Abstract
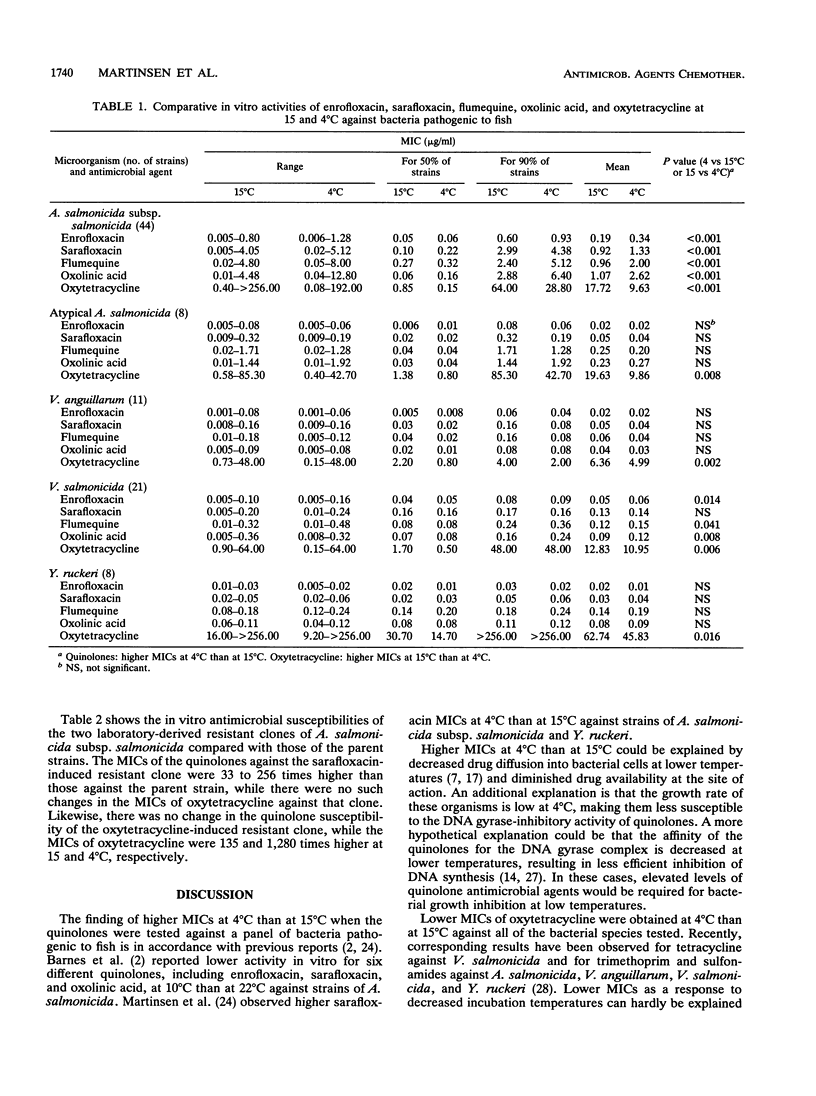
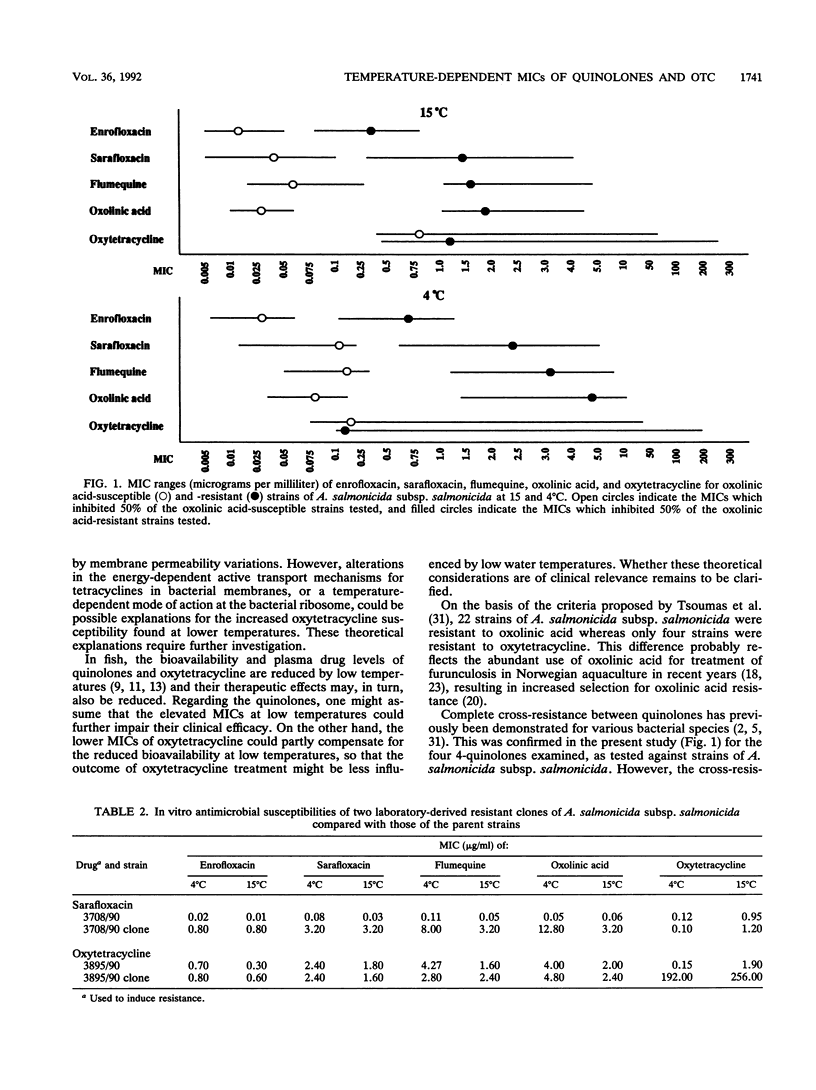
The in vitro antimicrobial activities of oxolinic acid, flumequine, sarafloxacin, enrofloxacin, and oxytetracycline against strains of bacteria pathogenic to fish (Aeromonas salmonicida subsp. salmonicida, atypical A. salmonicida, Vibrio salmonicida, Vibrio anguillarum, and Yersinia ruckeri) were determined at two different incubation temperatures, 4 and 15 degrees C, by a drug microdilution method. The main objective of the study was to examine the effect of incubation temperature on the in vitro activities of 4-quinolones and oxytetracycline against these bacteria. When tested against A. salmonicida subsp. salmonicida, all of the quinolones examined had MICs two- to threefold higher at 4 degrees C than at 15 degrees C. Similarly, 1.5- to 2-fold higher MICs were recorded for all of the quinolones except sarafloxacin at 4 degrees C than at 15 degrees C when the drugs were tested against V. salmonicida. In contrast to those of the quinolones, the MICs of oxytetracycline were two- to eightfold lower at 4 degrees C than at 15 degrees C against all of the bacterial species tested. Of the antimicrobial agents tested against the bacterial species included in the study, enrofloxacin was the most active and oxytetracycline was the least active. Sarafloxacin was slightly more active than flumequine and oxolinic acid, especially against oxolinic acid-resistant A. salmonicida subsp. salmonicida strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baquero F. Resistance to quinolones in gram-negative microorganisms: mechanisms and prevention. Eur Urol. 1990;17 (Suppl 1):3–12. doi: 10.1159/000464084. [DOI] [PubMed] [Google Scholar]
- Barnes A. C., Lewin C. S., Hastings T. S., Amyes S. G. In vitro activities of 4-quinolones against the fish pathogen Aeromonas salmonicida. Antimicrob Agents Chemother. 1990 Sep;34(9):1819–1820. doi: 10.1128/aac.34.9.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Gardiner R. V., Packer R. R. Bactericidal activities of ten different fluoroquinolones against selected Enterobacteriaceae. Diagn Microbiol Infect Dis. 1987 Jan;6(1):81–83. doi: 10.1016/0732-8893(87)90119-2. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Cross-resistance among cinoxacin, ciprofloxacin, DJ-6783, enoxacin, nalidixic acid, norfloxacin, and oxolinic acid after in vitro selection of resistant populations. Antimicrob Agents Chemother. 1984 Jun;25(6):775–777. doi: 10.1128/aac.25.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C., Ayers L. W., Gerlach E. H., Sommers H. M. Antibacterial activities of ciprofloxacin, norfloxacin, oxolinic acid, cinoxacin, and nalidixic acid. Antimicrob Agents Chemother. 1984 May;25(5):633–637. doi: 10.1128/aac.25.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgan A., Odegaard S., Bergsjø T. Temperature related absorption and excretion of sulphadimidine in rainbow trout, Salmo gairdneri. Acta Vet Scand. 1981;22(2):211–217. doi: 10.1186/BF03547510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser P. R., Wooster G. A., St Leger J., Babish J. G. Pharmacokinetics of enrofloxacin in fingerling rainbow trout (Oncorhynchus mykiss). J Vet Pharmacol Ther. 1992 Mar;15(1):62–71. doi: 10.1111/j.1365-2885.1992.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Chow R. T., Dougherty T. J., Fraimow H. S., Bellin E. Y., Miller M. H. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob Agents Chemother. 1988 Aug;32(8):1113–1118. doi: 10.1128/aac.32.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W., Stieglitz M., Baars B., Opferkuch W. Comparative evaluation of recently developed quinolone compounds--with a note on the frequency of resistant mutants. Chemotherapy. 1985;31(1):19–28. doi: 10.1159/000238309. [DOI] [PubMed] [Google Scholar]
- Diver J. M., Piddock L. J., Wise R. The accumulation of five quinolone antibacterial agents by Escherichia coli. J Antimicrob Chemother. 1990 Mar;25(3):319–333. doi: 10.1093/jac/25.3.319. [DOI] [PubMed] [Google Scholar]
- Snyder M., Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979 Jun 25;131(2):287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- Søorum H., Poppe T. T., Olsvik O. Plasmids in Vibrio salmonicida isolated from salmonids with hemorrhagic syndrome (Hitra disease). J Clin Microbiol. 1988 Sep;26(9):1679–1683. doi: 10.1128/jcm.26.9.1679-1683.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



