Abstract
Purpose
To define the role of molecular interaction between the UV-induced JNK (c-Jun N-terminal kinase) cascade and corneal epithelial cell apoptosis and protection against apoptosis by caffeine.
Methods
Rabbit and human corneal epithelial cells were cultured in DMEM/F12 medium containing 10% FBS and 5 μg/mL insulin at 37°C in 5% CO2. DNA fragmentation and ethidium bromide/acridine orange (EB/AO) nuclear staining were performed to detect cell death. Western blot, immunoprecipitation, and kinase assays were used to measure UV-induced mitogen-activated protein (MAP) kinase activity.
Results
UV irradiation–induced apoptosis through apoptosis signal-regulating kinase 1 (ASK1) and MAKK4 (SEK1) upstream from JNK was caffeine sensitive. Caffeine (1,3,7-trimethylxanthine), an agent that is one of the most popular additions to food consumed in the world and a potential enhancer of chemotherapy, effectively protected corneal epithelial cells against apoptosis by its specific effect on the JNK cascade. Theophylline (1,3-dimethylxanthine) exhibited an effect similar to that of caffeine on prevention of UV irradiation–induced apoptosis. However, alterations of either intracellular cAMP or Ca2+ levels did not alter the effect of caffeine on the JNK signaling pathway. In addition, the blockade of PI3K-like kinases by wortmannin had no impact on the protective effect of caffeine against UV irradiation–induced apoptosis, suggesting that the protective effect of caffeine acts through a specific mechanism involving UV irradiation–induced activation of ASK1 and SEK1. In contrast, caffeine had no effects on melphalan-, hyperosmotic stress–, or IL-1 β-induced activation of the JNK signaling pathway in these cells.
Conclusions
UV irradiation stress–induced activation of the ASK1-SEK1-JNK signaling pathway leading to apoptosis is a caffeine-sensitive process, and caffeine, as a multifunctional agent in cells, can specifically interact with the pathway to protect against apoptosis.
Exposure of mammalian cells to UV irradiation causes programmed cell death (apoptosis) and cancer. For example, intensities of UV-C and -B irradiation sufficient to induce corneal epithelial cell death are on the order of microjoules per square centimeter and millijoules per square centimeter, respectively. These intensities of UV light are comparable to that which people are exposed to on a daily basis.1–3 In response to UV irradiation, mammalian cells initiate a series of intracellular signaling cascades by activating a variety of protein kinases. UV irradiation–induced activation of signal transduction shares common signaling pathways that are induced by cytokines/stress stimulation and that are related to programmed cell death. The complex cellular events triggered by UV irradiation are initiated in the membrane and include activation of non–receptor tyrosine kinase, G proteins, transcription factors, and ligand-independent membrane receptors, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), TNFα, and Fas ligand.4–6 In addition, these cytokine receptors can be activated by hypertonic stress.7 The effect of UV irradiation on activation of growth factors and other cytokine receptors in the cell membrane has been verified further by microarray experiments.8 Several signaling components have been identified in response to UV-induced cytokine receptor activation, including Ras-Raf, Src, and the caspases.9–12 UV irradiation induces clustering and phosphorylation of membrane receptors and subsequently activates mitogen activated protein (MAP) kinase cascades, leading to programmed cell death in various cell types.13–18 More important, portions of the MAP kinase signaling pathway, including the c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) and p38 signaling pathways, mediate UV irradiation–induced apoptosis in various cell types, including corneal epithelial cells.17,19–22
Recent studies indicate that stimulation of K+ channel activity causes the rapid loss of intracellular K+, which results in the activation of the caspase cascade.18,23–27 The effect of the rapid loss of intracellular K+ on caspase activation suggests that crosstalk occurs between activation of the K+ channel and intracellular signaling pathways. We found that UV irradiation–induced activation of caspase 1 (ICE) and JNK-1 occur subsequent to the stimulation of K+ channel activity and the loss of intracellular K+ in corneal epithelial cells.17 This finding is consistent with the report that the activation of caspase 1 can affect upstream events in the JNK pathway at the JNK level.28,29 Activation of the JNK signaling pathway by UV irradiation eventually results in apoptosis. UV irradiation–induced JNK-1 activation is markedly increased after ~60 minutes, and JNK-1 activation is initially observed within 5 to 15 minutes after the end of the UV-exposure period.17
Caffeine, a natural component of coffee and tea, is used in medicine for respiratory stimulation, relief of headache, suppression of the appetite, among other uses. It also is one of the world’s most popular additives to food products. Caffeine plays multiple roles in the regulation of cell function. It is a blocker of adenosine 3,5-cyclic monophosphate (cAMP) phosphodiesterase, resulting in an increase in concentration of intracellular cAMP.30 It promotes the release of Ca2+ from intracellular stores, thus increasing cytosolic Ca2+.31 It acts as an adenosine receptor antagonist to induce dopamine release from neuronal cells in the central nervous system.32,33 It has been shown that caffeine is a direct inhibitor of members of PI3K-like kinase family, such as ATM (ataxia telangiectasia mutated kinase, ATR (ATM and Rad3-related kinase), and DNA-PK (DNA-dependent protein kinase). Suppression of ATM and ATR by caffeine affects regulation of the cell cycle checkpoint, signal transduction of DNA repair, and apoptosis.34–37 However, the role of caffeine in cell survival is still not fully understood and is rather controversial because of its multiple cellular effects.
In previous studies, we found that suppression of K+ channel hyperactivity as a early response to UV irradiation (at a dosage of 42 μJ/cm2) with specific channel blockers prevents p53 phosphorylation in corneal epithelial cells. K+ channel blockers have no inhibitory effect on UV irradiation–induced p53 phosphorylation in the later response induced by continuous exposure of cells to UV irradiation.38 However, caffeine blocks both early and later phosphorylation of p53 induced by UV irradiation, indicating that there is an involvement of UV irradiation–induced DNA damage distinguishable in the effect of UV irradiation–induced activation of the membrane Kv channel-linked signaling pathway. It has been suggested that the sensors in response to DNA damage resulting in phosphorylation of p53 involve ATM and ATR.39,40 Caffeine is able to block DNA damage-induced ATM and ATR responses to inhibit subsequent phosphorylation of p53.34,41 Thus, suppression of the DNA damage–related signaling pathway by caffeine can effectively prevent UV irradiation–induced phosphorylation of p53. In the present study, we report that the action of caffeine to suppress the early cellular response induced by UV irradiation is the result of an interaction of upstream events in UV irradiation–induced activation of the JNK signaling pathway and prevents apoptosis of corneal epithelial cells. The mechanism involving cell protection by caffeine acts through the inhibition of UV-induced ASK1-SEK-JNK activation.
Materials and Methods
Culture of Corneal Epithelial Cells
A human corneal epithelial (HCE) cell line was used in the study. The cells were grown in DMEM/F-12 culture medium containing 10% fetal bovine serum and 5 μg/mL insulin and maintained in an incubator supplied with 95% air and 5% CO2 at 37°C. The culture medium was replaced every 2 days. HCE cells were passaged by treating cells with 0.05% trypsin-EDTA.
Apoptosis Induction
For UV irradiation experiments, confluent cells or corneas were placed in a tissue culture hood at a distance of 60 cm from the UV-C light source and exposed at an intensity of 42 μJ/m2. For exposure of cells to melphalan (an antitumor drug), a stock solution of 100 mg/mL was added to the culture medium at a final concentration of 100 μg/mL. After melphalan and UV treatments, cells were incubated at 37°C in 5% CO2 for 15 to 24 hours followed by measurements of cell viability and DNA fragmentation.
Measurements of Cell Death with DNA Fragmentation and Nuclear Staining
HCE cells were washed twice with PBS and resuspended in lysis buffer (200 mM Tris-HCl [pH 8.0] 100 mM EDTA, 1% SDS, and 100 μg/mL proteinase-K). Cell mixtures were incubated at 55°C for 4 hours. Lysates were extracted with an equal volume of phenol (pH 8.0) and then extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1). Nuclear DNA was precipitated with 0.3 M NaCl and 2 volumes of absolute ethanol and stored at −20°C overnight. After centrifugation at 13,000g at 4°C for 10 minutes, the DNA pellet was dried and dissolved in TE buffer (10 mM Tris-HCl [pH 7.5] and 1 mM EDTA) containing 20 μg/mL RNase A and incubated at 37°C for 1 hour. DNA samples were analyzed by electrophoresis on 1.5% agarose gels, and the results were visualized by staining with 1 μg/mL ethidium bromide for 30 minutes.
For nuclear staining, HCE cells were stained with 8 μg/mL ethidium bromide and acridine orange (EB/AO), to determine cell viability and apoptosis by detecting nuclear DNA condensation. Cell populations were scored according to their staining color and intensity by using a UV-fluorescence microscope (Nikon, Tokyo, Japan). HCE cells with green nuclear staining were deemed to be viable with membrane integrity. In contrast, cells with orange nuclear staining were judged to be apoptotic, distinguishable because of the presence of chromatin condensation and DNA fragmentation.
Immunoprecipitation and Kinase Assay
Cell lysates were incubated on ice for 10 minutes and then were precleared by centrifugation at 13,000g for 10 minutes. JNK-1 and ASK1 proteins were immunoprecipitated with 0.5 μg of rabbit polyclonal antibody against JNK-1 or ASK1 and protein A-Sepharose beads. The immunocomplex was washed three times with lysis buffer and twice with kinase buffer (20 mM HEPES [pH 7.6], 20 mM MgCl2, 25 mM μ-glycerophosphate, 100 mM sodium orthovanadate, and 2 mM dithiothreitol [DTT]) and resuspended in 50 μL of kinase buffer. To determine JNK activity, 0.5 μg of GST-ATF-2 (CST, MA) was added to 30 μL of the immunocomplex. The kinase reaction was initiated by adding 2 μL of an adenosine triphosphate (ATP) mixture (20 μM ATP and 10 μCi γ-P32-ATP; GE Healthcare, Piscataway, NJ). For ASK1 activity, 1.5 μg of myelin basic fusion protein (MBP; Santa Cruz Biotechnology, Santa Cruz, CA) was added to 30 μL of the immunocomplex. The kinase reaction was initiated by adding 1 μL of γ-P32-ATP (10 μCi). The reaction proceeded at room temperature for 10 minutes before it was terminated by adding 30 μL of 2× Laemmli buffer. Phosphorylation of ATF-2 or MBP was visualized by autoradiography after SDS-polyacrylamide gel electrophoresis. Phosphorylation of ATF-2 and MBP was quantified by densitometry.
Western Blot Analysis
The cells (2 × 105) were rinsed twice with ice-cold PBS and solubilized in SDS-polyacrylamide sample buffer containing 62.5 mM Tris-HCl (pH 6.8), 2% wt/vol SDS, 10% glycerol, 50 mM DTT, and 0.01% wt/vol bromophenol blue or phenol red. The resultant suspensions were denatured by boiling for 5 minutes. After fractionation of cell lysates by with 12% polyacrylamide gel (PAGE), proteins were electrotransferred to polyvinylidene difluoride (PVDF) membranes. Membranes were exposed to blocking buffer containing 5% nonfat milk in TBS-0.1% Tween 20 (TBS-T) for 1 hour at room temperature (RT, 21–23°C), and then incubated overnight with antibodies of interest at 4°C or for 1 hour. All antibodies used in the study were obtained from Santa Cruz Biotechnology. After three washes with TBS-T buffer, the membranes were incubated with alkaline phosphatase (AP)–linked secondary antibody for 1 hour at room temperature, and the proteins were detected (Phototope-Star Western Blot Detection kit; Cell Signaling Technology, Beverly, MA).
Results
Protective Effect of Caffeine against UV-Induced Cell Death
The effect of UV-induced apoptosis has been well defined. To investigate whether caffeine protects against UV irradiation–induced apoptosis in corneal epithelial cells, we treated HCE cells with caffeine in a range of concentrations. HCE cells were exposed to UV irradiation (42 μJ/cm2) in the absence and presence of caffeine. After additional incubation of cells for 8 to 12 hours, the cells were subjected to apoptotic assays by detecting nuclear condensation and DNA fragmentation. Nuclear staining with EB/AO revealed that UV irradiation–induced apoptosis was markedly blocked by caffeine at concentrations of 1 to 20 mM (Fig. 1A). UV irradiation–induced apoptosis detected with a DNA fragmentation assay was also diminished by the addition of caffeine to the cell culture 20 minutes before UV exposure (Fig. 1B). Control experiments showed that caffeine itself did not affect HCE cell viability. These results indicate that caffeine is a strong inhibitor of UV irradiation–induced apoptosis in these cells.
Figure 1.
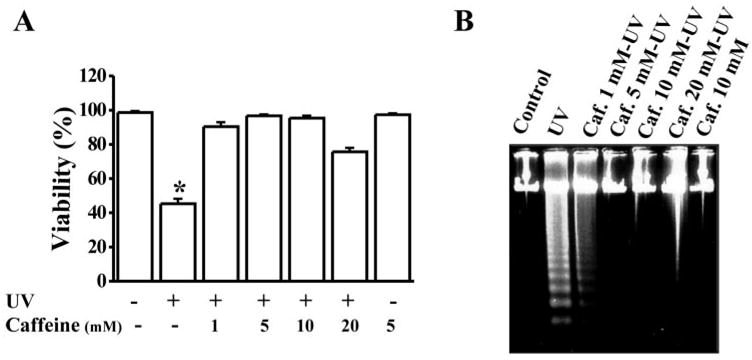
Protection of corneal epithelial cells from UV irradiation–induced apoptosis by caffeine. (A) Viability of HCE cells treated with UV irradiation in the absence or presence of caffeine. Cell viability was examined 16 hours after UV irradiation by using trypan blue exclusion. (B) Effect of caffeine on UV-induced DNA fragmentation. HCE cells were incubated with caffeine for 30 minutes at the concentration shown and then exposed to UV irradiation (42 μJ/cm2). DNA fragmentation was detected in HCE cells 8 hours after UV exposure. Statistical significance was determined at a CI level of P < 0.05 (n = 6).
Inhibition of UV-Induced JNK Activation by Caffeine
In a previous report, we found that UV irradiation–induced apoptosis in corneal epithelial cell occurs through activation of the JNK cascade.17 It is a logical extension for us to investigate whether UV irradiation–induced JNK activation can be blocked by caffeine that protects corneal epithelial cells from apoptosis. HCE cells were exposed to UV irradiation in the absence and presence of different concentrations of caffeine. JNK activities were determined by Western blot analysis and an immunocomplex kinase assay. Western blot showed that UV irradiation elicited JNK phosphorylation in 5 minutes and that caffeine markedly blocked UV irradiation–induced JNK phosphorylation in a dose-dependent fashion (Figs. 2A, 2B). UV irradiation–induced JNK kinase activity was determined by an immunocomplex kinase assay, and the hypophosphorylated fusion protein ATF was used as the substrate (Fig. 2C). Caffeine (1–10 mM) was applied to corneal epithelial cells and UV irradiation–induced JNK kinase activity was completely suppressed by 5 mM caffeine (Fig. 2D). These results demonstrate for the first time that caffeine prevents UV irradiation–induced JNK activation in these cells.
Figure 2.
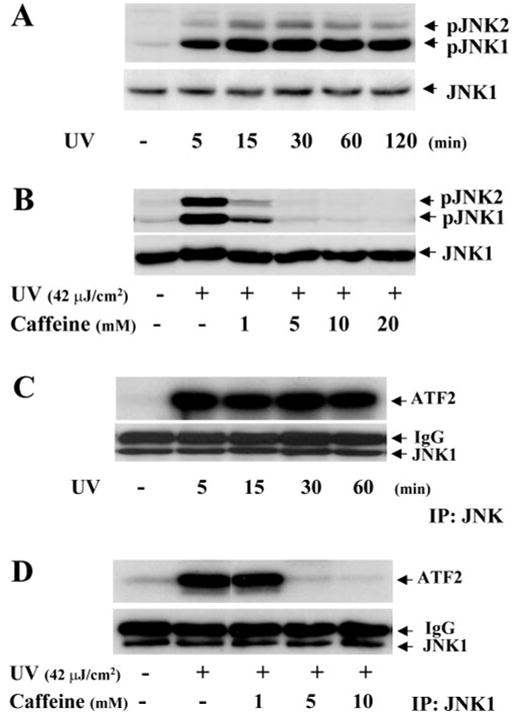
Inhibition of UV irradiation–induced JNK activation by caffeine. (A) Time course of UV irradiation–induced JNK phosphorylation. (B) Inhibition of UV irradiation–induced JNK phosphorylation by caffeine in a dose-dependent pattern. (C) Time course of UV irradiation–induced kinase activity of JNK. (D) Dose-dependent suppression of UV irradiation–induced kinase activity of JNK. HCE cells were lysed and JNK phosphorylation and kinase activities were detected by Western blot using specific anti-phospho-JNK antibodies or by immunocomplex kinase assay using the fusion protein ATF-2 as a substrate.
Effect of cAMP on UV-Induced JNK Activation
It has been shown that one of the important effects of caffeine is that it can inhibit cAMP phosphodiesterase, resulting in the elevation of intracellular cAMP levels and an increase in PKA activity. To examine whether the inhibitory effect of caffeine on UV irradiation–induced JNK activity is due to cAMP–PKA activation, we manipulated intracellular cAMP levels by using cAMP analogues and antagonists to interfere with caffeine’s effect. Various dosages of the membrane-permeable cAMP analogue 8-cpt-cAMP (50 –200 μM) and the adenylcyclase activator forskolin (10 –100 μM) failed to block UV irradiation–induced JNK activation, indicating that increases in intracellular cAMP levels did not inhibit the effect of UV irradiation on JNK activation (Figs. 3A, 3B). In contrast, decreases in intracellular cAMP levels by Rp-cAMP (50 –200 μM), a membrane-permeable cAMP analogue, neither affected UV irradiation–induced JNK activation, nor inhibited the effect of caffeine on the blockade the activation (Fig. 3C). In addition, H-89, a specific inhibitor of PKA, showed no effects on UV irradiation–induced JNK activation or caffeine protection against the activation (data not shown). Our data reveal that the protective effect of caffeine against UV irradiation–induced JNK activation is not through cAMP-PKA activation in corneal epithelial cells.
Figure 3.
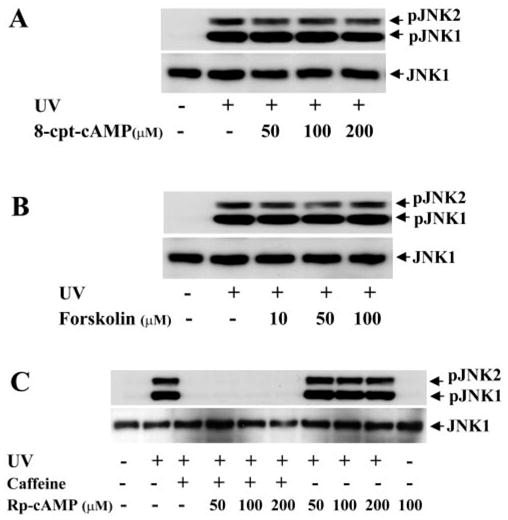
Effect of altered intracellular cAMP levels on UV irradiation–induced JNK activation. (A) Effect of 8-cpt-cAMP on UV irradiation–induced JNK phosphorylation. (B) Effect of forskolin on UV irradiation–induced JNK phosphorylation. (C) Effect of Rp-cpt-cAMP on UV irradiation–induced JNK phosphorylation. Intracellular cMAP levels were altered by exposure of HCE cells to 8-cpt-cAMP, forskolin or Rp-cpt-cAMP at indicated concentrations in the absence and presence of 5 mM caffeine 30 minutes before UV irradiation. HCE cells were harvested 15 minutes after UV irradiation for Western blot analysis.
Effect of Calcium on UV-Induced JNK Activation
It has been reported that caffeine induces intracellular Ca2+ concentration by activation of receptor-linked Ca2+-release channels.32,33 To determine whether caffeine blocks UV irradiation–induced JNK activation through a Ca2+ mechanism, different doses of ryanodine and A23187 (a Ca2+ ionophore) were applied to HCE cells, to increase the intracellular Ca2+ concentration. We found that neither ryanodine nor A23187 blocked UV irradiation–induced JNK activation (Figs. 4A, 4B). In addition, TMB-8 (10 –100 μM), a blocker of Ca2+ release from the intracellular Ca2+ store, failed to block the effect of caffeine on UV irradiation–induced JNK activation (Fig. 4C). These results do not support the notion that the inhibitory effect of caffeine on UV irradiation–induced JNK activation is due to the elevation of intracellular Ca2+ levels.
Figure 4.
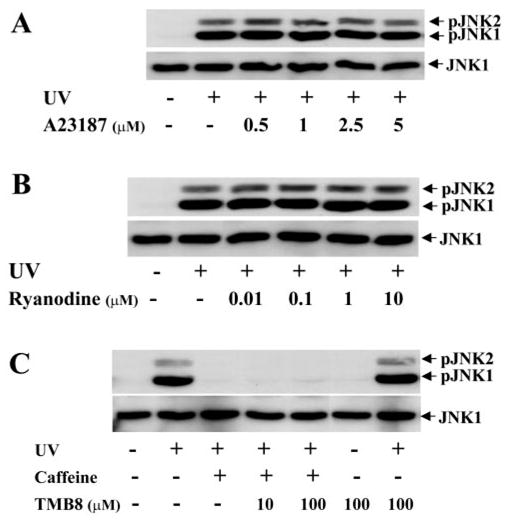
Effect of altered intracellular Ca2+ concentration on UV irradiation–induced JNK phosphorylation. (A) Effect of Ca2+ influx induced by the calcium ionophore A23187 on UV irradiation–induced JNK phosphorylation. (B) Effect of increase in intracellular Ca2+ release by ryanodine stimulation on UV irradiation–induced JNK phosphorylation. (C) Effect of reducing intracellular Ca2+ release by TMB-8 on UV irradiation–induced JNK phosphorylation. Intracellular Ca2+ levels were altered by exposure of HCE cells to A23187, ryanodine, or TMB-8 at the indicated concentrations in the absence and presence of 5 mM caffeine 30 minutes before UV irradiation. HCE cells were harvested for Western blot analysis 15 minutes after UV irradiation.
Effect of PI3K-like Kinases on UV-Induced JNK Activation
In other reports, it has been suggested that caffeine exerts its cellular effects through blocking phosphatidylinositol 3′-kinase PI3K-like kinases, such as ATM, ATR, and DNA-PK.34–37 To test whether caffeine suppresses UV irradiation–induced JNK activation through inhibition of PI3K-like kinase activities, HCE cells were treated for 30 minutes with wortmannin (a inhibitor of PI3K) before UV exposure. We found that application of wortmannin (100 –1000 μM) to UV-exposed HCE cells had no significant effect on UV-induced JNK activation or on caffeine-induced blockade of the activation (Fig. 5A). However, a significant enhancement of UV-induced cell death was observed in wortmannin-treated cells (Fig. 5B). These results indicate that PI3K-like kinases may not play a role in the protective effect of caffeine against UV irradiation–induced JNK activation. Further experiments were performed to confirm the effect of caffeine on DNA damage–induced JNK activation, since DNA damage activates JNK through PI3K-like kinases. Melphalan, an anti-tumor drug that forms DNA intrastrand crosslinks causing DNA damage, was used to activate the JNK cascade in HCE cells. Application of caffeine (5 mM) effectively suppressed UV irradiation–induced JNK activation; however, caffeine (up to 20 mM) did not inhibit melphalan-induced JNK activation (Fig. 5C). Furthermore, the application of caffeine failed to inhibit melphalan-induced cell death (Fig. 5D). These results suggest that the effect of caffeine on inhibition of JNK activity is through a specific mechanism and that activation of the JNK signaling pathway is essential for stress-induced corneal epithelial cell apoptosis.
Figure 5.

Effect of caffeine on UV irradiation– and melphalan-induced JNK phosphorylation and apoptosis. (A) Effect of caffeine in the presence of wortmannin (a inhibitor of PI3K) on UV irradiation–induced JNK phosphorylation. (B) Effect of suppressing PI3K activity with wortmannin on UV irradiation–induced HCE cell apoptosis. HCE cells were treated with caffeine in the absence and presence of wortmannin for 30 minutes before exposure of cells to UV irradiation. Western blot analysis was performed to detect JNK activation 15 minutes after UV irradiation. Cell viability was determined by using EB/AO staining 6 hours after UV exposure. (C) Effect of caffeine on melphalan-induced JNK activation. (D) Effect of caffeine on melphalan-induced HCE cell apoptosis. Melphalan (100 μg/mL) was added to culture medium in the presence or absence of caffeine, and cells were harvested for Western blot analysis 1 hour after treatment. HCE cell viability was measured by EB/AO staining 18 hours after the drug treatment. Statistical significance was determined by Student’s t-test (P < 0.001, n = 6).
Effect of Theophylline on UV-Induced JNK Activation
Theophylline is another member of the methylxanthines. Caffeine (1,3,7-trimethylxanthine) can be converted to theophylline (1,3 dimethylxanthine) in the liver. We found that theophylline had the same inhibitory effect as caffeine on UV-induced JNK activation. Theophylline was applied to cultured HCE cells 30 minutes before UV exposure. UV-induced JNK activation was markedly blocked by theophylline at concentrations of 1 to 10 mM, similar to the effect of caffeine (Fig. 6A). Theophylline also significantly protected against UV irradiation–induced apoptosis in corneal epithelial cells (Fig. 6B). The finding that theophylline prevents UV irradiation–induced JNK activation and cell apoptosis further confirms the inhibitory effect of caffeine on UV irradiation–induced JNK activation and apoptosis.
Figure 6.
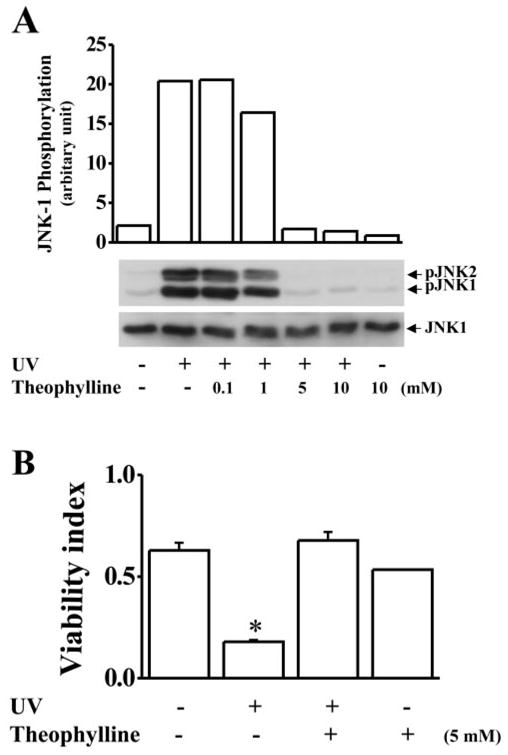
Effect of theophylline on UV irradiation–induced JNK phosphorylation and cell apoptosis. (A) Effect of theophylline (Theoph) on UV irradiation–induced JNK phosphorylation. (B) Effect of theophylline on UV irradiation–induced cell death. HCE cells were preincubated with theophylline at the indicated concentrations for 30 minutes and then exposed to UV irradiation. The cells were harvested for Western blot analysis 15 minutes after UV irradiation. Cell viability was tested by EB/AO staining 20 hours after UV exposure. Statistical significance was determined by Student’s t-test (P < 0.001, n = 6).
Effect of Caffeine on Other Stress-Induced JNK Activation
To study the specificity of caffeine’s effects on UV irradiation–induced JNK activation, JNK was activated by other stress stimuli, including IL-1β, hyperosmotic pressure, and H2O2. We found in HCE cells that hyperosmotic pressure created by the addition of 600 mM sorbitol to the culture medium induced an increase in the phosphorylation level of JNK and that treating cells with 10 nM IL-1β and 0.5 mM induced a rather H2O2 weaker JNK phosphorylation (Fig. 7). HCE cells were treated with 5 mM caffeine 30 minutes before the stress stimulation. We found that caffeine totally blocked UV irradiation–induced JNK phosphorylation, but it had no effect on hyperosmotic pressure–, IL-1β-, or H2O2-induced JNK activation (Fig. 7). These results indicate that the inhibitory effect of caffeine is only specific to UV irradiation–induced JNK activation.
Figure 7.

Effects of caffeine on hyperosmotic pressure–, IL-1β- and H2O2-induced JNK activation. HCE cells were incubated with 600 mM sorbitol (A), 10 ng/mL IL-1β (B), or 0.5 mM H2O2 (C), in the absence or presence of 5 mM caffeine (Caf ). HCE cells were harvested 15 minutes after the treatments and subjected to Western blot analysis.
Effect of Caffeine on UV-Induced ERK and p38 Phosphorylation
We have shown that in corneal epithelial cells, UV irradiation can also activate the ERK and p38 limbs. To examine the effect of caffeine on UV irradiation–induced ERK and p38 activation, caffeine and theophylline were administrated to the culture medium 30 minutes before UV irradiation. UV irradiation–activated ERK and p38 in human corneal epithelial cell were indeed blocked by caffeine and theophylline (Fig. 8A). In contrast, EGF-induced ERK activation and hyperosmotic pressure–induced p38 activation were not blocked by either caffeine or theophylline (Fig. 8). The results demonstrate that caffeine can specifically interact with upstream components in the UV irradiation–induced MAP kinase pathways resulting in protection from UV irradiation–induced apoptosis.
Figure 8.
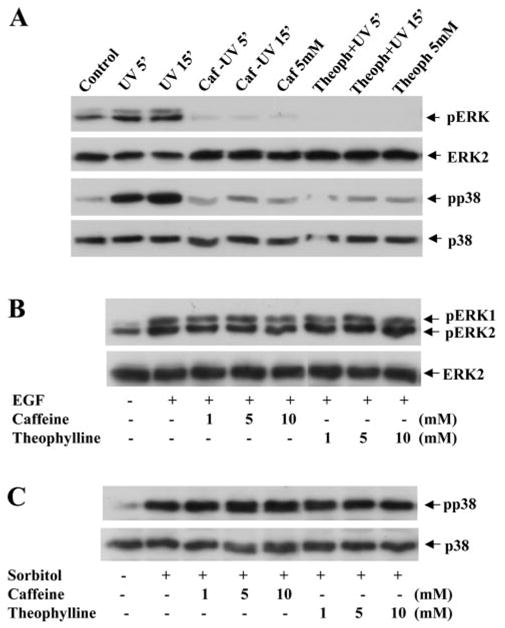
Effects of caffeine and theophylline on ERK and p38 activation induced by UV irradiation, EGF, or hyperosmotic pressure. (A) Effects of caffeine and theophylline on UV irradiation–induced ERK and p38 phosphorylations. (B) Effects of caffeine and theophylline on EGF-induced ERK phosphorylation. (C) Effect of caffeine and theophylline on sorbitol-induced p38 activation. ERK and p38 phosphorylations were measured 15 minutes after treating cells with 20 ng/mL EGF or 600 mM sorbitol in the absence or presence of 5 mM caffeine or theophylline. The lysates were analyzed by Western blot with specific antibodies against phospho-ERK or phospho-p38.
Effect of Caffeine on UV-Induced ASK1 and SEK1 Activation
ASK1 and SEK1 are the upstream components in the JNK signaling pathway. The effect of UV irradiation–induced stress on activation of ASK1 and SEK1 in corneal epithelial cells is still not completely understood. To investigate whether these upstream kinases play roles in UV irradiation–induced JNK activation, HCE cells were exposed to UV light and harvested for tests of kinase activity and protein phosphorylation status for ASK1 and SEK1. First, SEK1 phosphorylation was determined by Western blot analysis with an anti-phospho-SEK1 antibody. UV irradiation markedly increased SEK1 phosphorylation levels in corneal epithelial cells in 5 minutes (Fig. 9A). Second, ASK1 kinase activity determined by an immunocomplex kinase assay was strongly elevated in 5 minutes in response to UV irradiation (Fig. 9B). MBP was used as the substrate in the immuno-complex kinase assays. It is notable that application of caffeine (5 mM) effectively inhibited both UV irradiation–induced SEK1 phosphorylation and ASK1 kinase activities. Results of these experiments indicate that the effect of caffeine on prevention of UV irradiation–induced activation of the JNK cascade is through interaction with the upstream components in the ASK1-SEK1-JNK pathway.
Figure 9.
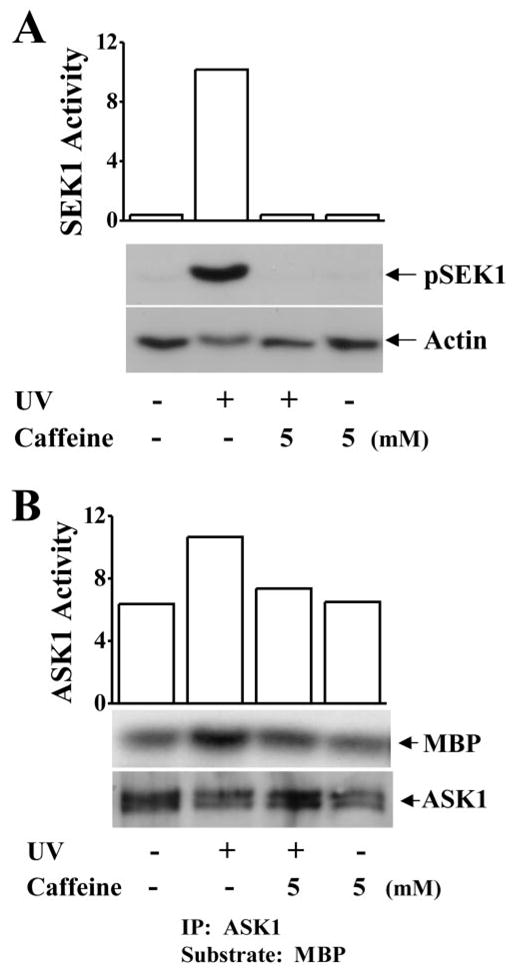
Effect of caffeine on UV irradiation–induced activation of SEK-1 and ASK1. (A) Suppression of UV irradiation–induced SEK1 phosphorylation by 5 mM caffeine. (B) Inhibition of UV irradiation–induced ASK1 kinase activation by 5 mM caffeine. SEK1 phosphorylation was determined by Western blot analysis with the anti-phospho-SEK1 antibody. ASK1 kinase activity was measured by and immunocomplex kinase assay. MBP was used as a substrate in the kinase assay.
Discussion
In various mammalian cell types, exposure of cells to UV irradiation can cause programmed cell death and cancer, depending on the intensity and wavelength of UV stimulation. The cellular effect of UV irradiation involves a variety of intra-cellular events from cell membrane to nuclei. UV irradiation affects cellular events including activation of membrane receptors in the absence of ligand binding, ion channels, nonreceptor tyrosine kinases, G proteins, MAP kinases, and some transcription factors. Caffeine has been described as an agent that can enhance the effect of radiation and chemotherapy in treating cancers because it retards cell proliferation and cell death.42,43 It has also been reported that caffeine inhibits the activation of DNA damage repair kinases, such as ATM and ATR, that leads to the failure of transition in the G2/M check-point and results in cell death. Recently, there have been a few reports suggesting that caffeine may protect against cell death in neurons in neurologic disorders.32,44 Therefore, studying the role of caffeine in the prevention of cell death is of clinical significance. We found that UV irradiation–induced programmed cell death in corneal epithelial cell is dependent on the ability to activate the JNK cascade in these cells. Caffeine prevents UV-induced cell apoptosis that was detected by DNA fragmentation and nuclear condensation staining through inhibition of UV irradiation–induced activation of the JNK cascade. We also found that caffeine can induce corneal epithelial cell apoptosis at concentrations greater than 40 mM (data not shown). We used caffeine dosages that were less than 20 mM in the present study, to demonstrate for the first time the protective effect of caffeine against UV irradiation–induced apoptosis.
In the present study, we report that caffeine specifically blocks UV irradiation–induced activation of the ASK1-SEK1-JNK signaling pathway, resulting in protection from UV irradiation–induced apoptosis in corneal epithelial cells. Because of the multiple interactions of caffeine with intracellular components, it was important for us to examine the many possible effects of caffeine on these components. We found that caffeine blocked specifically JNK activation in response to UV irradiation, but had no effect on JNK activation induced by other stimuli, including melphalan (DNA damage drug), sorbitol (hyperosmotic pressure), and IL-1β (cytokine; Figs. 5, 7). It has been shown that melphalan causes DNA damage by forming DNA intrastrand crosslinks resulting in cell death.45 However, activation of JNK by medphalan has not been reported, and the mechanism remains to be investigated. Sorbitol induces hyperosmotic stress in cells and activates MKK3/6 leading to p38 and JNK activation.4,46,47 Cytokines, such as TGF-β and IL-1β, can activate TAK1, an MAPKK, leading to activation of the JNK cascade.48,49 It is likely that different stimulators induce JNK activation through different signaling pathways. We found that caffeine suppressed only UV irradiation–induced JNK activation, indicating that caffeine is not a universal blocker of the ASK1-SEK1-JNK signaling pathway.
It has been reported that UV irradiation induces activation of EGF and PDGF receptors and subsequently activates MAPK signaling pathways including activation of the ERK, JNK, and p38 pathways.4,13,50 In addition, UV irradiation–induced up-regulation of growth factor receptors has been confirmed by microarray experiments.8 It is consistent with previous results that UV irradiation can activate MAP kinases in corneal epithelial cells similar to the effect of UV irradiation stress in other cell types. Caffeine can block UV irradiation–induced JNK, ERK, and p38 activation, but caffeine cannot prevent EGF-induced ERK activation and sorbitol-induced p38 activation (Fig. 8). These results suggest that UV irradiation–induced activation of the JNK, ERK, and p38 signaling pathways may be through different components upstream from these pathways and indicates that caffeine is able to interact with the upstream components. In fact, SEK1 is one of the kinases immediately upstream from JNK, and ASK1 can phosphorylate both MAKK3/6 and MKK1/4 (SEK1), resulting in activation of the p38 and JNK cascades.51
It is known that caffeine inhibits cAMP phosphodiesterase, resulting in accumulation of intracellular cAMP and activates ryanodine receptors to promote the release of Ca2+ from the cellular stores leading to increases in intracellular Ca2+.52–54 To investigate the mechanisms through which caffeine inhibits the UV irradiation–induced activation of JNK, experiments were designed to examine whether the effect of caffeine on the JNK cascade is related to the levels of intracellular cAMP and Ca2+ release. We found that increases in the intracellular cAMP level or increases in the intracellular Ca2+ concentration stimulated by either forskolin and 8-cpt-cAMP or ryanodine and A23187 had no effect on the interaction between caffeine and UV irradiation–induced JNK activation. In contrast, suppression of the intracellular cAMP level or intracellular Ca2+ concentration by Rp-cpt-cAMP or TMB-8 did not reverse the effect of caffeine on UV irradiation–induced JNK activation. These results provide strong evidence that intracellular cAMP and Ca2+ levels induced by caffeine do not play roles in the inhibitory effect of caffeine on the activation of the UV irradiation–induced JNK cascade.
It has also been reported that caffeine may interact with three distinct classes (I, II, and III) of phosphoinositide 3-kinases (PI3Ks) and PI3K-like kinases, such as DNA-PK, ATM, and ATR. These kinases share a similar ATP- and substrate-binding site in the catalytic core. Common blockers that target the ATP-binding site of all PI3K isoforms and interferes with PI3K-related kinase activities are wortmannin and LY294002.55,56 Caffeine has an effect similar to wortmannin on the inhibition of PI3K-like kinases, including ATM and ATR.35,37 In the present study, we found that wortmannin failed to mimic the effect of caffeine on blockage of UV irradiation–induced JNK activation. Instead, it enhanced UV irradiation–induced apoptosis in corneal epithelial cells (Fig. 5B). This finding is consistent with those in a previous report that activation of PI3K at the membrane promotes cell growth and survival and that inhibition of PI3K suppresses cell proliferation and induces apoptosis.57
Functioning as adenosine receptor antagonists, caffeine and theophylline have similar effects on the protection against UV irradiation–induced JNK activation and cell death. This action implies a new role for adenosine receptors in UV irradiation–induced cell death. Adenosine receptors belong to the G protein– coupled receptor family and activate MAP kinases.58,59 Subsequently, activation of G proteins mediates UV irradiation–induced p38 and JNK activation.59–61 However, a link between the UV irradiation–induced activation of the adenosine receptor and JNK activation has not been reported. It is very likely that the adenosine receptor mediates UV irradiation–induced JNK activation, and this area is of great interest for our further studies.
The other possible target of caffeine is interaction with ion channels in the cell membrane, including KATP, KCa, and other Kv channels. 62,63 We have reported that UV irradiation evokes hyperactivation of Kv channels in corneal epithelial and hematopoietic ML-1 cells.18,27 Suppression of Kv channel activity by specific channel blockers effectively prevents UV irradiation–induced JNK activation and apoptosis. Thus, the possibility that caffeine interacts with channels to prevent UV irradiation–induced JNK activation and cell death is another topic for future study.
Footnotes
Disclosure: L. Wang, None; L. Lu, None
Supported by National Eye Institute Grants EY12953 and EY15282 (LL).
References
- 1.Andrady AL, Hamid HS, Torikai A. Effects of climate change and UV-B on materials. Photochem Photobiol Sci. 2003;2:68–72. doi: 10.1039/b211085g. [DOI] [PubMed] [Google Scholar]
- 2.Sinha RP, Hader DP. Life under solar UV radiation in aquatic organisms. Adv Space Res. 2002;30:1547–1556. doi: 10.1016/s0273-1177(02)00370-8. [DOI] [PubMed] [Google Scholar]
- 3.Tronnier H. Hazards due to UV-light (in German) Strahlenschutz Forsch Prax. 1980;20:36–49. [PubMed] [Google Scholar]
- 4.Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 5.Bender K, Blattner C, Knebel A, Iordanov M, Herrlich P, Rahmsdorf HJ. UV-induced signal transduction (review) J Photochem Photobiol B Biology. 1997;37:1–17. doi: 10.1016/s1011-1344(96)07459-3. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz T. UV light affects cell membrane and cytoplasmic targets. J Photochem Photobiol B. 1998;44:91–96. doi: 10.1016/S1011-1344(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill LA. Interleukin-1 signal transduction. Int J Clin Lab Res. 1995;25:169–177. doi: 10.1007/BF02592694. [DOI] [PubMed] [Google Scholar]
- 8.Koch-Paiz CA, Amundson SA, Bittner ML, Meltzer PS, Fornace AJ., Jr Functional genomics of UV radiation responses in human cells. Mutat Res. 2004;549:65–78. doi: 10.1016/j.mrfmmm.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Engelberg D, Klein C, Martinetto H, Struhl K, Karin M. The UV response involving the Ras signaling pathway and AP-1 transcription factors is conserved between yeast and mammals. Cell. 1994;77:381–390. doi: 10.1016/0092-8674(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 10.Iordanov MS, Choi RJ, Ryabinina OP, Dinh TH, Bright RK, Magun BE. The UV (Ribotoxic) stress response of human keratinocytes involves the unexpected uncoupling of the Ras-extracellular signal-regulated kinase signaling cascade from the activated epidermal growth factor receptor. Mol Cell Biol. 2002;22:5380–5394. doi: 10.1128/MCB.22.15.5380-5394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitailo LA, Tibudan SS, Denning MF. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J Biol Chem. 2002;277:19346–19352. doi: 10.1074/jbc.M200401200. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Li G. ING3 promotes UV-induced apoptosis via Fas/caspase-8 pathway in melanoma cells. J Biol Chem. 2006;281:11887–11893. doi: 10.1074/jbc.M511309200. [DOI] [PubMed] [Google Scholar]
- 13.Derijard B, Hibi M, Wu IH, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 14.Bulavin DV, Saito S, Hollander MC, et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18:6845–6854. doi: 10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritz G, Kaina B. rhoB encoding a UV-inducible Ras-related small GTP-binding protein is regulated by GTPases of the Rho family and independent of JNK, ERK, and p38 MAP kinase. J Biol Chem. 1997;272:30637–30644. doi: 10.1074/jbc.272.49.30637. [DOI] [PubMed] [Google Scholar]
- 16.Seo M, Cho CH, Lee YI, et al. Cdc42-dependent mediation of UV-induced p38 activation by G protein betagamma subunits. J Biol Chem. 2004;279:17366–17375. doi: 10.1074/jbc.M312442200. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Wang L, Shell B. UV-induced signaling pathways associated with corneal epithelial cell apoptosis. Invest Ophthalmol Vis Sci. 2003;44:5102–5109. doi: 10.1167/iovs.03-0591. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Xu D, Dai W, Lu L. An ultraviolet-activated K+ channel mediates apoptosis of myeloblastic leukemia cells. J Biol Chem. 1999;274:3678–3685. doi: 10.1074/jbc.274.6.3678. [DOI] [PubMed] [Google Scholar]
- 19.Adler V, Polotskaya A, Kim J, et al. Dose rate and mode of exposure are key factors in JNK activation by UV irradiation. Carcinogenesis. 1996;17:2073–2076. doi: 10.1093/carcin/17.9.2073. [DOI] [PubMed] [Google Scholar]
- 20.Bodero AJ, Ye R, Lees-Miller SP. UV-light induces p38 MAPK-dependent phosphorylation of Bcl10. Biochem Biophys Res Commun. 2003;301:923–926. doi: 10.1016/s0006-291x(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 21.Merienne K, Jacquot S, Zeniou M, Pannetier S, Sassone-Corsi P, Hanauer A. Activation of RSK by UV-light: phosphorylation dynamics and involvement of the MAPK pathway. Oncogene. 2000;19:4221–4229. doi: 10.1038/sj.onc.1203712. [DOI] [PubMed] [Google Scholar]
- 22.Price MA, Cruzalegui FH, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate Ternary Complex Factors and c-fos transcription in response to UV light. EMBO J. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L. Stress-induced corneal epithelial apoptosis mediated by K(+) channel activation. Prog Retin Eye Res. 2006;25:151–538. doi: 10.1016/j.preteyeres.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bortner CD, Hughes FM, Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem. 1997;272:32436–32442. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- 25.Frisch SM, Vuori K, Kelaita D, Sicks S. A role for Jun-N-terminal kinase in anoikis: suppression by bcl-2 and crmA. J Cell Biol. 1996;135:1377–1382. doi: 10.1083/jcb.135.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Li T, Lu L. UV-induced corneal epithelial cell death by activation of potassium channels. Invest Ophthalmol Vis Sci. 2003;44:5095–5101. doi: 10.1167/iovs.03-0590. [DOI] [PubMed] [Google Scholar]
- 28.Kong AN, Yu R, Lei W, Mandlekar S, Tan TH, Ucker DS. Differential activation of MAPK and ICE/Ced-3 protease in chemical-induced apoptosis: the role of oxidative stress in the regulation of mitogen-activated protein kinases (MAPKs) leading to gene expression and survival or activation of caspases leading to apoptosis. Restor Neurol Neurosci. 1998;12:63–70. [PubMed] [Google Scholar]
- 29.Lei W, Yu R, Mandlekar S, Kong AN. Induction of apoptosis and activation of interleukin 1beta-converting enzyme/Ced-3 protease (caspase-3) and c-Jun NH2-terminal kinase 1 by benzo(a)pyrene. Cancer Res. 1998;58:2102–2106. [PubMed] [Google Scholar]
- 30.Kemp RG, Huang YC. Purification and characterization of cyclic nucleotide phosphodiesterase from skeletal muscle. Methods Enzymol. 1974;38:240–244. doi: 10.1016/0076-6879(74)38037-8. [DOI] [PubMed] [Google Scholar]
- 31.Islam MS, Leibiger I, Leibiger B, et al. In situ activation of the type 2 ryanodine receptor in pancreatic beta cells requires cAMP-dependent phosphorylation. Proc Natl Acad Sci USA. 1998;95:6145–6150. doi: 10.1073/pnas.95.11.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarzschild MA, Xu K, Oztas E, et al. Neuroprotection by caffeine and more specific A2A receptor antagonists in animal models of Parkinson’s disease. Neurology. 2003;61:S55–S61. doi: 10.1212/01.wnl.0000095214.53646.72. [DOI] [PubMed] [Google Scholar]
- 33.Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costanzo V, Shechter D, Lupardus PJ, Cimprich KA, Gottesman M, Gautier J. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol Cell. 2003;11:203–213. doi: 10.1016/s1097-2765(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 35.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 36.Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc Natl Acad Sci USA. 2001;98:9092–9097. doi: 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkaria JN, Busby EC, Tibbetts RS, et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 38.Wang L, Dai W, Lu L. Ultraviolet irradiation-induced K(+) channel activity involving p53 activation in corneal epithelial cells. Oncogene. 2005;24:3020–3027. doi: 10.1038/sj.onc.1208547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie S, Wang Q, Wu H, et al. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem. 2001;276:36194–36199. doi: 10.1074/jbc.M104157200. [DOI] [PubMed] [Google Scholar]
- 40.Ye R, Bodero A, Zhou BB, Khanna KK, Lavin MF, Lees-Miller SP. The plant isoflavenoid genistein activates p53 and Chk2 in an ATM-dependent manner. J Biol Chem. 2001;276:4828–4833. doi: 10.1074/jbc.M004894200. [DOI] [PubMed] [Google Scholar]
- 41.Ito K, Nakazato T, Miyakawa Y, Yamato K, Ikeda Y, Kizaki M. Caffeine induces G2/M arrest and apoptosis via a novel p53-dependent pathway in NB4 promyelocytic leukemia cells. J Cell Physiol. 2003;196:276–283. doi: 10.1002/jcp.10289. [DOI] [PubMed] [Google Scholar]
- 42.Waldren CA, Rasko I. Caffeine enhancement of X-ray killing in cultured human and rodent cells. Radiat Res. 1978;73:95–110. [PubMed] [Google Scholar]
- 43.Yao SL, Akhtar AJ, McKenna KA, et al. Selective radiosensitization of p53-deficient cells by caffeine-mediated activation of p34cdc2 kinase. Nat Med. 1996;2:1140–1143. doi: 10.1038/nm1096-1140. [DOI] [PubMed] [Google Scholar]
- 44.Maia L, de Mendonca A. Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 45.Bauer GB, Povirk LF. Specificity and kinetics of interstrand and intrastrand bifunctional alkylation by nitrogen mustards at a G-G-C sequence. Nucleic Acids Res. 1997;25:1211–1218. doi: 10.1093/nar/25.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhlik MT, Abell AN, Johnson NL, et al. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 47.Tokiwa G, Dikic I, Lev S, Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- 48.Shim JH, Xiao C, Paschal AE, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takaesu G, Kishida S, Hiyama A, et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- 50.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 51.Ichijo H, Nishida E, Irie K, ten Dijke P, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 52.Pan Z, Damron D, Nieminen AL, Bhat MB, Ma J. Depletion of intracellular Ca2+ by caffeine and ryanodine induces apoptosis of chinese hamster ovary cells transfected with ryanodine receptor. J Biol Chem. 2000;275:19978–19984. doi: 10.1074/jbc.M908329199. [DOI] [PubMed] [Google Scholar]
- 53.Fredholm BB, Hjemdahl P, Hammarstrom S. Stimulation and inhibition of cyclic AMP formation in isolated rat fat cell by prostacyclin (PGI2) Biochem Pharmacol. 1980;29:661–663. doi: 10.1016/0006-2952(80)90396-2. [DOI] [PubMed] [Google Scholar]
- 54.McPherson PS, Kim YK, Valdivia H, et al. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991;7:17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- 55.Wymann MP, Marone R. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr Opin Cell Biol. 2005;17:141–149. doi: 10.1016/j.ceb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Wymann MP, Zvelebil M, Laffargue M. Phosphoinositide 3-kinase signaling: which way to target? Trends Pharmacol Sci. 2003;24:366–376. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 57.Fresno Vara JA, Casado E, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55:726–734. [PubMed] [Google Scholar]
- 59.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 60.Seo M, Lee YI, Cho CH, Bae CD, Kim IH, Juhnn YS. Bi-directional regulation of UV-induced activation of p38 kinase and c-Jun N-terminal kinase by G protein beta gamma-subunits. J Biol Chem. 2002;277:24197–24203. doi: 10.1074/jbc.M201717200. [DOI] [PubMed] [Google Scholar]
- 61.Coso OA, Teramoto H, Simonds WF, Gutkind JS. Signaling from G protein-coupled receptors to c-Jun kinase involves beta gamma subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J Biol Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- 62.Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol. 1998;30:13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- 63.Akaike N, Sadoshima J. Caffeine affects four different ionic currents in the bull-frog sympathetic neurone. J Physiol. 1989;412:221–244. doi: 10.1113/jphysiol.1989.sp017612. [DOI] [PMC free article] [PubMed] [Google Scholar]


