Abstract
In a previous study (B. P. Lockhart, H. Tsiang, P. E. Ceccaldi, and S. Guillemer, Antiviral Chem. Chemother. 2:9-15, 1991), we demonstrated an antiviral effect of the general anesthetic ketamine for rabies virus in neuronal cultures and in rat brain. This report describes an attempt to determine at what level ketamine acts on the rabies virus cycle in rat cortical neuron cultures. Immunofluorescence and [35S]methionine labelling of infected neurons showed that ketamine (1 to 1.5 mM) inhibited viral nucleoprotein and glycoprotein syntheses. Northern (RNA) blots of total RNA from drug-treated neurons, hybridized with 32P-labelled oligonucleotide probes for rabies virus nucleoprotein, matrix protein, and glycoprotein genes, showed a marked reduction (5- to 11-fold) in the levels of rabies virus mRNAs, relative to those in untreated neurons. No significant change in the levels of cellular beta-actin mRNA were detected in ketamine-treated cells. A similar antiviral effect was observed with MK-801; however, no inhibition of rabies virus synthesis was observed with the general anesthetic chloral hydrate. The antiviral effect was not complete; a time-dependent recovery of viral transcription and rabies virus protein synthesis was observed, but no infectious virus was released into the culture supernatant. The lack of any modification of cellular protein or mRNA synthesis by ketamine suggests an antiviral mechanism acting at the level of rabies virus genome transcription.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson Tommy, Schultzberg Marianne, Schwarcz Robert, Löve Arthur, Wickman Charlotte, Kristensson Krister. NMDA-Receptor Antagonist Prevents Measles Virus-induced Neurodegeneration. Eur J Neurosci. 1991 Oct;3(1):66–71. doi: 10.1111/j.1460-9568.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Arya S. C. Failures of postexposure rabies and other immunotherapies in developing countries. Vaccine. 1989 Aug;7(4):372–372. doi: 10.1016/0264-410x(89)90222-3. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedows E., Davidson B. A., Knight P. R. Effect of halothane on the replication of animal viruses. Antimicrob Agents Chemother. 1984 Jun;25(6):719–724. doi: 10.1128/aac.25.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedows E., Davidson B. A., Williams B. A., Knight P. R. Characterization of a halothane-resistant strain of measles virus. Antimicrob Agents Chemother. 1989 Mar;33(3):400–403. doi: 10.1128/aac.33.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussereau F., Picard M., Blancou J., Sureau P. Treatment of rabies in mice and foxes with antiviral compounds. Acta Virol. 1988 Jan;32(1):33–49. [PubMed] [Google Scholar]
- Cohen M. L., Trevor A. J. On the cerebral accumulation of ketamine and the relationship between metabolism of the drug and its pharmacological effects. J Pharmacol Exp Ther. 1974 May;189(2):351–358. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Harmon M. W., Janis B. Effects of cytosine arabinoside, adenine arabinoside, and 6-azauridine on rabies virus in vitro and in vivo. J Infect Dis. 1976 Jan;133(1):7–13. doi: 10.1093/infdis/133.1.7. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Knight P. R., Nahrwold M. L., Bedows E. Anesthetic action and virus replication: inhibition of measles virus replication in cells exposed to halothane. Antimicrob Agents Chemother. 1980 May;17(5):890–896. doi: 10.1128/aac.17.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P. R., Nahrwold M. L., Bedows E. Inhibiting effects of enflurane and isoflurane anesthesia on measles virus replication: comparison with halothane. Antimicrob Agents Chemother. 1981 Sep;20(3):298–306. doi: 10.1128/aac.20.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafon M., Edelman L., Bouvet J. P., Lafage M., Montchâtre E. Human monoclonal antibodies specific for the rabies virus glycoprotein and N protein. J Gen Virol. 1990 Aug;71(Pt 8):1689–1696. doi: 10.1099/0022-1317-71-8-1689. [DOI] [PubMed] [Google Scholar]
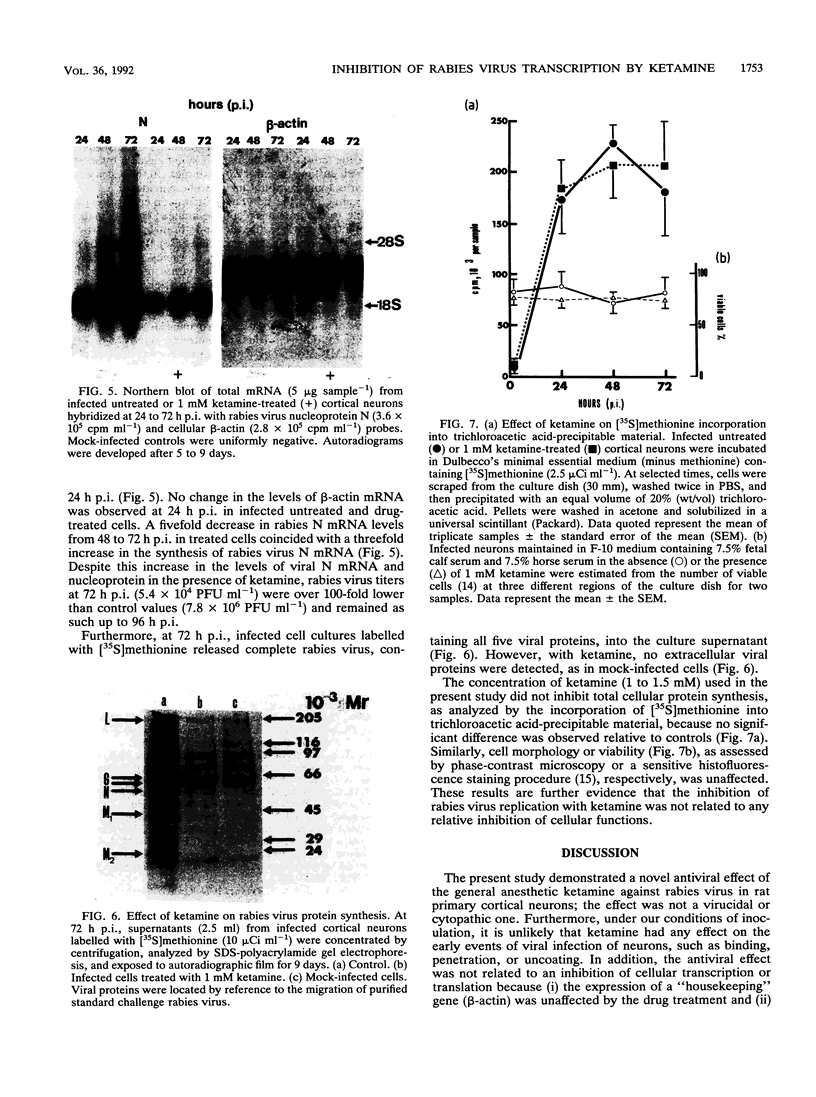
- Lockhart B. P., Tsiang H. Actin-independent maturation of rabies virus in neuronal cultures. J Gen Virol. 1991 Sep;72(Pt 9):2257–2261. doi: 10.1099/0022-1317-72-9-2257. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A. Rabies pathogenesis. Arch Virol. 1977;54(4):279–297. doi: 10.1007/BF01314774. [DOI] [PubMed] [Google Scholar]
- Nevins M. E., Arnolde S. M. A comparison of the anticonvulsant effects of competitive and non-competitive antagonists of the N-methyl-D-aspartate receptor. Brain Res. 1989 Nov 27;503(1):1–4. doi: 10.1016/0006-8993(89)91695-8. [DOI] [PubMed] [Google Scholar]
- Sacramento D., Bourhy H., Tordo N. PCR technique as an alternative method for diagnosis and molecular epidemiology of rabies virus. Mol Cell Probes. 1991 Jun;5(3):229–240. doi: 10.1016/0890-8508(91)90045-l. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H., Mifune K., Motohashi T. Isolation and assay of rabies serogroup viruses in CER cells. Intervirology. 1977;8(2):92–99. doi: 10.1159/000148883. [DOI] [PubMed] [Google Scholar]
- Tas P. W., Kress H. G., Koschel K. General anesthetics can competitively interfere with sensitive membrane proteins. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5972–5975. doi: 10.1073/pnas.84.16.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Nucleoside diphosphate kinase activity in purified cores of vesicular stomatitis virus. J Biol Chem. 1979 Sep 25;254(18):9075–9079. [PubMed] [Google Scholar]
- Tordo N., Poch O., Ermine A., Keith G. Primary structure of leader RNA and nucleoprotein genes of the rabies genome: segmented homology with VSV. Nucleic Acids Res. 1986 Mar 25;14(6):2671–2683. doi: 10.1093/nar/14.6.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordo N., Poch O., Ermine A., Keith G., Rougeon F. Walking along the rabies genome: is the large G-L intergenic region a remnant gene? Proc Natl Acad Sci U S A. 1986 Jun;83(11):3914–3918. doi: 10.1073/pnas.83.11.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang H., Ceccaldi P. E., Ermine A., Lockhart B., Guillemer S. Inhibition of rabies virus infection in cultured rat cortical neurons by an N-methyl-D-aspartate noncompetitive antagonist, MK-801. Antimicrob Agents Chemother. 1991 Mar;35(3):572–574. doi: 10.1128/aac.35.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang H., Koulakoff A., Bizzini B., Berwald-Netter Y. Neurotropism of rabies virus. An in vitro study. J Neuropathol Exp Neurol. 1983 Jul;42(4):439–452. doi: 10.1097/00005072-198307000-00006. [DOI] [PubMed] [Google Scholar]
- Warrell D. A., Warrell M. J. Human rabies and its prevention: an overview. Rev Infect Dis. 1988 Nov-Dec;10 (Suppl 4):S726–S731. doi: 10.1093/clinids/10.supplement_4.s726. [DOI] [PubMed] [Google Scholar]
- Wilde H., Choomkasien P., Hemachudha T., Supich C., Chutivongse S. Failure of rabies postexposure treatment in Thailand. Vaccine. 1989 Feb;7(1):49–52. doi: 10.1016/0264-410x(89)90010-8. [DOI] [PubMed] [Google Scholar]
- Yanagi K., Harada S. Destabilization of herpes simplex virus type 1 virions by local anesthetics, alkaline pH, and calcium depletion. Arch Virol. 1989;108(1-2):151–159. doi: 10.1007/BF01313753. [DOI] [PubMed] [Google Scholar]