Abstract
Dopaminergic and glutamatergic systems are critical components responsible for prefrontal signal-to-noise tuning in working memory. Recent functional MRI (fMRI) studies of genetic variation in these systems in catechol-O-methyltransferase (COMT) and in metabotropic glutamate receptor mgluR3 (GRM3), respectively, suggest that these genes influence prefrontal physiological signal-to-noise in humans. Here, using fMRI, we extend these individual gene findings to examine the combined effects of COMT and GRM3 on dissociable components of the frontoparietal working memory network. We observed an apparent epistatic interaction of these two genes on the engagement of prefrontal cortex during working memory. Specifically, the GRM3 genotype putatively associated with suboptimal glutamatergic signaling was significantly associated with inefficient prefrontal engagement and altered prefrontal-parietal coupling on the background of COMT Val-homozygous genotype. Conversely, COMT Met-homozygous background mediated against the effect of GRM3 genotype. These findings extend putative brain dopaminergic and glutamatergic relationships indexed by COMT and GRM3 to a systems-level interaction in human cortical circuits implicated in working memory dysfunction such as in schizophrenia.
Keywords: dopamine, functional MRI, genetics, schizophrenia
Dopaminergic and glutamatergic abnormalities have long served as major theoretical frameworks for understanding the pathophysiology as well as pharmacology of schizophrenia and its cognitive deficits, particularly working memory (1–4). They are also central in the neural dynamics mediating sustained activity of prefrontal neurons during working memory, and in optimizing cortical signal-to-noise (5–10). Locally sustained activity of prefrontal neurons crucial in the maintenance of information during the delay period of working memory tasks (11) seems to be protected against distracters and instability by dopamine-mediated mechanisms (12). This mechanism is likely to be through dopamine D1-receptors (9) and through their role in enhancing NMDA receptor-mediated postsynaptic currents in prefrontal pyramidal neurons active during the delay period (7, 10, 13). Concurrently, D1-receptors seem to cause a tonic increase in the firing of GABAergic inhibitory interneurons, allowing a focused increase in task-relevant activity while reducing that which is not, thus optimizing signal-to-noise (14). Therefore, dopaminergic and glutamatergic (and GABAergic) systems act together in signal-to-noise tuning critical for information processing and should have important interactions of relevance at the in vivo systems level.
Less is known, however, about how these molecular and single-neuron paradigms resonate in vivo during prefrontally mediated human executive and cognitive control processes (15, 16). Biophysically based neural network models suggest that the reverberatory complement of excitatory and inhibitory NMDA and dopaminergic (and GABA) mechanisms underlie both persistent activity in working memory as well as its executive control (17, 18). Emerging integrate-and-fire neural network models have further argued that the dorsolateral prefrontal cortex [DLPFC; Brodmann areas (BA) 9 and 46] more critically engages cognitive control during the manipulation and temporal ordering of information in memory than related regions in the ventrolateral prefrontal cortex (VLPFC; BA 44, 45, and 47) (19, 20). These models are consistent with human neuroimaging studies that mapped to the DLPFC higher-order processes such as manipulation of information or applying them in context; on the other hand, simpler operations such as rehearsal of information in working memory tended to engage the VLPFC (21–24). Thus, prefrontal dopaminergic and NMDA receptor (and GABA) systems, through their roles in prefrontal signal-to-noise processing, should interact biologically to affect the tuning of these neural signals critical for working memory. Moreover, executive aspects of working memory should be more susceptible to variation in these interacting systems at the level of DLPFC.
Here, we sought to further evaluate these predictions in terms of how human prefrontal cortical interactions might relate to genetic variation modulating aspects of brain dopaminergic and glutamatergic systems in vivo. Specifically, we examined the interplay between candidate single-nucleotide polymorphisms (SNPs) in catechol-O-methyltransferase (COMT) and the type II metabotropic glutamate receptor 3 (GRM3) on frontoparietal networks engaged by working memory. There is ample prior evidence that these genes impact specific brain function to a degree. COMT plays a critical role in dopamine catabolism within the PFC because of the lack of dopamine transporters in this region (25, 26). A common polymorphism in the COMT gene resulting in a valine-to-methionine Val(108/158)Met substitution gives rise to a significant reduction in its enzymatic activity in the PFC and in peripheral tissues (25). This polymorphism impacts prefrontal working memory and correlated neurophysiology (27–33).
In the case of GRM3, a G protein-linked receptor modulating synaptic glutamate and located on chromosome 7q21-22 (34, 35), a polymorphism rs6465084 in intron 2 was associated, even in normal subjects, with a pattern of inefficient PFC engagement during working memory studied with functional MRI (fMRI) analogous to that found with COMT. The same GRM3 allele was associated with lower expression in postmortem human brain of the prefrontal glial glutamate transporter EAAT2, a protein modulating synaptic glutamate, and with reduced N-acetyl-aspartate signals on magnetic resonance spectroscopy, a measure correlated with tissue glutamate concentrations (36, 37). GRM3 was recently associated with an abnormal fMRI response in another independent study of episodic memory (38). A potential molecular mechanism for these genetic associations may involve alternative splicing of GRM3 (39).
Thus, whereas variation in the COMT and GRM3 SNPs have individually been implicated in the neurophysiology of working memory using functional neuroimaging (27, 31, 32, 37, 40), they have not previously been evaluated together, or within a framework where their influence on the systems level prefrontal functional organization of specific higher-order working memory processing is examined. Here, we investigated the prediction that, if indeed prefrontal cortical functions critically involve at least in part dopaminergic and glutamatergic interactions, then higher-order working memory processes assumed to tax DLPFC would be especially vulnerable to the combined effect of suboptimal dopaminergic and glutamatergic influence at the DLPFC during such an executive task. In addition to examining the prefrontal cortex, we extended our observations to the frontoparietal working memory network, with the prediction that DLPFC activation, if inefficient, would be accompanied by relatively reduced functional connectivity (41, 42). Finally, apart from hypotheses driven by fundamental models of neural function, we also examined an empirical observation that additional VLPFC engagement occurred in the background of relatively deleterious COMT alleles (33) and in patients with schizophrenia (41, 43–45). This VLPFC engagement apparently diverges from DLPFC models of optimal executive function, possibly signifying a systems-level response to deficits at the DLPFC (41). We explored how this VLPFC response could be related to combined variation in COMT and GRM3. Specifically, if the additional VLPFC engagement played an adaptive or compensatory role to allow task performance indices to be maintained despite physiological inefficiencies in the deleterious dopaminergic and glutamatergic states, we might then observe increased VLPFC activation as well as increased VLPFC-parietal functional connectivity during the executive task in these individuals.
Results
We studied 29 right-handed healthy subjects of European ancestry who were either homozygous for the Val or Met allele at the COMT Val(108/158)Met SNP, to increase the variation of associated brain activity (40). Variation at GRM3 SNP rs64650844 in intron 2 was also analyzed. Acquisition of working memory N-back fMRI data was similar to previous descriptions (43). There were no significant differences in age, gender, education, and behavioral performance (accuracy and reaction time) across genotype groups [Table 1 and supporting information (SI) Text].
Table 1.
Demographics and behavioral performance across groups of healthy subjects according to genotype
| COMT-GRM3 Genotypes |
||||||||
|---|---|---|---|---|---|---|---|---|
| VV + AA (n = 9) |
VV + G-carriers (n = 7) |
MM + AA (n = 6) |
MM + G-carriers (n = 7) |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age, yr | 33.0 | 10.8 | 36.9 | 8.2 | 35.3 | 11.9 | 33.4 | 13.9 |
| Gender, no. of male | 3 | 5 | 2 | 5 | ||||
| Education, yr | 15.1 | 2.2 | 17.3 | 3.0 | 14.7 | 2.3 | 15.9 | 2.0 |
| WAIS IQ | 110 | 8.1 | 110 | 10.9 | 107 | 11.1 | 110 | 6.6 |
| 1-Back accuracy | 0.956 | 0.041 | 0.894 | 0.188 | 0.950 | 0.079 | 0.897 | 0.130 |
| 1-Back reaction time, s | 0.566 | 0.137 | 0.581 | 0.186 | 0.577 | 0.146 | 0.520 | 0.131 |
| 2-Back accuracy | 0.807 | 0.180 | 0.736 | 0.206 | 0.872 | 0.180 | 0.783 | 0.233 |
| 2-Back reaction time, s | 0.592 | 0.113 | 0.601 | 0.208 | 0.556 | 0.164 | 0.657 | 0.125 |
VV, COMT-Val homozygotes; MM, COMT-Met homozygotes; AA, GRM3-A homozygotes; G, GRM3-G carriers.
Interaction between COMT and GRM3 Variation at the DLPFC.
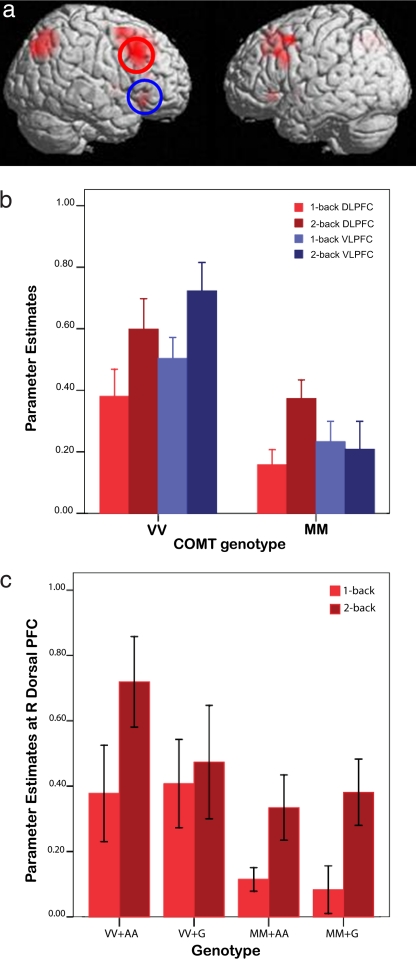
As we sought to examine how the prefrontal functional organization might be altered with genetic variation during executive aspects of working memory, we focused on the differential activation between 1-back and 2-back tasks. This activation reflected incremental working memory load (number of target items stored) and information updating (the executive process of continuous selection and updating of target items), and putatively regions that tend to engage more DLPFC than VLPFC (22, 28, 41, 46). Here, the higher-order working memory task engaged by 2-back > 1-back was associated with bilateral prefrontal cortical activation (Fig. 1a and SI Table 2). We then examined parameter estimates from orthogonal regions-of-interest (ROIs) with the largest cluster size in the DLPFC (BA9: 45 20 32, z = 4.82) and VLPFC (BA47: 49 25 −6, z = 3.39) for gene effects. We first examined COMT effects, which showed a load-by-region-by-COMT interaction (F1,27 = 6.80, P = 0.015; Fig. 1b), as well as a main effect of COMT-genotype (F1,27 = 12.99, P = 0.001). Thus, COMT-Met homozygote (COMT-MM) individuals were found to engage the DLPFC but not the VLPFC during the higher-order working memory task. On the other hand, COMT-Val homozygote (COMT-VV) individuals, who presumably have relatively reduced cortical synaptic dopamine (25, 26) exhibited disproportionately increased, therefore “inefficient,” DLPFC as well as VLPFC activation.
Fig. 1.
Working memory task activations. (a) Surface rendering of regions activated by subjects as they engaged 2-back > 1-back (P < 0.05 corrected for false discovery rate). The red circle shows the functional ROI at the DLPFC, and the blue circle shows the functional ROI at the VLPFC. (b) Parameter estimates extracted from ROIs defined in a, showing that COMT-VV individuals used both DLPFC and VLPFC regions to process working memory load, whereas COMT-MM individuals used the DLPFC but not the VLPFC, and had relatively lower activation magnitude. Error bars are ±1 standard error. (c) GRM3 A homozygosity (AA) interacted to give disproportionately greater load-dependent DLPFC activation in COMT Val-homozygotes (VV) but not in Met homozygotes (MM). Error bars are ±1 standard error.
Examining for combined COMT and GRM3 gene effects at the DLPFC ROI, we found a load-by-COMT-by-GRM3 interaction (F1,25 = 4.47, P = 0.045), as well as a COMT main effect (F1,25 = 4.72, P = 0.039) (Fig. 1c). Thus, within COMT-VV but not COMT-MM individuals, those homozygous for the relatively more deleterious GRM3 A-allele (37) had disproportionately greater, therefore inefficient, working memory load-dependent activation relative to GRM3 G-carriers at the DLPFC. At the VLPFC ROI, there was a main effect of COMT-genotype (F1,25 = 18.89, P < 0.001), and a trend for load-by-COMT interaction (F1,25 = 4.01, P = 0.056), consistent with the COMT effects reported above (Fig. 1b), but no significant load-by-COMT-by-GRM3 interaction (F1,25 = 1.68, P > 0.2).
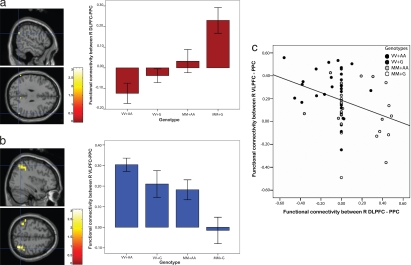
The findings of interacting dopaminergic and glutamatergic gene effects were extended by means of the frontoparietal functional connectivity analyses. Seed regions were derived from the same ROIs at the DLPFC and VLPFC (Fig. 1a), and used to evaluate working memory task-related coactivity with the posterior parietal cortex (PPC; BA7 and 40) across genotypes. Consistent with the hypothesis that combined relatively deleterious dopaminergic and glutamatergic states would be inefficient and thus associated with regions of reduced DLPFC-PPC functional connectivity, conjunction contrasts of DLPFC functional connectivity where COMT-MM > VV and GRM3 G-carriers > A-homozygotes engaged a region in the right inferior parietal lobule (BA40: 55 −61 31, z = 4.56, P < 0.05 corrected) (Fig. 2a). Extracted parameter estimates from this peak showed that individuals who were COMT-VV and GRM3-A homozygotes had disproportionately decreased DLPFC-PPC functional connectivity, whereas the converse occurred for those who had more optimal COMT-MM and GRM3-G carrier genotypes (F3,53 = 8.94, P < 0.001).
Fig. 2.
Frontoparietal functional connectivity. (a) Regions in the PPC where 1- and 2-back functional connectivity with the DLPFC ROI were activated in the conjunction contrast of COMT Met-homozygote > Val homozygote and GRM3 G-carrier > A-homozygote (shown at P < 0.001 for display only). Extracted parameter estimates demonstrated COMT Met-homozygotes and GRM3 G-carriers had disproportionately increased functional connectivity. Error bars are ±1 standard error. (b) Regions in the PPC where functional connectivity with the VLPFC ROI were activated in the conjunction contrast of COMT Val-homozygote > Met homozygote and GRM3 A-homozygote > G-carriers (shown at P < 0.001 for display only). Extracted parameter estimates showed COMT Val and GRM3 A homozygotes had disproportionately greater functional connectivity. (c) There was a reciprocal relationship whereby decreasing DLPFC-PPC functional connectivity was associated with increasing VLPFC-PPC functional connectivity (P < 0.01).
VLPFC Activation and Functional Connectivity.
Whereas the DLPFC might successfully drive higher-order working memory performance in the more efficient COMT-MM individuals, subjects with less efficient prefrontal dopaminergic and glutamatergic function engaged VLPFC in addition to DLPFC (Fig. 1b). Consistent with the hypothesis that the VLPFC engagement would be associated with corresponding regions of much greater VLPFC-PPC connectivity relative to more optimal COMT and GRM3 states, conjunction contrasts of VLPFC functional connectivity where COMT-VV > MM and GRM3 A-homozygotes > G-carriers engaged a region in the right superior parietal lobule (BA7, 34 −60 53, z = 4.51, P < 0.05 corrected) (Fig. 2b). Parameter estimates extracted from this peak showed that COMT-VV and GRM3-A homozygotes had disproportionately increased frontoparietal functional connectivity between the VLPFC and PPC (F3,53 = 6.83, P = 0.001). The overall inverse relationship between functional connectivity at the ventral and dorsal PFC-PPC in the combined sample (r = −0.34, P = 0.005) (Fig. 2c) further underlines the possibility that there might exist a continuum, whereby increasing ventral PFC-PPC connectivity compensates for decreasing dorsal PFC-PPC connectivity.
Discussion
Extending previous work implicating prefrontal cortical efficiency as a function of the individual SNPs tested in COMT and GRM3 (27, 37, 40), we found that the COMT-Val allele putatively associated with reduced prefrontal dopamine (25, 26) and the GRM3-A allele putatively related to suboptimal glutamatergic function (35–37) interacted to give disproportionately inefficient DLPFC activation in the higher-order working memory contrast. This inefficiency was further associated with reduced frontoparietal functional integration. In an apparent compensation for these deficits, we found reciprocal additional engagement of the VLPFC, and increased VLPFC-PPC functional connectivity. Conversely, the COMT-MM genotype associated with relatively increased prefrontal dopamine seemed to mediate against the relatively deleterious GRM3 genotype.
Dopaminergic and Glutamatergic Influence on DLPFC Activation and Functional Connectivity.
Appreciative of the limited resolving power fMRI has in terms of definitively linking cognitive process, neurophysiology and the biological effects of genetic variation, we nevertheless posited several possibilities based on converging findings in cellular and in vivo systems models. Beginning with the COMT Val(108/158)Met polymorphism, a number of independent fMRI, PET and pharmacogenetic imaging studies have demonstrated that Val-allele load associated with decreased prefrontal dopamine (25) shifted individuals along an inverted-U curve toward lower physiological efficiency (or increased activation to perform the task at the same level), whereas the converse held for Met alleles (27, 31, 32, 40). Thus, the cortical efficiency measured with neuroimaging potentially mirrors that observed at the molecular level in signal-to-noise tuning by dopamine D1 receptors (9, 47, 48). Our present findings support these conceptualizations as COMT-MM individuals performing similarly with their COMT-VV counterparts engaged relatively less prefrontal activity. Furthermore, optimized dopamine signaling through the D1 receptors were previously associated with increased neural firing synchronicity (10, 49), which might facilitate cortical communication within the working memory network. Our finding of enhanced functional connectivity between the DLPFC and task-critical regions in the PPC in COMT-MM relative to COMT-VV individuals supports this possibility. Conversely, lower synaptic dopamine in the COMT-VV state might be associated with more noise in neural firing or field potentials (47), with resultant increased spread of activation that is ultimately inefficient at recruiting key regions in the PPC, thus manifesting as reduced functional connectivity, as in our findings.
The NMDA receptor is a critical partner in these working memory processes (8, 10, 50), and by extension GRM3, because it regulates synaptic glutamate. The latter relationship was established through various lines of investigations. Stimulation of mgluR3 receptors in primary glial culture regulates the expression of the glial glutamate transporter (EAAT2), the protein responsible for the removal of synaptic glutamate (51). In vivo measurements of synaptic glutamate levels and cognitive performance were maintained after the application of an NMDA antagonist and a group II metabotropic glutamate receptor agonist in rodents (34); analogous cognitive observations have been made in marmosets (52) and human subjects (53), reflecting the intimate dependencies between the NMDA and group II metabotropic glutamate receptors. Concerning the effects of relatively deleterious GRM3 alleles, postmortem prefrontal tissue from these individuals were associated with reduced EAAT2 levels (37); and magnetic resonance spectroscopy found reduced N-acetylaspartate/creatine levels in the prefrontal cortex of these individuals, presumably reflecting reduced tissue glutamate and/or neuronal integrity (36, 37). Whereas these human brain measures do not directly assay change in synaptic glutamate or NMDA receptor function, and may not simply reduce to models of increased or decreased glutamate levels, there is emerging evidence that GRM3 genetic variation, perhaps through the expression of splice variants (39), could well affect the finely tuned synaptic glutamate balance and consequent NMDA-dependent neuronal firing dynamics critical in working memory function (7). Building on this evolving framework, our present findings that genetic variation in COMT and GRM3 interacted at the level of efficiency of cortical executive processing is remarkably consistent with evidence that relatively reduced synaptic dopamine (27, 31, 40, 54), NMDA receptor antagonists (55), as well as suboptimal synaptic glutamatergic function in GRM3 A-homozygotes (37) were all previously associated with inefficient DLPFC activation during working memory tasks. Furthermore, electrophysiological studies indicate that the NMDA receptor hypofunction leads to increased irregular spike activity, but decreased coordinated burst activity within PFC (50), results that would be consistent with our findings of increased DLPFC activation but reduced DLPFC-PPC connectivity. More optimal dopaminergic states, as postulated to occur in the COMT-MM individuals in our cohort, seemed to moderate these activation and functional connectivity inefficiencies, thus highlighting the critical cross-talk between these neurotransmitter systems at the DLPFC during higher order working memory processing (1, 2, 8, 56).
VLPFC Activation and Functional Connectivity.
Individuals with putatively lower synaptic dopamine (COMT-VV individuals) and relatively deleterious glutamatergic states (GRM3 A-homozygotes) were found to engage additional VLPFC networks, consistent with cortical regions identified in related work investigating multiple COMT haplotypes (33). The VLPFC is associated with hierarchically simpler cognitive control, inhibitory and set-shifting processes relative to the DLPFC (22, 24, 57), and so, seems endowed with computational properties relevant to the working memory task in general (58). Therefore, in the presence of relatively dysfunctional DLPFC activation and functional integration, as might be the case with relatively deleterious COMT and GRM3 alleles, VLPFC processing might supplement or compensate for the dysfunctional DLPFC executive processes to an extent. This conjecture is supported by our finding of the reciprocal relationship between increasing VLPFC-PPC and decreasing DLPFC-PPC functional connectivity as a function of more deleterious dopaminergic and glutamatergic genotypes (Fig. 2). Normal or increased engagement of more ventral prefrontal regions during executive tasks taxing DLPFC have also previously been observed in relatively high-performing schizophrenia patients (41, 43–45, 59). These results suggest that physiological inefficiency relevant to schizophrenia could be influenced by prefrontal dopaminergic and glutamatergic states to involve first, a relative loss of function at higher-order processing regions in the DLPFC, despite intact cognitive performance and increased activation that is inefficient, and second, compensatory activation from the VLPFC. The possibility that similar suboptimal dopaminergic and glutaminergic states might occur in schizophrenia patients who have analogous cortical macrocircuit activation profiles resonates with hypotheses linking dysfunction in these interacting neurotransmitter systems to psychosis (1, 4, 47, 56).
It is also intriguing to note that parietal networks were differentially engaged as a function of prefrontal neural efficiency. The more optimal frontoparietal network for higher-order working memory as engaged by the COMT-MM individuals seemed to involve more integrated DLPFC and ventral PPC activation (see above and Fig. 2a). This region of the PPC has been suggested to mediate spatial reorientating to new targets (60), and is empirically consistent with the effective negotiation of increased executive demands between 1-back and 2-back of our version of the spatial N-back task. Conversely, the putative compensatory network engaged by COMT-VV and GRM3 A-homozygote individuals involved more VLPFC and dorsal PPC functional integration (see above and Fig. 2b). This parietal region has been suggested to mediate general attentional demands (60, 61), and is consistent with our suggestion that their engagement within a less effective working memory neural strategy could serve to counterbalance the nosier PFC in these individuals. Thus, future work might investigate whether modulating attentional processes could indeed help.
Linking Genes to Cognitive Neurophysiology.
In this study, we extended recent “imaging genetics” work suggesting links between genes that influence neurotransmitter systems and working memory neurophysiology (27, 29, 31–33, 37, 40, 54). Here, we considered the biologic interaction between these genes. We studied these effects, arguably, within a more specific framework of higher order working memory processing, which allowed us to make tentative suggestions regarding gene effects on dissociable regions within the prefrontal cortex. Through functional connectivity analyses, we were able to further parse cortical efficiencies within the prefrontal cortex, and suggest regions of possible dysfunctional and compensatory responses. Nevertheless, it should be no surprise that links between neurotransmitter genetic variations, regional hemodynamic responses and specific cognitive processes are still incomplete, and our findings are therefore exploratory. In searching for brain regions possibly associated with gene effects, we have elected a principled approach: first, we examined neurotransmitter SNPs previously shown to have working memory effects; and second, we used an ROI approach in which gene effects were explored from unbiased contrasts that potentially isolated the specific cognitive processes studied. This approach maximized the prior probability of gene effects, and constrained the search to regions identified under statistical control. Moreover, findings from brain activation and functional connectivity were converging and mutually supportive. However, statistical thresholds of higher-order analyses and sample sizes needed for inferences within “imaging genetics” are still controversial (62), and our results need to be cautiously interpreted, replicated, and extended by using other specific task manipulations.
In this “imaging genetics” approach, we have attempted to juxtapose molecular models, the neurophysiology of COMT and GRM3 SNP effects, and empirical findings from schizophrenia patients, to illustrate suggestive parallels including the possibility that some form of compensation occurred in response to relative deficits in cortical function. However, it remains to be determined how genetic variation in dopaminergic and glutamatergic contributions to cortical signal-to-noise could directly effect the differential engagement of these frontoparietal networks at a cellular level. We speculate that neuroplasticity involving prefrontal dopamine (8, 25) and the type II metabotropic glutamate receptor (37, 39) are key nodes in these processes. This contention needs to be further explored in work on molecular and neural models (19), as well as emerging in vivo genetic and neuropsychopharmacological imaging methods using fMRI (40, 54, 55).
Conclusion
In this study on executive aspects of working memory, we found that variants in two genes implicated in cortical glutamate and dopamine signaling interacted to modulate the efficiency of cortical activation and critical frontoparietal integration at the DLPFC, and differential engagement of the VLPFC. We conclude that these findings potentially extend putative brain dopaminergic and glutamatergic cross-talk to the in vivo systems-level interactions in cortical circuits implicated in working memory dysfunction and potentially in schizophrenia.
Materials and Methods
Subjects.
Healthy subjects were recruited from a larger group that included previously reported data sets establishing the effects of COMT and GRM3 SNPs on 2-back working memory efficiency in the prefrontal cortex (33, 37). However, the subjects reported here were additionally asked to perform the 1-back working memory task in the same setting, which was critical in the present effort to examine differential load effects and higher-order working memory function. Genotyping was by the Taqman 5′-exonuclease assay previously described (25, 37). Additionally, our sample was genotyped with a panel of 96 unlinked SNPs having minor allele frequencies of >30% in 95 genes to survey for occult genetic stratification. We found no significant variation in frequency of these SNPs across the COMT or GRM3 genotype groups (results available on request).
This study was approved by the Institutional Review Board of the Intramural Program of the National Institute of Mental Health. Details of subject recruitment and ascertainment are in SI Text.
Neuroimaging Experiment.
Acquisition of N-back fMRI data was similar to previous descriptions (43). Briefly, the N-back task consisted of continual presentation of visual stimuli in which every number was both a probe and a target (i.e., 100% target). The numbers 1–4 appeared randomly every 1.8 s for 500 ms at set locations at the points of a diamond-shaped box. Subjects were to recall the stimulus seen “N” previously by means of a fiberoptic response box with buttons arrayed in the same configuration as the stimuli presented on the screen. The task was presented as two counterbalanced runs in which 30-s epochs of 0-back alternated with either 1-back or 2-back.
Whole brain blood oxygen level-dependent fMRI data were collected on a 3-T scanner (General Electric Systems, Milwaukee, WI) with a GE-EPI pulse sequence acquisition of 24 contiguous slices (echo time = 30 ms, repetition time = 2 s, flip angle = 90°, field of view = 24 cm, matrix = 64 × 64, voxel dimensions = 3.75 × 3.75 × 6 mm). All fMRI data were preprocessed and spatially normalized to a common stereotaxic space (Montreal Neurologic Institute template) with SPM 99 software (Wellcome Department of Cognitive Neurology, London; www.fil.ion.ucl.ac.uk/spm), and individually examined for motion artifacts as described (43).
Analysis of Imaging Data.
Single-subject contrast images were entered into a second level of analysis with subject as a random factor. We examined the effect of working memory load (2-back vs. 1-back) in the sample, adopting a voxel-wise statistical threshold corrected for false discovery rate for the whole brain search volume (63). The coordinates were reported in standard space (64). Given our strong prior hypotheses regarding the DLPFC and VLPFC regions implicated in the higher-order working memory task, we chose functional ROIs from this contrast for subsequent testing of orthogonal gene effects. A peak from the largest cluster in the DLPFC and another from the VLPFC then defined centers of 10-mm-diameter spheres from which we examined for interacting working memory load, COMT Val(108/153)Met SNP, GRM3 rs64650844 SNP, and prefrontal regional effects using repeated-measures ANOVA. Results of higher-order analyses where P < 0.05 were reported.
In the functional connectivity analyses, the same DLPFC and VLPFC ROIs were used as seed volumes. From each of these ROIs, the median time series was computed for each individual at 1-back and 2-back, and cross-correlated with the time series of all other brain voxels by using Pearson's correlation coefficients. Group differences in functional connectivity were subsequently computed by combining the correlation maps with group as a random factor (42). In accordance with our focus upon frontoparietal integration on the basis of known distributed functional and structural anatomy of working memory (23, 65), we reported regions activated in the PPC (BAs 7 and 40), and in hypothesized genotype conjunction contrasts (66) involving either the combined deleterious or more optimal COMT and GRM3 genotype groups (COMT Val and GRM3 A homozygotes, or COMT Met homozygote and GRM3 G-carriers, respectively). These contrasts were thresholded at P < 0.05 corrected for the reduced parietal search volume (2,043 voxels) by using Gaussian Random Field theory (67). To minimize the number of voxel-wise statistical tests, subsequent ANOVAs examined for group effects within ROIs identified as the maxima in these conjunction contrasts.
Supplementary Material
Acknowledgments
We thank R. E. Straub, B. Kolachana, and R. Vakkalanka for genotyping support. This work was supported by the National Institute of Mental Health Intramural Research Program, and a National Institutes of Health fellowship (to H.-Y.T.).
Abbreviations
- fMRI
functional MRI
- COMT
catechol-O-methyltransferase
- GRM3
type II metabotropic glutamate receptor 3
- DLPFC
dorsolateral prefrontal cortex
- VLPFC
ventrolateral prefrontal cortex
- PPC
posterior parietal cortex
- BA
Brodmann Area
- COMT-VV
COMT-Val homozygote
- COMT-MM
COMT-Met homozygote
- ROI
region-of-interest.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610125104/DC1.
References
- 1.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 2.Laruelle M, Kegeles LS, Abi-Dargham A. Ann NY Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DR, Berman KF, Illowsky BP. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 5.Lisman JE, Fellous JM, Wang XJ. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- 6.Sawaguchi T, Goldman-Rakic PS. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 7.Wang X-J. J Neurosci. 1999;19:9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seamans JK, Yang CR. Prog Neurobiol. 2004;74:1–57. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Williams GV, Goldman-Rakic PS. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, O'Donnell P. Cereb Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- 11.Goldman-Rakic PS. Prog Brain Res. 1990;85:325–336. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- 12.Durstewitz D, Seamans JK, Sejnowski TJ. Nat Neurosci. 2000:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- 13.Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Proc Natl Acad Sci USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seamans JK, Gorelova N, Durstewitz D, Yang CR. J Neurosci. 2001;21:3628. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller EK, Cohen JD. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 16.Curtis C, D'Esposito M. Trends Cognit Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang XJ. Neuron. 2002;36:955–968. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 18.Lo CC, Wang XJ. Nat Neurosci. 2006;9:956–963. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- 19.Deco G, Rolls ET, Horwitz B. J Cognit Neurosci. 2004;16:683–701. doi: 10.1162/089892904323057380. [DOI] [PubMed] [Google Scholar]
- 20.Deco G, Rolls ET. J Cognit Neurosci. 2005;17:294–307. doi: 10.1162/0898929053124875. [DOI] [PubMed] [Google Scholar]
- 21.Paulesu E, Frith CD, Frackowiak RS. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 22.D'Esposito M, Postle BR, Ballard D, Lease J. Brain Cognit. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- 23.Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. Philadelphia: Lippincott–Raven; 1997. [Google Scholar]
- 24.Koechlin E, Ody C, Kouneiher FT. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, et al. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tunbridge EM, Harrison PJ, Weinberger DR. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg T, Egan M, Gscheidle T, Coppola R, Weickert T, Kolachana B, Goldman D, Weinberger D. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- 29.Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Kwon H, Jin S, Jo B, Antonarakis SE, et al. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- 30.Nolan KA, Bilder RM, Lachman HM, Volavka J. Am J Psychiatry. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- 31.Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, et al. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 33.Meyer-Lindenberg AS, Nichols T, Callicott JH, Ding J, Kolachana BS, Buckholtz J, Mattay VS, Egan MF, Weinberger DR. Mol Psychiatry. 2006;11:867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- 34.Moghaddam B, Adams BW. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 35.Harrison PJ, Weinberger DR. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 36.Marenco S, Steele SU, Egan MF, Goldberg TE, Straub RE, Sharrief AZ, Weinberger DR. Am J Psychiatry. 2006;163:740–742. doi: 10.1176/appi.ajp.163.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, et al. Proc Natl Acad Sci USA. 2004;101:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Quervain DJF, Papassotiropoulos A. Proc Natl Acad Sci USA. 2006;103:4270–4274. doi: 10.1073/pnas.0510212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartorius LJ, Nagappan G, Lipska BK, Lu B, Sei Y, Ren-Patterson R, Li Z, Weinberger DR, Harrison PJ. J Neurochem. 2006;96:1139–1148. doi: 10.1111/j.1471-4159.2005.03609.x. [DOI] [PubMed] [Google Scholar]
- 40.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg AS, Egan MF, Weinberger DR, Callicott JH. Am J Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Lindenberg A, Poline J-B, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 43.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 44.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan HY, Choo WC, Fones CSL, Chee MWL. Am J Psychiatry. 2005;162:1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- 46.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 47.Winterer G, Weinberger DR. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 49.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson ME, Homayoun H, Moghaddam B. Proc Natl Acad Sci USA. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Eur J Neurosci. 2003;17:2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- 52.Spinelli S, Ballard T, Gatti-McArthur S, Richards GJ, Kapps M, Woltering T, Wichmann J, Stadler H, Feldom J, Pryce CR. Psychopharmacology (Berlin) 2005;179:292–302. doi: 10.1007/s00213-004-2126-x. [DOI] [PubMed] [Google Scholar]
- 53.Krystal JH, Abi-Saab W, Perry E, D'Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, et al. Psychopharmacology (Berlin) 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- 54.Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, et al. Neuropsychopharmacology. 2007;32:1011–1020. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 55.Honey RAE, Honey GD, O'Loughlin C, Sharar SR, Kumaran D, Bullmore ET, Menon DK, Donovan T, Lupson VC, Bisbrown-Chippendale R, et al. Neuropsychopharmacology. 2004;29:1203–1214. doi: 10.1038/sj.npp.1300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, MacDougall L, Abi-Saab W, D'Souza DC. Arch Gen Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- 57.Liddle P, Kiehl K, Smith A. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deco G, Rolls ET. Prog Neurobiol. 2005;76:236–256. doi: 10.1016/j.pneurobio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 60.Corbetta M, Shulman GL. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 61.Ravizza SM, Delgado MR, Chien JM, Becker JT, Feiz JA. NeuroImage. 2004;22:563–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 62.Meyer-Lindenberg AS, Weinberger DR. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 63.Genovese CR, Lazar NA, Nichols T. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 64.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 65.Goldman-Rakic PS. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 66.Nichols T, Brett M, Andersson J, Wager T, Poline J-B. NeuroImage. 2005;25:653. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Worsley KJM, S., Neelin P, Vandal AC, Friston KJ, Evans AC. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.