Abstract
Establishment of functional and stable collaterals in the ischemic myocardium is crucial to restoring cardiac function after myocardial infarction. Here, we show that only dual delivery of a combination of angiogenic and arteriogenic factors to the ischemic myocardium could significantly reestablish stable collateral networks and improve myocardial perfusion and function. A combination of FGF-2 with PDGF-BB, two factors primarily targeting endothelial cells and vascular smooth muscle cells, remarkably promotes myocardial collateral growth and stabilizes the newly formed collateral networks, which significantly restore myocardial perfusion and function. Using various members of the PDGF family together with FGF-2 in an angiogenesis assay, we demonstrate that PDGFR-α is mainly involved in angiogenic synergism, whereas PDGFR-β mediates vessel stability signals. Our findings provide conceptual guidelines for the clinical development of proangiogenic/arteriogenic factors for the treatment of ischemic heart disease.
Keywords: angiogenesis, growth factor, ischemia, myocardial infarction, neovascularization
Atherosclerosis-induced coronary artery disease is the leading cause of morbidity and mortality in Western societies and increases at an alarming rate in developing countries (1). Usually, progressive atherosclerosis results in plaque rupture and thrombosis of major coronary arteries, leading to angina, myocardial infarction, and heart failure (2). Except for surgical interventions, an effective therapeutic method for treatment has been lacking. Although delivery of proangiogenic factors to the ischemic myocardium to stimulate collaterogenesis and to improve myocardial perfusion and function is a straightforward idea proposed for >30 years, clinical evaluation of these individual proangiogenic molecules has produced unfulfilled promises (3–5). After more than a decade of clinical practice with different single proangiogenic factors for the treatment of ischemic disorders, almost all large randomized, double-blinded, and placebo-controlled human trials have proven to be nonbeneficial (6–8).
The clinical failures with this attractive approach have raised several unresolved fundamental issues regarding the basic mechanisms of cardiovascular biology. These include the underlying mechanisms of angiogenesis versus arteriogenesis, choice of proangiogenic agents, monotherapy versus combinatorial therapy, appropriate animal models for preclinical evaluation, optimal drug release systems, and time line of delivery. For improvement of perfusion of high-oxygenated blood in ischemic tissues, it is essential to reestablish functional arterial vascular networks, which should remain stable long-term. Most previous preclinical and clinical studies on the development of proangiogenic therapies for treating ischemic myocardium have been based on monotherapeutic approaches (9–20). These approaches usually lack rationales for understanding the molecular mechanisms of arteriogenesis, for defining molecular targets of the deliverable proangiogenic factors, and for selection of optimal angiogenic/arteriogenic agents. In addition, most studies have focused on evaluation of the therapeutic efficacy of vascular endothelial growth factor (VEGF) A, which primarily targets the endothelial cell compartment (5, 12, 17, 21, 22). Unlike rodents and dogs, pigs usually lack sufficient native collaterals to prevent major infarction after coronary artery occlusion (23–25). Thus, the pig myocardial infarction model is the most relevant and ideal animal system to human coronary artery disease.
We have recently shown that a combination of two non-VEGF growth factors, fibroblast growth factor 2 (FGF-2) and platelet-derived growth factor BB (PDGF-BB), induces angiogenic synergy and vessel stability in rodents (26). FGF-2 is the prototype of a structurally related growth factor family consisting of 24 known members (27–29). Although the release of the signal peptide-less FGF-2 from its synthetic cells remains enigmatic, this growth factor plays a crucial role extracellularly in the regulation of the growth of its target cells including endothelial cells. Interestingly, administration of FGF-2 in vivo selectively stimulates angiogenesis but not other tissue growth (29). In contrast to FGF-2, members of the PDGF family act mainly on vascular mural cells including pericytes and vascular smooth muscle cells (VSMC) (30–32). Whereas PDGF-AA specifically interacts with the homodimeric cell-surface tyrosine kinase receptor, PDGF receptor α (PDGFR-α), PDGF-BB interacts with both homodimeric and heterodimeric PDGFR-α and PDGFR-β complexes (30). It is known that PDGFR-β plays an essential role in the stabilization of blood vessels via the recruitment of mural cells/VSMC onto the nascent vasculature (33). Thus, PDGFs, and particularly PDGF-BB, are important arteriogenic factors for stabilization of the newly formed vasculature. PDGFR-α is also expressed in blood-vessel endothelial cells (34). The rationale of our present work is to combine arteriogenic factors, such as PDGF-BB, with angiogenic factors, such as FGF-2, for therapeutic evaluation in a pig myocardial infarction model.
Results
Dual Delivery of PDGF-BB/FGF-2 and PDGF-AB/FGF-2 but Not PDGF-AA/FGF-2 Promotes Vessel Stability.
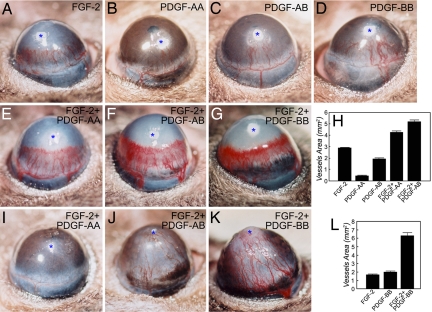
To study the role of various members of the PDGF family in a combination with FGF-2 in promoting angiogenic synergisms and the formation of stable vessels, a slow-release polymer containing PDGF-AA/FGF-2, PDGF-AB/FGF-2, or PDGF-BB/FGF-2 was implanted into the mouse cornea. Both FGF-2 and PDGFs are strong heparin-binding growth factors, which can be released by slow-release polymers with heparin-binding properties. Sucrose aluminum octosulfate possesses a strong heparin-binding affinity that binds to both factors and serves as a slow-release material. All three combinations of growth factors released by this polymeric delivery system synergistically induced angiogenesis as compared with single growth factor-induced angiogenesis (Fig. 1 A–H). These data validate our previous findings that a combination of FGF-2 with PDGF-BB synergistically induces angiogenesis (26). The avascular feature of the corneal tissue allowed us to noninvasively follow blood vessel stability long-term. At day 70 after implantation, PDGF-AA/FGF-2-induced vessels were almost completely regressed, suggesting that this combination is unable to stabilize the newly formed vasculature (Fig. 1I). Interestingly, both PDGF-AB/FGF-2- and PDGF-BB/FGF-2-induced corneal vascular networks remained stable at this and later time points (Fig. 1 J and K). It should be emphasized that the primitive nascent vasculatures induced by both combinations underwent remarkable remodeling, resulting in well defined, stable, and mature vascular networks at later time points. These findings demonstrate that only PDGF-AB/FGF-2 or PDGF-BB/FGF-2, but not PDGF-AA/FGF-2, is able to stabilize the newly formed vasculature, although all three combinations promote angiogenic synergisms. We thus chose a combination of PDGF-BB/FGF-2 for the therapeutic evaluation and further studies.
Fig. 1.
Angiogenic synergism and vessel stability promoted by various members of the PDGF family alone or in combinations with FGF-2. Micropellets containing FGF-2 (A), PDGF-AA (B), PDGF-AB (C), PDGF-BB (D), PDGF-AA/FGF-2 (E and I), PDGF-AB/FGF-2 (F and J), or PDGF-BB/FGF-2 (G and K) together with the slow-release polymer sucrose aluminum octosulfate were implanted into the corneal micropockets of mice. Corneal neovascularization was examined on days 5 (A–H) and 70 (I–K) after growth factor implantation. The corneal neovascularization was quantitatively measured as clock hours and vessel lengths, and vascularization areas were calculated (H and L). Asterisks indicate the implanted pellets.
Establishment of Stable Collateral Networks by PDGF-BB/FGF-2 in the Ischemic Myocardium.
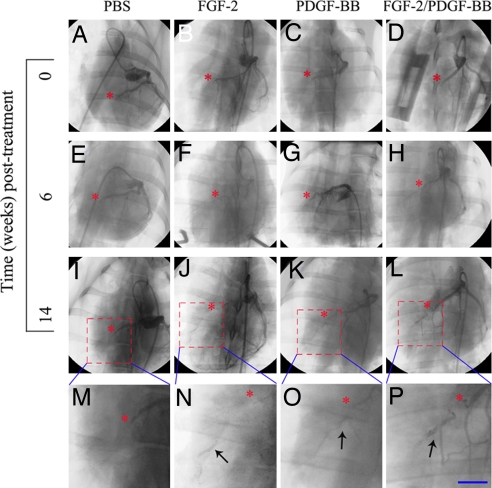
To evaluate the combinatorial therapeutic approach in a clinically relevant animal model, we developed a pig myocardial infarction model based on a modified protocol in which left anterior descending (LAD) coronary artery was ligated to induce myocardial infarction. Single or combinatorial angiogenic/arteriogenic factors were delivered to the ischemic region of the myocardium at the time of infarction by using a slow-release polymer consisting of sucrose aluminum octosulfate and hydron possessing heparin binding activity. This slow-release polymer composition was the same as that previously used in our mouse corneal micropocket assay and proven to effectively release PDGF-BB and FGF-2 (Fig. 1). Coronary angiographic analysis showed that at week 6 PDGF-BB/FGF-2 significantly induced myocardial collateral growth as compared with various individual angiogenic or arteriogenic factor-treated myocardium. These newly established collaterals formed vascular networks proximally and distally from the ligation site (Fig. 2 E–H). To study the stability of these newly established collateral networks, coronary angiography was again performed on week 14 after treatment. Remarkably, the PDGF-BB/FGF-2-induced collaterals had developed into well established coronary arterials appearing as corkscrew-shaped vessels forming vascular networks downstream from the ligation site, indicating the reestablished collaterals remained stable (Fig. 2 L and P). Although FGF-2 alone seemed to induce marginal collateral growth, PDGF-BB alone did not induce any detectable collateral growth at either time point in the ischemic myocardium as compared with those promoted by buffer alone (Fig. 2 I–K and M–O).
Fig. 2.
Angiographic analysis of ischemic myocardium treated by various factors. Angiography was performed on myocardium treated with PBS (A, E, I, and M), FGF-2 (B, F, J, and N), PDGF-BB (C, G, K, and O), or FGF-2 and PDGF-BB together (D, H, L, and P) at day 0 (A–D), week 6 (E–H), and week 14 (I–P). Asterisks indicate the LAD coronary artery ligation sites. Arrows indicate the newly established vascular networks downstream from the ligation sites. (Scale bar: 10 μm.)
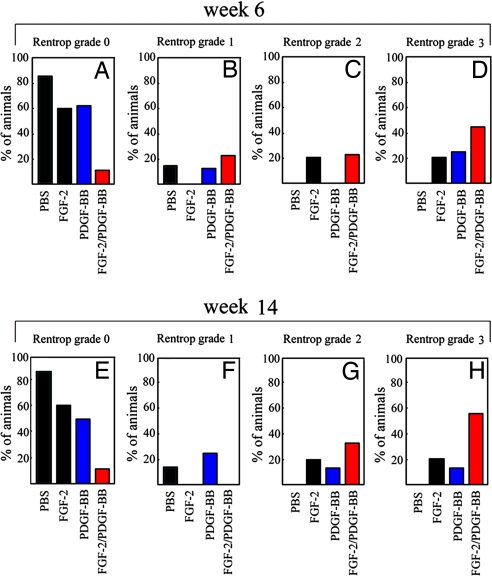
For quantitative evaluation, collateral index analysis was performed at weeks 6 and 14 according to a standard protocol using Rentrop's grading scales (35). The baseline collateral indexes in all four groups were indistinguishable. At week 6, both FGF-2- and PDGF-BB/FGF-2-treated groups exhibited remarkable increases of collaterals indexes (P = 0.018 and P = 0.001, respectively; Fig. 3 A–D) as compared with their baseline indexes. Although the PDGF-BB-treated group showed a trend toward an improvement of the collateral index, this marginal increase was statistically insignificant as compared with its baseline index (P = 0.058). Additionally, the collateral index was significantly higher in the PDGF-BB/FGF-2 together-treated group as compared with those of FGF-2- or PDGF-BB alone-treated groups (FGF-2, P = 0.037; PDGF-BB, P = 0.021) or PBS alone (P = 0.002). Long-term follow-up analysis showed that the collateral index continued to increase and remained stable in the PDGF-BB/FGF-2-treated group (Fig. 3 E–H). These data are consistent with the results obtained in our corneal angiogenesis model demonstrating that PDGF-BB and FGF-2 together promote the establishment of long-lasting blood vessels.
Fig. 3.
Collateral index. The collateral index was assessed by using the Rentrop grading scales. The Rentrop scoring is based on the following grades: 0 = no filling in any branches of the LAD coronary artery; 1 = filling of only side branches of the LAD coronary artery; 2 = partial filling of the LAD coronary artery; and 3 = complete filling of the LAD coronary artery. The collateral index was scored at weeks 6 (A–D) and 14 (E–H), and the percentages of animals per grade are presented.
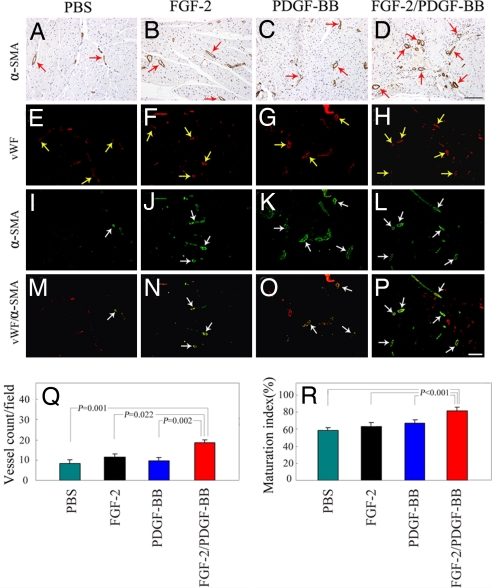
To further validate the presence of newly formed collaterals, various growth factor-treated ischemic myocardium were immunologically stained with an anti-α smooth muscle cell actin (α-SMA) antibody. Consistent with the coronary angiographic analysis, high numbers of arterial vessels were detected in the PDGF-BB/FGF-2-treated myocardium at week 14 posttreatment as compared with those of single factor-treated or control samples (Fig. 4 A–D and Q). Although PDGF-BB- or FGF-2-treated myocardium seemed to contain higher numbers of collaterals as compared with the buffer-treated group, there were no statistically significant differences between these groups (Fig. 4 A–C and Q). To determine the degree of vessel maturation and correlate association between blood vessels with VSMC, various factor-treated myocardium were examined by double immunohistochemistry staining for von Willebrand factor (vWF) and α-SMA. At week 14 after treatment, >80% of all of the myocardial vessels were α-SMA positive in the PFGF-BB/FGF-2 together-treated group, whereas significantly fewer α-SMA-positive vessels were detected in the PDGF-BB, FGF-2, or buffer alone-treated myocardium (Fig. 4 E–P and R). These results demonstrate that dual delivery of PDGF-BB/FGF-2 synergistically promotes arteriogenesis, arterial maturation, and stabilization of myocardial collateral networks.
Fig. 4.
Immunohistochemical analysis of collaterals in the ischemic myocardium. An anti-α-SMA antibody was used to stain VSMC on paraffin-embedded (A–D) and frozen (green signals, I–L) sections of myocardium treated with single or combinatorial factors. The total number of blood vessels was revealed with an anti-vWF antibody (red signals, E–H), and the vWF/α-SMA double-positive vessel structures (yellow signals) were shown by superimposing both positive signals in the same sections (M–P). (Scale bars: 100 μm.) Myocardial collaterals were quantified from 10 random fields (×20 magnification), and the maturation index was quantified as the percentages of α-SMA-positive vessels vs. the total number of vessels (Q and R). The data are presented as means of determinants (±SEM).
Improvement of Regional Myocardial Blood Flow.
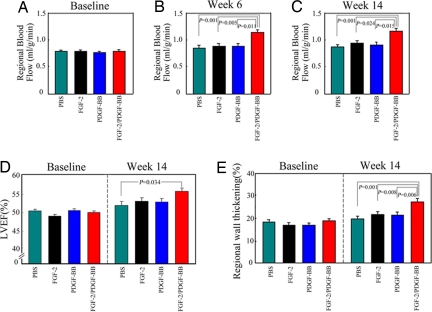
To study the improvement of cardiac function after monotherapy or combinatorial therapy, the myocardial blood flow (MBF) was assessed by a colored microsphere method. Before treatment, the basal levels of MBF in the LAD territory were indistinguishable among all groups (Fig. 5A). At week 6, there were no differences in MBF in single growth factor-treated myocardium as compared with the buffer-treated group (Fig. 5B). Notably, the PDGF-BB/FGF-2 together-treated group exhibited remarkably higher MBF than either factor alone-treated myocardium (Fig. 5B). The high level of MBF persisted long-term in the PDGF-BB/FGF-2-treated group, and no reduction of MBF was observed at the end point of the experiment (14 weeks) (Fig. 5C). Statistical analysis of these data by factorial analysis of variance showed that MBF in the PDGF-BB/FGF-2 together-treated group was markedly greater than the sum effects obtained from two factors alone-treated myocardium (F = 7.317, P = 0.011, at week 6; and F = 4.930, and P = 0.034, at week 14). These findings indicate that dual delivery of PDGF-BB/FGF-2 synergistically improves local blood flow in the ischemic myocardium, indicating that the high numbers of newly formed collateral networks are functional.
Fig. 5.
Regional blood flow and echocardiographic evaluation of global and regional cardiac functions. Regional blood flow was analyzed at day 0 (A), week 6 (B), and week 14 (C) by using a colored microsphere method, and the data are presented as mean values (±SEM) of the ischemic myocardial flow (ml/g/min). LVEF was measured to monitor the improvement of global function of the left ventricle after delivery of growth factors for 14 weeks (D). The values of regional wall thickening were obtained by using the open-chest echocardiography at day 0 and week 14 after treatments (E).
Improvement of Global and Regional Myocardial Functions.
Echocardiography was performed to monitor global and regional myocardial function. Measurement of left ventricular ejection fraction (LVEF), a standard parameter to monitor global myocardial contractile function, showed that the basal cardiac dysfunction was indistinguishable among all groups before treatment (Fig. 5D). At week 14 after treatment, a significant improvement of LVEF was observed in the PDGF-BB/FGF-2-treated group as compared with the buffer-treated group (Fig. 5D). In contrast, neither single growth factor-treated group showed significant improvements of LVEF, consistent with the MBF findings. To assess regional wall motion, left ventricular systolic wall thickening (WT%) was calculated. Similar to LVEF results, PDGF-BB and FGF-2 alone-treated groups did not show any significant improvement in WT% as compared with buffer controls. In contrast, the PDGF-BB/FGF-2-treated group significantly improved the WT% as compared with either buffer- or single factor-treated groups (Fig. 5E). These findings demonstrate that dual delivery of PDGF-BB/FGF-2 remarkably stimulates myocardial collaterogenesis, leading to a significant improvement of regional as well as global myocardial contractile functions.
Discussion
We have shown here that a protein-based angiotherapeutic approach, based on dual delivery of arteriogenic and angiogenic factors, significantly improves myocardial collateral growth, blood perfusion, and cardiac function in a pig ischemic myocardial model. Both therapeutic angiogenesis and antiangiogenic therapies have received significant attentions as they hold great promises for the treatment of the most common and severe human diseases including malignant, ophthalmological, and cardiovascular disorders. After the first FDA-approved antiangiogenesis drug, bevacizumab, several other antiangiogenic drugs targeting VEGF or its signaling components, including pegaptanib, sunitinib, and ranibizumab, have very recently been approved (36–39). The failures of clinical trials with proangiogenic factors for the treatment of ischemic diseases have left the therapeutic angiogenesis field far behind (4, 6). Although targeting single angiogenic factors such as VEGF for suppression of angiogenesis is an effective approach for therapy, the delivery of single angiogenic factors to ischemic tissues has failed to conclusively show a therapeutic benefit, suggesting that single angiogenic or arteriogenic factors are unable to accomplish the complex process of arteriogenesis (3, 4, 6, 40). Among many possible reasons for these clinical failures, the choice of optimal biological agents is a crucial issue for successful therapy.
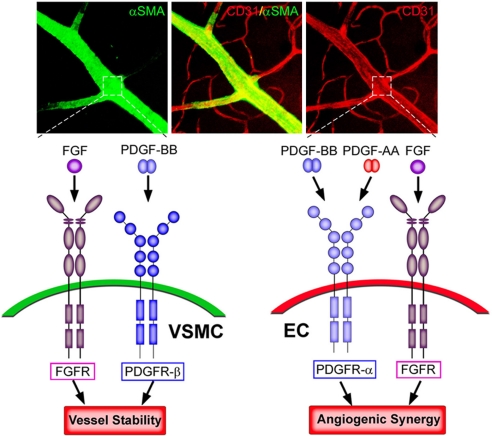
Selection of optimal angiogenic/arteriogenic molecules should consider the basic process of arteriogenesis, which builds up functional arterial networks. Unlike angiogenesis, arteriogenesis refers to the process of maturation, de novo formation and/or remodeling of collateral conduits (41). Previous studies in rodents have suggested that remodeling of preexisting vessels is pivotal to the establishment of arterial networks (42). Because arterial blood vessels are composed of both endothelial cells and VSMC, the rationale of our present study was to combine both angiogenic and arteriogenic factors, thus targeting endothelial cells and VSMC for de novo formation and remodeling of myocardial collaterals (Fig. 6). For this purpose, we have investigated various combinations of different angiogenic and arteriogenic factors for promoting angiogenesis and arteriogenesis in our in vivo animal models. Our previous studies have indicated that only the combination PDGF-BB/FGF-2, but not PDGF-BB/VEGF or FGF-2/VEGF, is able to synergistically induce angiogenesis and arteriogenesis (26). This work further suggests that the interplay between various angiogenic and arteriogenic factors for promoting arteriogenesis is specifically limited to certain combinations, and that each factor has its own specific molecular partners.
Fig. 6.
Schematic diagram of possible mechanisms of angiogenic synergisms and vessel stability promoted by various members of the PDGF family and FGF-2. Arteries consist of endothelial cells expressing specific markers such as CD31 (red) and VSMC expressing specific markers such as α-SMA (green). FGF-2 primarily acts on FGFRs expressed on endothelial cells in vivo to promote angiogenesis. Both PDGF-AA and PDGF-BB may activate PDGFR-α expressed on endothelial cells to induce angiogenesis. When both FGFRs- and PDGFR-mediated-signaling pathways are coactivated simultaneously in endothelial cells by their respective ligands, these two independent signaling systems lead to an angiogenic synergism. VSMC play a crucial role in vessel stability and maturation. PDGF-BB, but not PDGF-AA, activates PDGFR-β predominantly expressed on VSMC. FGFRs could also be expressed in VSMC and cooperatively promote vessel stability signals with the PDGFR-β. Therefore, although both PDGF-AA and PDGF-BB are able to synergistically stimulate angiogenesis together with FGF-2, only PDGF-BB, but not PDGF-AA, in the presence with FGF-2 is able to stabilize the newly formed vasculature and to function as an arteriogenic factor.
FGF-2 is one of the most potent angiogenic factors, primarily targeting vascular endothelial cells as a mitogenic and survival factor (29). FGF-2 has been reported to up-regulate the expression levels of PDGFRs in endothelial cells (26). Although PDGF-BB primarily acts on VSMC and pericytes via PDGFR-β, the PDGFR-α for this ligand is also expressed in endothelial cells, suggesting its direct role in regulation of angiogenesis (34, 43). In this regard, it is not surprising that a combination of FGF-2 with PDGF-AA promotes angiogenic synergism (Fig. 6). Unlike PDGFR-α, PDGFR-β is mainly distributed in VSMC and controls the growth and recruitment of VSMC to endothelial cells (31). Together with angiopoietin-1, PDGF-BB is considered as a vascular stabilizer (32, 34, 44). Unexpectedly and surprisingly, our present results show that PDGF-BB is unable to stabilize its own vessels, suggesting that additional factors are required for vessel stability. Our in vivo studies provide compelling evidence that PDGF-BB is required to cooperatively work with FGF-2 for vessel stability (26). These findings imply that the establishment of stable arterial networks requires a cooperative effort between angiogenic and arteriogenic factors. A simplified model of the interplay between PDGFs and FGF-2 in stimulation of arteriogenesis and vessel stability is presented in Fig. 6, although it is likely that PDGFR-β and PDGFR-α also are expressed in endothelial cells and VSMC, respectively.
The underlying cellular and molecular mechanisms of arteriogenesis might involve both de novo formation of new collaterals and remodeling of preexisting collaterals. Whereas coronary angiographic analysis clearly showed the reestablishment of occluded coronary collaterals, immunohistological analysis demonstrated increased numbers of new collaterals in the dual factor-treated group. The simultaneous events of growth and remodeling of arterial vessels require intimate cooperation between PDGF-BB and FGF-2, which should be bioavailable on the same cellular targets. Our dose studies show that the optimal ratio between FGF-2 and PDGF-BB released by sucrose aluminum octosulfate polymers is 4:1. The issue regarding the optimal time line of delivery warrants further study. To the best of our knowledge, the delivery of angiogenic and arteriogenic factors in combination to the ischemic myocardium in big animals has not previously been reported. Because of the lack of native collaterals, the pig myocardial infarction model is probably one of the most relevant preclinical systems to human coronary artery disease (25). Unfortunately, for several reasons, including high costs, difficulty in obtaining ethics approval, sophisticated operation procedures, and the need for relatively high dosages of angiogenic factors, most previous preclinical studies have been carried out in smaller animals such as rodents and rabbits, which often have native collaterals that prevent major myocardial infarction and lead to significant spontaneous recuperation (45, 46). Based on our findings in pigs, we speculate that dual delivery of PDGF-BB/FGF-2 to ischemic myocardium would produce beneficial effects in humans, although future studies are needed to validate this assumption.
Taken together, our present study provides an example of applying a combination of arteriogenic and angiogenic factors to the pig ischemic myocardium for establishing stable collateral networks, leading to a remarkable improvement of both blood flow and myocardial function. These findings may open new avenues and serve as a guiding principle for the development of future proangiogenic and arteriogenic therapies for the clinical treatment of myocardial infarction.
Materials and Methods
Mouse Corneal Neovascularization Model.
The mouse corneal assay was performed as described in ref. 47 [see supporting information (SI) Methods for detailed information].
Pig Myocardial Infarction Model and Growth Factor Implantation.
Chinese experimental minipigs weighing between 25 and 30 kg each were used for the myocardial infarction model (China Agricultural University, Beijing, China). All animal care and experimental protocols complied with the Animal Management Rules of the Ministry of Health of the People's Republic of China (document no. 55, 2001) and the guidelines for the care and use of laboratory animals of Qi Lu Hospital.
Animals were randomly divided into four groups (n = 10 pigs per group). Before all experimental procedures, all animals were anesthetized by intramuscular administration of ketamine hydrochloride (20 mg/kg; Heng Rui Medicine, Jiang Su, China) together with i.v. injection of sodium pentobarbital (30 mg/kg; Beijing Chemical Reagent Company, Beijing, China) and were mechanically ventilated with a volume respirator (E100m ventilator; Newport Medical Instruments, Costa Mesa, CA). Median sternotomy was performed to expose the heart followed by incision of the pericardium. Acute myocardial infarction was produced by ligation of the LAD coronary artery distal to its third diagonal branch using a 7.0-prolene suture. Selective left and right angiography, performed through a standard procedure (see below), was made to confirm complete occlusion of the LAD coronary artery and to assess baseline levels of collaterals to assess the collateral index. Immediately after this procedure, 5 × 106 red microspheres (10 ± 2 μm diameter; E-Z Trac, Los Angeles, CA) were injected into the left ventricle for measurement of regional blood flow levels. Additionally, echocardiography was performed in all animals to evaluate baseline global and regional left ventricle function. A slow-releasing pellet of sucrose aluminum sulfate coated with hydron polymer (0.2 × 0.2 cm) containing PBS, 5 μg of FGF-2, 10 μg of PDGF-BB, or 5 μg of FGF-2/10 μg of PDGF-BB was attached to an aseptic application (0.5 × 0.5 cm), which was sutured (7.0-prolene) onto the border zone adjacent to the mid and distal LAD coronary artery. After implantation, the pericardium and chest were closed and the animals were allowed to recover from the anesthesia.
Angiography and Quantification Analysis of Coronary Collateral Circulation.
Six and 14 weeks after treatments, repeated, selective angiography was performed. Under general anesthesia, the animals received a contrast agent, meglumine diatrizoate, through a standard femoral puncture by using digital subtraction angiography (CGO-2100; Wandong Medical Equipment). To precisely assess collateral filling of LAD territory, semiquantification of the collateral coronary circulation was performed with Image ProPlus 5.1 according to the manufacturer's instruction (Media Cybernetics, Silver Spring, MD). Images with the best contrast were selected for analysis for each animal, and the angiographic index was calculated by using a standard protocol based on Rentrop's grading scales. The area of interest (AOI) was defined as a left ventricular region that included neo-collaterals and a distal segment of the ligated LAD coronary artery. The profile of the LAD and collateral artery reperfusion were delineated carefully, and total collateral vascular areas were calculated. Each image was recorded three times.
Regional Myocardial Blood Flow.
Assessment of regional MBF was carried out before treatment and at the end of weeks 6 and 14 by catheter-guided injection into the left ventricle of 5 × 106 red, yellow, and green microspheres. Reference blood samples were simultaneously collected. Recovery of microspheres from tissues and blood was performed according to the manufacturer's instructions.
Echocardiographic Analysis.
Two-dimensional echocardiography was used to measure global and regional left ventricular function in all animals with open chest after angiographic assessment before and 14 weeks after treatment using an ultrasound scanner (SONOS 7500; Philips Medical Systems, Andover, MA). Analyses of LVEF and systolic wall thickening (WT%) were performed to determine the global function of the left ventricle and the regional function of the myocardium, respectively. LVEF was determined from the apical two- and four-chamber views by using a modified Simpson's algorithm. Regional wall thickness was measured at end-diastole (the peak of R wave of the ECG) and end-systole (the end of T wave of the ECG) individually on two-dimensional echocardiograms. Left ventricular wall thickness at both end-diastole (DWT) and end-systole (SWT) was measured, and left ventricular systolic wall thickening (WT%) was calculated as WT% = (SWT − DWT)/DWT × 100%.
Immunohistochemistry.
The treated areas of the left ventricle myocardium in the LAD territory were fixed with 3% paraformaldehyde or immediately frozen in liquid nitrogen. To determine vessel density, paraffin-embedded, 5-μm sections were incubated with a rabbit anti-vWF antibody or a mouse anti-αSMA antibody, followed by incubation with secondary antibodies labeled with horseradish peroxidase. Ischemic myocardial sections were also used for detection of vWF/αSMA double positivity by immunofluorescent analysis. A maturation index (percentage of smooth muscle cell-positive vessels vs. total vessel numbers) was calculated (see SI Methods for detailed information).
Statistical Analysis.
Data are presented as mean determinants (±SEM) and were analyzed with the SPSS package (Version 11.5; SPSS, Chicago, IL) (see SI Methods for detailed information).
Supplementary Material
Acknowledgments
We thank Drs. Xiaolu Li, Zhaoqiang Liu, Fenglei Zhang, and Xiaojing Yu for help in establishing the pig myocardial infarction model; Dr. Fuhai Li for help with the image editing; and Dr. Yuan Xue for the artistic work presented in Fig. 6. This work was supported by National Natural Science Foundation of China Grant 30428005, National 973 Basic Research Program Grant 2006CB503803, the Swedish Heart and Lung Foundation, European Union integrated project VascuPlug Contract STRP 013811, the Söderberg Foundation, and the Swedish Medical Research Council.
Abbreviations
- αSMA
α smooth muscle actin
- LAD
left anterior descending
- LVEF
left ventricle ejection fraction
- MBF
myocardial blood flow
- WT
wall thickening
- VSMC
vascular smooth muscle cells
- vWF
von Willebrand factor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704966104/DC1.
References
- 1.Okrainec K, Banerjee DK, Eisenberg MJ. Am Heart J. 2004;148:7–15. doi: 10.1016/j.ahj.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Maseri A. Cardiovasc Drugs Ther. 1990;4(Suppl 4):827–831. doi: 10.1007/BF00051288. [DOI] [PubMed] [Google Scholar]
- 3.Epstein SE, Kornowski R, Fuchs S, Dvorak HF. Circulation. 2001;104:115–119. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, Laham RJ, Li W, Pike M, Sellke FW, et al. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 5.Ware JA, Simons M. Nat Med. 1997;3:158–164. doi: 10.1038/nm0297-158. [DOI] [PubMed] [Google Scholar]
- 6.Simons M. Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 7.Khurana R, Simons M, Martin JF, Zachary IC. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 8.Annex BH, Simons M. Cardiovasc Res. 2005;65:649–655. doi: 10.1016/j.cardiores.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 10.Sellke FW, Laham RJ, Edelman ER, Pearlman JD, Simons M. Ann Thorac Surg. 1998;65:1540–1544. doi: 10.1016/s0003-4975(98)00340-3. [DOI] [PubMed] [Google Scholar]
- 11.Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, Hachamovitch R, Szulc M, Kligfield PD, Okin PM, et al. Circulation. 1999;100:468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 12.Fortuin FD, Vale P, Losordo DW, Symes J, DeLaria GA, Tyner JJ, Schaer GL, March R, Snell RJ, Henry TD, et al. Am J Cardiol. 2003;92:436–439. doi: 10.1016/s0002-9149(03)00661-1. [DOI] [PubMed] [Google Scholar]
- 13.Sivakumar B, Harry LE, Paleolog EM. J Am Med Assoc. 2004;292:972–977. doi: 10.1001/jama.292.8.972. [DOI] [PubMed] [Google Scholar]
- 14.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, et al. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher B, Pecher P, von Specht BU, Stegmann T. Circulation. 1998;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 16.Grines CL, Watkins MW, Mahmarian JJ, Iskandrian AE, Rade JJ, Marrott P, Pratt C, Kleiman N. J Am Coll Cardiol. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 17.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, et al. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 18.Seiler C, Pohl T, Wustmann K, Hutter D, Nicolet PA, Windecker S, Eberli FR, Meier B. Circulation. 2001;104:2012–2017. doi: 10.1161/hc4201.097835. [DOI] [PubMed] [Google Scholar]
- 19.Udelson JE, Dilsizian V, Laham RJ, Chronos N, Vansant J, Blais M, Galt JR, Pike M, Yoshizawa C, Simons M. Circulation. 2000;102:1605–1610. doi: 10.1161/01.cir.102.14.1605. [DOI] [PubMed] [Google Scholar]
- 20.Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Bonow RO. Circulation. 2000;101:118–121. doi: 10.1161/01.cir.101.2.118. [DOI] [PubMed] [Google Scholar]
- 21.Gyongyosi M, Khorsand A, Zamini S, Sperker W, Strehblow C, Kastrup J, Jorgensen E, Hesse B, Tagil K, Botker HE, et al. Circulation. 2005;112:I157–I165. doi: 10.1161/01.CIRCULATIONAHA.105.525782. [DOI] [PubMed] [Google Scholar]
- 22.Losordo DW, Dimmeler S. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 23.Gregg DE, Patterson RE. New Engl J Med. 1980;303:1404–1406. doi: 10.1056/NEJM198012113032406. [DOI] [PubMed] [Google Scholar]
- 24.Campbell CD, Takanashi Y, Laas J, Meus P, Pick R, Replogle RL. J Thorac Cardiovasc Surg. 1981;81:288–296. [PubMed] [Google Scholar]
- 25.Schaper W, Jageneau A, Xhonneux R. Cardiologia. 1967;51:321–335. doi: 10.1159/000165875. [DOI] [PubMed] [Google Scholar]
- 26.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 27.Ornitz DM, Itoh N. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klint P, Claesson-Welsh L. Front Biosci. 1999;4:D165–D177. doi: 10.2741/klint. [DOI] [PubMed] [Google Scholar]
- 29.Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Nature. 1987;325:257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 30.Heldin CH, Westermark B. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 31.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Development (Cambridge, UK) 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 32.Armulik A, Abramsson A, Betsholtz C. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 33.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Tjwa M, Moons L, Fons P, Noel A, Ny A, Zhou JM, Lennartsson J, Li H, Luttun A, et al. J Clin Invest. 2005;115:118–127. doi: 10.1172/JCI19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen M, Rentrop KP. Circulation. 1986;74:469–476. doi: 10.1161/01.cir.74.3.469. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. New Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 37.Pieramici DJ, Avery RL. Exp Opin Biol Ther. 2006;6:1237–1245. doi: 10.1517/14712598.6.11.1237. [DOI] [PubMed] [Google Scholar]
- 38.Marx J. Science. 2005;308:1248–1249. doi: 10.1126/science.308.5726.1248. [DOI] [PubMed] [Google Scholar]
- 39.Ireson CR, Kelland LR. Mol Cancer Ther. 2006;5:2957–2962. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 40.Post MJ, Simons M. Minerva Cardioangiol. 2003;51:421–432. [PubMed] [Google Scholar]
- 41.Carmeliet P, Conway EM. Nat Biotechnol. 2001;19:1019–1020. doi: 10.1038/nbt1101-1019. [DOI] [PubMed] [Google Scholar]
- 42.Helisch A, Schaper W. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 43.Edelberg JM, Aird WC, Wu W, Rayburn H, Mamuya WS, Mercola M, Rosenberg RD. J Clin Invest. 1998;102:837–843. doi: 10.1172/JCI3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brindle NP, Saharinen P, Alitalo K. Circ Res. 2006;98:1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaper W, Piek J, Munoz-Chapuli R, Wolf C, Ito W. In: Collateral Circulation of the Heart. Ware JA, Simons M, editors. New York: Oxford Univ Press; 1999. pp. 159–198. [Google Scholar]
- 46.Maxwell MP, Hearse DJ, Yellon DM. Cardiovasc Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 47.Cao R, Brakenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, Eriksson U, Cao Y. FASEB J. 2002;16:1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.