. T., Hansch C., Kaufman B. T., Kraut J. Crystal structure of avian dihydrofolate reductase containing phenyltriazine and NADPH. J Biol Chem. 1982 Mar 10;257(5):2528–2536. [PubMed] [Google Scholar]
- Wanger A. R., Murray B. E., Echeverria P., Mathewson J. J., DuPont H. L. Enteroinvasive Escherichia coli in travelers with diarrhea. J Infect Dis. 1988 Sep;158(3):640–642. doi: 10.1093/infdis/158.3.640. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Podzorski R. P., Peterson P. K., Ramsay N. K., Simmons R. L., Rhame F. S. Incidence of trimethoprim-sulfamethoxazole-resistant enterobacteriaceae among transplant recipients. J Infect Dis. 1984 Nov;150(5):699–706. doi: 10.1093/infdis/150.5.699. [DOI] [PubMed] [Google Scholar]
- Wylie B. A., Koornhof H. J. Nucleotide sequence of dihydrofolate reductase type VI. J Med Microbiol. 1991 Oct;35(4):214–218. doi: 10.1099/00222615-35-4-214. [DOI] [PubMed] [Google Scholar]
- Young H. K., Jesudason M. V., Koshi G., Amyes S. G. Unusual expression of new low-level-trimethoprim-resistance plasmids. J Clin Microbiol. 1986 Jul;24(1):61–64. doi: 10.1128/jcm.24.1.61-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]