Abstract
Finding a sexually receptive partner of the opposite sex is a challenge; one solution is to advertise. That advertising is usually the province of males has shaped scenarios for sexual selection, especially the ardent active male courting the passive but choosy female. Herein we consider an unusual case in which constraints on reproduction may have led to fertility advertisement by female frogs. When oviposition is imminent, female South African clawed frogs swim to an advertising male and produce an aphrodisiac call, rapping, that stimulates both male vocalization and approach. Males respond to rapping with a distinctive answer call. The rapping–answer interaction thus forms a duet between partners of a receptive pair.
Keywords: Xenopus laevis, courtship songs, fertility advertisement, vocal behaviors, sexual selection
In most vertebrates, perhaps due to the greater investment of females in gametes and offspring (1), it is the male that competes for receptive conspecific females (2, 3). Many males find mates by advertising; prominent examples are mating leks and the songs of birds and frogs. This simple solution works well when the responding female can be identified by the signaling male. However, if the signaling environment is noisy or if population density is high, it may be difficult for the signaler to distinguish the responder. Under these conditions, we might expect a reciprocal signaling system involving both sexes. Herein we present evidence for a system of auditory recognition—receptive duets—in the South African clawed frog.
We have studied male–female courtship vocalizations in Xenopus laevis, a member of a large genus of totally aquatic frogs from southern Africa (4). Xenopus inhabit murky ponds and mating occurs at night so that few if any visual cues are available to identify potential mates. Instead, it is believed that Xenopus, like other frogs (5), relies on auditory cues to broadcast receptivity and location. At the time we began our studies, two courtship vocalizations had been examined in this species. The advertisement or mating call (6) given only by sexually receptive males (7) is loud and prolonged with distinct fast and slow trill phases (7, 8). Sexually receptive females display positive phonotaxis to tapes of advertisement calling (8). Ticking (6), given by sexually unreceptive females (9), is a quiet, slow, and monotonous trill (10) believed to function as a release call (6, 11).
It has been assumed that male Xenopus find females by producing prolonged bouts of advertisement calling to which females are attracted; the male then clasps the nearest moving animal, releasing those that tick (12). The natural breeding conditions for Xenopus, high population density and low visibility, suggest that finding a mate may not be this simple. Unless the calling male could isolate himself from the rest of the group, clasping every animal in his vicinity would be disadvantageous; how then does he discriminate a responder?
We have examined populations of Xenopus near Cape Town during the prolonged breeding season (about 6 months). Most (88%) adult males taken from these ponds were sexually receptive whereas relatively few (20%) adult females were sexually receptive. The sexual receptivity that accompanies ovulation and oviposition is of relatively short duration (<24 h when hormonally induced; ref. 11). Xenopus females cannot store eggs in the oviduct and will release them, unfertilized, if mating does not occur. These constraints may put a premium on rapidly and accurately locating the calling male.
We report herein a vocalization, rapping, given by receptive female Xenopus laevis. Rapping stimulates males and elicits an answer call; the result is a duet between receptive partners. Female receptive calls and courtship duets are rare in vertebrates and have seldom been reported in amphibians (for review, see ref. 13). In Xenopus laevis, the combination of low visibility, high population density, and short periods of female receptivity may have necessitated the production of female receptive signals.
MATERIALS AND METHODS
Individual adults were obtained from natural ponds in the vicinity of Cape Town, Republic of South Africa, from July to December 1995. Bone-baited funnel traps were set on nine nonconsecutive nights in 10 ponds. Traps were examined the following morning, and the receptive state of adult individuals was determined: receptive males were recognized by well-developed forearm nuptial pads and receptive females were recognized by swollen red cloacae. Sexually receptive adults were transported to the laboratory, housed in plastic aquaria (one per tank) under natural light conditions, and fed beef liver three times per week. Behavior observations were carried out in a clear-water concrete pond (7.2 m wide by 9.6 m long by 0.4–3.2 m deep) at night under constant dim illumination. In the early afternoon of each test day, females to be observed were injected with human chorionic gonadotropin (Sigma, 500 IU); male frogs that did not call spontaneously were also injected (250 IU).
To observe courtship behaviors, a vocalizing male was placed behind an opaque but acoustically transparent barrier at one end and a receptive female was released at the opposite end of the artificial pond. Vocalizations were recorded with a hydrophone (Wilcoxon, model H505L, with an output sensitivity of −160 dB at 1 V/μPa and a frequency sensitivity of 0.002–10 kHz or Cornell Bioacoustics Program with an output sensitivity of −163 ± 3 dB at 1 V/μPa and a frequency sensitivity of 0.015–10 kHz) mounted at a depth of 0.3 m on the male side of the barrier.
To observe behavior between an unreceptive female and a receptive male, uninjected females were placed with the male and vocal behaviors were recorded as described above. Ticking females were recorded on the male side of the barrier and rapping females were recorded on the opposite side. Because the hydrophone was placed on the male side, any bias in sound amplitude would have favored ticking, which is generally lower in amplitude, rather than rapping. The furthest distance between the hydrophone and the vocalizing frog was 1 m. The responses of three males to a ticking female were recorded, and call durations were measured; acoustic features of male calls could only be analyzed from two of the three recordings.
To determine the effect of female vocalizations on male behavior, laboratory playback experiments were conducted in a 1 m by 2 m by 0.5 m fiberglass tank in New York City from June to August 1996. Sexually mature males were obtained from Nasco (Ft. Atkinson, WI), maintained in plastic aquaria under a 12-h light/12-h dark cycle and fed frog brittle three times per week. Frogs were injected with human chorionic gonadotropin (250–400 IU) 6 h before testing and placed in the phonotaxis tank; testing began at the beginning of the dark period of the day/night cycle. Only males that called prior to hearing female calls were used. Female calls were presented through an underwater speaker (University Sound, UW30; frequency response, 0.1–10 kHz). Stimulus tapes consisted of five samples, each of 12 clicks (a 1-s duration for rapping and a 3-s duration for ticking) taken from recordings (one female rapping and another ticking) from the artificial pond. Samples were separated by 1- or 3-min intervals to allow time for male vocal responses. For each male, the volume of playbacks was initially low and was gradually increased until the male responded; the maximum volume used in these experiments did not exceed the maximum volume of rapping (86 dB at 1 V/μPa). One hundred male calls were collected as baseline data and males were then stimulated until they stopped responding or until 100 additional calls had been collected. Vocalizations were recorded as described above, filtered (to reduce 60-Hz noise), and analyzed by using sound edit on a Macintosh (Quadra 800).
The durations of the fast and slow trill portions of the advertisement call and the percent amplitude modulation of the fast trill were determined. The fast trill was identified as the portion of the call during which clicks are partially superimposed (Fig. 1D). Duration of the fast trill was measured from the onset of the first click of the fast trill to the onset of the first click of the slow trill. Duration of the slow trill was measured from the onset of the first click to the end of the last click. Percent amplitude modulation was determined as: [(the amplitude of the last click of the fast trill − the amplitude of the first click of the fast trill)/the amplitude of the first] × 100. Clicks from male and female vocalizations were distinguished at slow sweep speeds by their duration; female clicks are shorter (see Fig. 1 A and D, for examples). For female vocalizations, the interclick interval from the beginning of one click to the beginning of the next and the amplitude above baseline were determined. All comparisons were carried out by using nonparametric statistics with the exception of the effects of ticking broadcasts on male calls where the n = 4 precluded a paired nonparametric test.
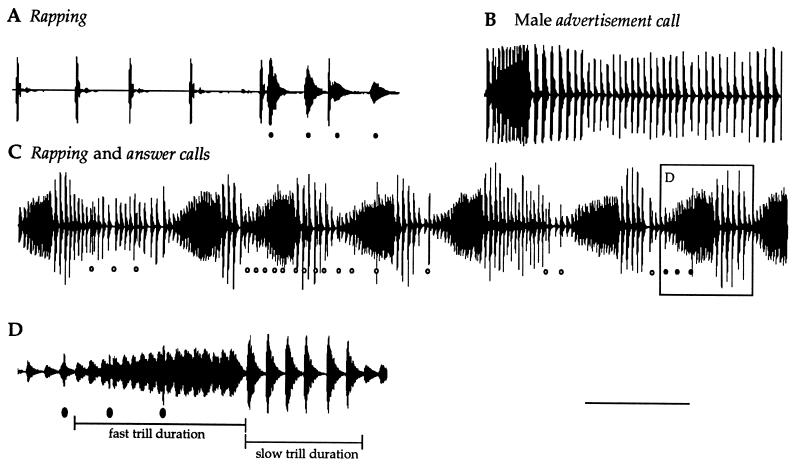
Figure 1.
Rapping and effects on male calls. (A) Oscillograph (amplitude envelope) of female rapping. After the fifth rap, the male is beginning to answer; male clicks are indicated. Note that male and female clicks are readily distinguishable by duration. (B) Oscillograph of a male advertisement call. Compared with the advertisement call, the male answer call (C, see box D) has a longer fast trill, a shorter slow trill, and increased amplitude modulation. (C) Oscillograph of a receptive duet; raps are indicated by circles. At the start of the trace the male is advertisement calling, as soon as the female raps, his vocalization changes to an answer call. (D) A portion of the receptive duet (C, box D) at a faster sweep speed illustrating raps and trill durations. [Bar = 500 ms (B and C) and 250 ms (A and D)]. Oscillographs illustrate amplitude (dB at 1 V/μPa) by time. Recordings were obtained in the artificial pond.
Component frequencies of individual clicks, randomly selected from different portions of recordings for three rapping and three ticking females in the artificial pond, were determined using a fast Fourier transform analysis (superscope; sampling rate, 142 kHz).
Vocalizations were recorded in a concrete artificial pond and a fiberglass tank. Although the spectral properties of individual clicks are known to be affected by the recording conditions (14), we only compared the spectral properties of ticks and raps in the artificial pond. In playback experiments, recordings obtained in the artificial pond were broadcast to males in the fiberglass tank. Changes in acoustic properties of the broadcast calls did not, however, prevent males from discriminating ticking from rapping.
RESULTS
Receptive females swam directly toward the vocalizing male and, on reaching the barrier, produced a vocalization that we call rapping, a rapid series of loud clicks (Fig. 1A). Rapping was only produced by receptive females and only in response to a calling male. Rapping had dramatic effects on males; they launched into prolonged intense bouts of calling (Fig. 1C) and swam about rapidly in the vicinity of the sound source. The effect of rapping was sufficiently powerful that it was rarely heard for more than 1 or 2 s unaccompanied by male vocalizations (Fig. 1A). Thus rapping stimulates both male vocal performance and activity. One rapping female was placed behind the barrier with the male and she continued rapping until he clasped her.
To determine whether rapping alone, in the absence of the female, is sufficient to elicit changes in male behavior, we played prerecorded tapes of rapping to receptive males in a fiberglass tank. Tapes of rapping produced the same marked effects on male behavior as did the vocalizing female; males launched into prolonged bouts of calling in response to tapes of rapping, a response similar to that elicited by calling females. Males were stationary while advertisement calling. Rapping playbacks induced all males to swim. Rapping induced positive phonotaxis: four of five males swam directly toward the speaker when rapping was played, and one male clasped the speaker in a misguided attempt at amplexus.
A rapping female also had a profound effect on the structure of male calls. The male advertisement call consists of alternating short–fast and long–slow trills (7); the fast portion is amplitude modulated, becoming louder throughout the trill (Fig. 1B). Trill durations and amplitude modulation changed in response to a rapping female (Figs. 1C and 2B). The fast trill was prolonged (194 ± 48 vs. 281 ± 64 ms; P < 0.02), the slow trill was shortened (806 ± 161 vs. 265 ± 105 ms; P < 0.02), and the amplitude modulation of the fast trill was increased (58 ± 42 vs. 221 ± 153%; P < 0.02; two-tailed Wilcoxon signed rank test comparing male calls before and during rapping in eight males). We have named the call the male produced in response to rapping the answer call. Because the sexes respond to each other’s calls and because their calls overlap, we refer to the vocal interactions between the sexes as “duets” (15).
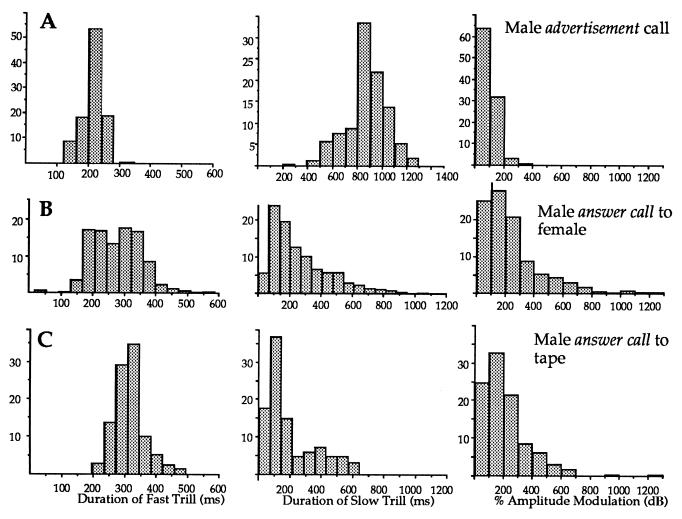
Figure 2.
Rapping alters male calling. The male advertisement call (A) is altered in response to a rapping female (B) and in response to a tape of rapping (C). Frequency histograms for fast and slow trill durations and percent amplitude modulation are shown for all male calls (A, n = 422; B, n = 729; C, n = 270).
Males also produced answer calls in response to tapes of rapping (Fig. 2C). Compared with advertisement calling (Fig. 2A), the duration of the fast trill increased (265 ± 65 vs. 315 ± 13 ms; P < 0.04), the duration of the slow trill decreased (1,005 ± 79 vs. 193 ± 88 ms; P < 0.04), and amplitude modulation increased (118 ± 36 vs. 196 ± 72%; P < 0.04; two-tailed Wilcoxon signed rank test on five males). The effects of rapping on male vocalizations in playback experiments were not distinguishable from effects observed in response to vocalizing females in the artificial pond (Fig. 2B; fast, P > 1.0; slow, P = 0.8; percent amplitude modulation; P = 0.3; Mann–Whitney U test). Thus rapping alone can induce the answer call, as well as positive phonotaxis toward and attempted copulation with the sound source.
To determine whether the male response to rapping is specific to that vocalization, male responses to tapes of ticking were also examined. Tapes of ticking did not induce the male to swim. Neither the duration of the fast trill portion (247 ± 8 to 288 ± 33 ms; P > 0.13) nor the amplitude modulation of the fast trill (93 ± 43 to 115 ± 56%; P > 0.21) were significantly altered (n = 4; paired t tests). The duration of the slow trill portion of the male call was significantly decreased (1,241 ± 298 to 273 ± 141 ms; P < 0.002) in response to ticking. We conclude that the male’s response to rapping is specific: ticking does not induce positive phonotaxis nor does it significantly alter most acoustic features of the call.
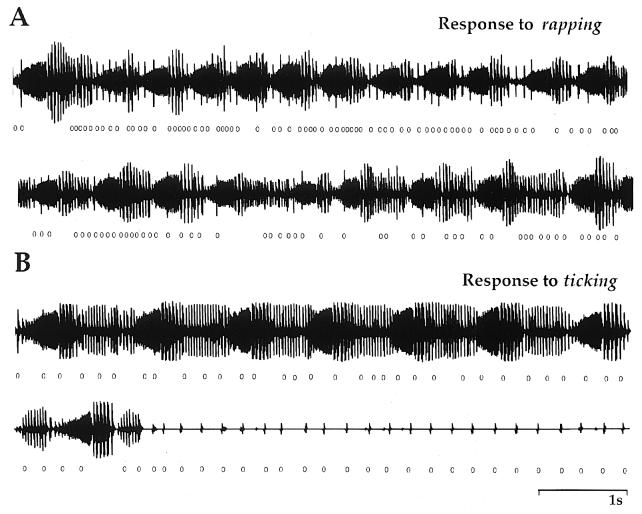
Rapping stimulates whereas ticking suppresses male calling (Fig. 3). During intense bouts of rapping (Fig. 3A, upper trace), the male responds with answer calls. If rapping slows or ceases, the male continues to call but reverts to advertisement calling (Fig. 3A, lower trace). In contrast, the kind of call the male produces is less affected by ticking (Fig. 3B); instead of stimulating, ticking inhibits male calling. Bouts of ticking continue after the male is silent (Fig. 3B, lower trace). The mean time males spent calling in response to a single bout of rapping was significantly longer (564 ± 775 s; n = 8 males) than the mean time spent calling in response to a bout of ticking (53 ± 43 s; n = 3 males; P < 0.01; Mann–Whitney U test). Because females tick for longer durations than they rap (45.4 ± 38.1 s vs. 0.5 ± 0.3 s; P = 0.02; Mann–Whitney U test), the mean ratio of calling in a receptive male–receptive female pair is 16:1 and in a receptive male–unreceptive female pair is 1:10 (P < 0.01; Mann–Whitney U test; n = 8 receptive and 3 unreceptive pairs).
Figure 3.
Male vocal responses to rapping and to ticking. (A) Oscillographs of rapping and the male’s response. At the onset of rapping (○), the male switches to the answer call (upper trace). Rapid bouts of rapping maintain answer calling. When raps decrease in frequency (lower trace), the male reverts to advertisement calling. (B) Oscillographs of ticking and the male’s response. The male continues advertisement calling during the first several seconds of ticking (○) but then falls silent (lower trace). The female continues ticking after male calling stops.
Playback experiments in the fiberglass tank also indicate that male calling is prolonged in response to tapes of rapping (1,414 ± 569 s; n = 5) compared with tapes of ticking (554 ± 397 s; n = 4; P < 0.05; Mann–Whitney U test). The suppression of male calling induced by ticking is less than that by a ticking female perhaps because the recording does not entirely reproduce a partner: the male is not clasping the female and ticking bout durations are not contingent on his behavior.
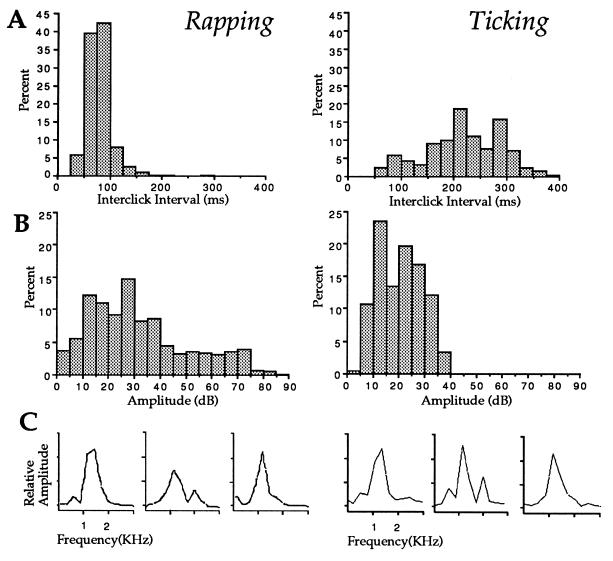
We next compared the acoustic characteristics of rapping and ticking in recordings from the artificial pond. Rapping was significantly faster than ticking (Fig. 4A; mean interclick interval, 80 ± 14 vs. 229 ± 14 ms; P = 0.01; Mann–Whitney U test). When raps are produced by the female calling alone, they can be low in amplitude; when produced during a duet (see Fig. 1 A and C) raps can be high amplitude, up to 86 dB at 1 V/μPa, levels equivalent to male clicks (7). Ticking, in contrast, is a low-amplitude call, less than 39 dB at 1 V/μPa. Thus, the frequency histogram for amplitude is considerably broader for raps than ticks (Fig. 4B). The spectral properties of raps and ticks do not differ; the mean peak frequency is 1.2 kHz for both (P = 0.8; Mann–Whitney U test). The acoustic cues that are available to males for distinguishing ticking from rapping thus include interclick interval and bout duration.
Figure 4.
Acoustic features of rapping and ticking. The distribution of interclick intervals (A) in milliseconds and amplitudes (B) in decibels (dB at 1 V/μPa) for each call are illustrated for 804 raps from eight females (96–109 samples per animal) and 208 ticks from three females (99–236 samples per animal). (C) Fast Fourier transform analyses of three representative clicks (one per animal) from rapping and ticking; frequency is 0–3 kHz for each graph and relative amplitudes (y axis) are measured in millivolts. Recordings were obtained in the artificial pond.
DISCUSSION
Female Fertility Advertisement.
Xenopus laevis is widely used in laboratory studies and its reproductive biology, including vocal behaviors, has been extensively studied in that setting (11). The typical habitat of the species is turbid freshwater ponds, and for this reason there are few field observations of reproductive activities, apart from recordings of vocal behaviors (16). Analogy to terrestrial frogs suggests that females locate males by using phonotaxis; the sexually receptive male attempts to clasp any nearby animal, distinguishing receptive females by their girth and silence and unreceptive females, or another male, by the release call (ticking). Phonotaxis to tapes of male calling by some receptive females (10–34%) can be observed in the laboratory (8, 12) but has not been observed in the wild (17). We show herein that receptive females actively signal as they approach a calling male. This female vocalization, rapping, may promote reproduction by unambiguously conveying receptive state and location.
The costs of advertisement, for both sexes, include energy expenditure, the undesirable attentions of predators, and intrasexual competition. The benefits of advertisement include the desirable attentions of a sexually receptive conspecific. That males usually advertise and females do not has been ascribed to greater investment by females in gametes and offspring and resultant male–male competition (2, 3). In midwife toads of the genus Alytes, the male contributes to parental care by wrapping the eggs around his thighs and incubating them until they hatch (18, 19). The occurrence of female calls in this species is thus understandably interpreted in light of the sex role “reversal” of parental care and a female-biased operational sex ratio (OSR, proportion of each sex available for reproduction). In another frog in which females initiate courtship, Rana blythi, male contribution to parental care (gravel nests) is also present and the OSR is also female-biased (20). In Xenopus no evidence of parental care is apparent from extensive laboratory studies and the OSR is more likely to be male than female biased (as judged from the higher proportion of receptive males in the ponds and the observation that all these males attempt to clasp females when transported to the laboratory). What Xenopus and Alytes share is marked difficulty in locating mates; in the Marjorcan species Alytes muletensis, adults live in dispersed cracks on cliff faces (13). This common function for female signaling may underlie its expression in both of these rare cases.
Female advertisement calls have also been described in the carpenter frog Rana virgatipes (21). This mating system is similar to Xenopus in that females display positive phonotaxis to a calling male and then produce a call (chirp) that attracts the male and elicits an answer call. However, the vocal advertisement system in R. virgatipes is distinct from Xenopus in that both sexes produce chirps upon entering the advertising male’s territory, whereas rapping in Xenopus is only given by females. Also the male R. virgatipes response is an aggressive call, whereas male–female interactions in Xenopus lack antagonistic qualities. Male R. virgatipes only answer the female call when it is accompanied by water displacement, whereas rapping alone, either played through a speaker or produced by a live female behind a barrier, elicits the male response in Xenopus.
Some female birds (22, 23) also produce receptive or fertility advertisement calls. Proposed functions for these calls are to incite male–male competition, to compete with other females, or to improve mate recognition or localization. A role in male–male competition is suggested by the observation that female advertisement is more common in birds with multimale mating systems (22). If rapping in Xenopus laevis incites male–male competition, it might evoke approaches of nonvocalizing males and/or male–male aggressive encounters. In alpine accentors, a polygynandrous bird in which females have overlapping periods of fertility, advertisement calls are used by females to compete for male parental care (23). However, absence of both parental care and long-term pair bonds in Xenopus together with the likelihood that females oviposit asynchronously (long breeding season, rare receptive females, and a short period of receptivity) argue against a role for rapping in female–female competition.
Given that advertisement is generally rare in females, what accounts for rapping in Xenopus laevis? The most likely function for rapping is to aid sexually active individuals in recognition and localization. We show herein that male advertisement calling induces approach by the female when oviposition is imminent. However, proximity alone may not be sufficient to actually locate a mate in crowded dark ponds. Once ovulated, eggs in female Xenopus laevis are oviposited as soon as they complete transit through the oviducts (11). The urgency of oviposition and difficulties in mate location may have shifted part of the task of mate location from the male to the female in Xenopus laevis; the result is a powerful intense call produced only by sexually receptive females.
Duetting in Xenopus.
Another unusual feature of Xenopus vocal interactions are male–female duets. Antiphonal calling is common in male frogs (5) but male–female duets are very rare (rapping in Xenopus and calling in Alytes muletensis; ref. 20). Male–female duets have, however, been described in more than 200 species of birds (15). Suggested functions for duets are the formation and maintenance of pair bonds, joint territory defense, and acoustic mate guarding. A recent study of duets in bay wrens (24, 25) provides a useful framework for considering possible functions of duetting in Xenopus. Mate removal and playback studies reveal that in Thryothorus nigrocapillus female songs are frequent and unrelated to pairing status, males (especially when unpaired) are attracted to the female song and females initiate male–female duets. Levin (24, 25) suggests that the primary function for female song is intrasexual aggression (territory defense). Female bay wrens may not be duetting at all but merely singing to keep other females away and it is the male response (a form of mate guarding) that forms the duet in this species.
In Xenopus laevis the primary function for the female song rapping is not intrasexual aggression but rather intersexual attraction. Females approach calling males and initiate duets by rapping; males are attracted to and stimulated by this call. We suggest that rapping assists in mate localization and evokes copulatory behaviors from the male. The duet that results from the male’s response to rapping may indicate to the female that he is the male she was attracted to; his answer call may discourage accepting clasps from silent males and ensure the singer’s success. Rapping always elicits a male answer and is potent enough that one bout will turn an unenthusiastic sporadic male caller into a robust persistent caller.
Amplitude Modulation of Male Calls.
A characteristic feature of Xenopus male calls is amplitude modulation of the rapid trill; the initial clicks are low amplitude and loudness increases progressively throughout the trill. Amplitude modulation relies on a sexually dimorphic feature of the laryngeal neuromuscular synapse: males have weaker synapses than females as a result of less transmitter release from the motor terminal (26, 27). Males use facilitation of the weak laryngeal synapse to progressively increase the number of muscle fibers contracting during nerve activity (28); facilitation is accompanied by amplitude modulation during the fast trill of the male call. Amplitude modulation is significantly increased in the answer call. Why is amplitude modulation such a distinctive feature of the male’s call? One possibility is that amplitude modulation helps to distinguish male and female calls during a duet because rapping can be as loud and as fast as the male call.
Conclusion.
In Xenopus females can produce both an unreceptive call and a receptive call; other female frogs typically produce only a release, or unreceptive, call (5). The type of call produced is tied to the female’s reproductive state; the nonovulating sexually unreceptive female ticks (9) and the gonadotropin injected sexually receptive female raps. It will be of interest now to determine which properties of the larynx are altered during the switch between ticking and rapping. The discovery of rapping provides a rich arena in which to examine the cellular control of vocal behaviors.
Acknowledgments
We particularly thank Mike Picker and the Department of Zoology, University of Cape Town, for scientific hospitality to M.L.T. and D.B.K. We thank T. Poulson for assistance in field experiments and A. Bass for the loan of a hydrophone. R. Bockman, S. Bottjer, J. Bradbury, S. Firestein, P. Marler, D. Reese, M. Ryan, J. Thornton, and A. Yamaguchi made helpful comments on the manuscript. Research in the authors’ laboratory is supported by a grant (NS23684) from the National Institutes of Health.
References
- 1.Trivers R L. In: Sexual Selection and the Descent of Man. Campbell B, editor. Chicago, IL: Aldine; 1972. [Google Scholar]
- 2.Darwin C. The Descent of Man and Selection in Relation to Sex. London: Murray; 1871. [Google Scholar]
- 3.Andersson M. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 4.Tinsley R, Kobel H, editors. The Biology of Xenopus. Oxford: Oxford Univ. Press; 1996. [Google Scholar]
- 5.Wells K. Anim Behav. 1977;25:666–693. [Google Scholar]
- 6.Russell W. Behavior. 1954;7:113–188. [Google Scholar]
- 7.Wetzel D, Kelley D B. Horm Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]
- 8.Picker M D. Behavior. 1983;86:74–90. [Google Scholar]
- 9.Weintraub A, Kelley D, Bockman R. Horm Behav. 1985;19:386–399. doi: 10.1016/0018-506x(85)90036-4. [DOI] [PubMed] [Google Scholar]
- 10.Hannigan P, Kelley D. J Comp Physiol A. 1986;158:17–528. doi: 10.1007/BF00603797. [DOI] [PubMed] [Google Scholar]
- 11.Kelley D B. In: The Biology of Xenopus. Tinsley R, Kobel H, editors. Oxford: Oxford Univ. Press; 1996. pp. 143–176. [Google Scholar]
- 12.Picker M. S Afr J Zool. 1980;15:150–158. [Google Scholar]
- 13.Bush S L. J Herpetol. 1997;31:251–257. [Google Scholar]
- 14.Yager D. Zool J Linn Soc. 1992;104:351–375. [Google Scholar]
- 15.Farabaugh S. In: Acoustic Communication in Birds. Kroodsma D, editor. New York: Academic; 1982. pp. 85–124. [Google Scholar]
- 16.Vigny C. J Zool (London) 1979;188:103–122. [Google Scholar]
- 17.Elepfant A, Ringeis A, Fischer W. In: Nervous Systems and Behaviors. Burrows M, Matheson T, Newland P, Schuppe H, editors. Stuttgart: Thieme; 1995. p. 343. (abstr.). [Google Scholar]
- 18.Heinzmann U. Oecologia. 1970;5:19–55. doi: 10.1007/BF00345974. [DOI] [PubMed] [Google Scholar]
- 19.Marquez R, Verrell P. J Zool (London) 1991;225:125–139. [Google Scholar]
- 20.Emerson S B. Copeia. 1992;1992:1123–1127. [Google Scholar]
- 21.Given M. Anim Behav. 1993;46:1139–1149. [Google Scholar]
- 22.Montgomerie R, Thornhill R. Ethology. 1989;81:209–220. [Google Scholar]
- 23.Langmore N, Davies N, Hatchwell B, Hartley I. Proc R Soc Lond B. 1996;263:141–146. [Google Scholar]
- 24.Levin R. Anim Behav. 1996;52:1093–1106. [Google Scholar]
- 25.Levin R. Anim Behav. 1996;52:1107–1117. [Google Scholar]
- 26.Tobias M, Kelley D B, Ellisman M. J Neurosci. 1995;15:1660–1668. doi: 10.1523/JNEUROSCI.15-03-01660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobias M, Kelley D B. J Neurosci. 1987;7:3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobias M, Kelley D B. J Neurosci. 1988;8:2422–2429. doi: 10.1523/JNEUROSCI.08-07-02422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]