Abstract
Synthetic agonists of Toll-like receptor 9 (TLR9), a class of agents that induce specific immune response, exhibit antitumor activity and are currently being investigated in cancer patients. Intriguingly, their mechanisms of action on tumor growth and angiogenesis are still incompletely understood. We recently discovered that a synthetic agonist of TLR9, immune modulatory oligonucleotide (IMO), acts by impairing epidermal growth factor receptor (EGFR) signaling and potently synergizes with anti-EGFR antibody cetuximab in GEO human colon cancer xenografts, whereas it is ineffective in VEGF-overexpressing cetuximab-resistant GEO cetuximab-resistant (GEO-CR) tumors. VEGF is activated by EGFR, and its overexpression causes resistance to EGFR inhibitors. Therefore, we used IMO and the anti-VEGF antibody bevacizumab as tools to study IMO's role on EGFR and angiogenesis and to explore its therapeutic potential in GEO, LS174T, and GEO-CR cancer xenografts. We found that IMO enhances the antibody-dependent cell-mediated cytotoxicity (ADCC) activity of cetuximab, that bevacizumab has no ADCC, and IMO is unable to enhance it. Nevertheless, the IMO-plus-bevacizumab combination synergistically inhibits the growth of GEO and LS174T as well as of GEO-CR tumors, preceded by inhibition of signaling protein expression, microvessel formation, and human, but not murine, VEGF secretion. Moreover, IMO inhibited the growth, adhesion, migration, and capillary formation of VEGF-stimulated endothelial cells. The antitumor activity was irrespective of the TLR9 expression on tumor cells. These studies demonstrate that synthetic agonists of TLR9 interfere with growth and angiogenesis also by EGFR- and ADCC-independent mechanisms affecting endothelial cell functions and provide a strong rationale to combine IMO with bevacizumab and EGFR inhibitory drugs in colon cancer patients.
Keywords: angiogenesis, cancer therapy, growth factor receptors
Activation of Toll-like receptor 9 (TLR9) by DNA containing unmethylated CpG motifs, its natural ligand, produces potent Th1-type innate and adaptive immune responses (1). TLR9-stimulated B cells and plasmacytoid dendritic cells secrete a number of Th-1-promoting cytokines and chemokines, including IL-12, IL-6, IFN-γ, Type 1 IFNs, MIP-1, and IP-10 (2–4). Agonists of TLR9 have shown antitumor activity, alone and in combination with chemotherapy and radiotherapy, and ability to enhance the antibody-dependent cell-mediated cytotoxicity (ADCC) of mAbs in a number of preclinical and early clinical trials (3, 5).
Based on extensive structure–activity relationship studies, synthetic agonists of TLR9 containing novel DNA structures and synthetic dinucleotide motifs, referred to as immune modulatory oligonucleotides (IMOs), have been synthesized, demonstrating distinct cytokine profiles in vitro and in vivo, compared with conventional TLR9 agonists (4, 6, 7) and higher metabolic stability due to the novel DNA structure present in them (8–10). Previous studies have demonstrated potent antitumor activity of IMOs as monotherapies and in combination with chemotherapeutic agents and mAbs (11, 12). Currently, a synthetic agonist of TLR9, IMO-2055, is under clinical evaluation, in combination with chemotherapy and other agents in cancer patients.
Intriguingly, although the TLR9 immunologic mechanisms are fairly well understood, and the clinical development of TLR9 agonists is very encouraging, the mechanisms by which they affect signaling proteins involved in tumor growth and angiogenesis, thus leading to tumor growth inhibition, have yet to be elucidated.
The epidermal growth factor receptor (EGFR) plays a pivotal role in the control of cell growth, apoptosis, and angiogenesis (13), and EGFR blockade by mAbs or small-molecule tyrosine kinase inhibitors has now entered clinical practice in patients affected by different types of cancer, including colorectal (14, 15).
We have recently demonstrated that an IMO inhibits the expression and function of activated EGFR and of other critical downstream proteins (12). IMO exhibited a synergistic antitumor effect with the anti-EGFR mAb cetuximab or the tyrosine kinase inhibitor gefitinib in GEO human colon cancer xenografts, whereas it was ineffective against the cetuximab-resistant tumors GEO cetuximab-resistant (GEO-CR) (12). These findings have opened the path to clinical studies combining IMO with EGFR inhibitors in cancer patients.
We and others have previously shown that colon tumors, including GEO-CR, that acquire resistance to the anti-EGFR drugs cetuximab or gefitinib overexpress and secrete VEGF, which acts as an escape pathway, overcoming the EGFR blockade (16, 17). Moreover, it has been shown that VEGF overexpression markedly impairs the activity of dendritic cells and the antitumor immune response (18, 19). These studies and our demonstration of a cooperative effect of cetuximab with a selective inhibitor of VEGF (20) have provided the basis for the ongoing clinical studies combining inhibitors of EGFR and of VEGF/VEGF receptors (21, 22). Currently, the anti-VEGF mAb bevacizumab is successfully used in combination with chemotherapy for the treatment of colorectal cancer (23) and has shown efficacy also in nonsmall-cell lung and breast cancer (24). Interestingly, the VEGF blockade by bevacizumab recovers the activity of dendritic cells, improving their antitumor function (18, 19). In the former study, we observed a potent inhibitory effect of IMO and cetuximab on VEGF and angiogenesis in wild-type but not in GEO-CR colon tumors, suggesting that the anti-VEGF effect is only EGFR-dependent. To address this issue, provide insight into the mechanism of action of IMO on signaling and angiogenesis, and explore the therapeutic potential of the association of IMO with a specific VEGF inhibitor, we have evaluated the effects of IMO in combination with bevacizumab on the growth, signaling, and angiogenesis of different wild-type and cetuximab-resistant colon cancer models.
Results
Bevacizumab Has No ADCC Activity, and IMO Is Unable to Affect It.
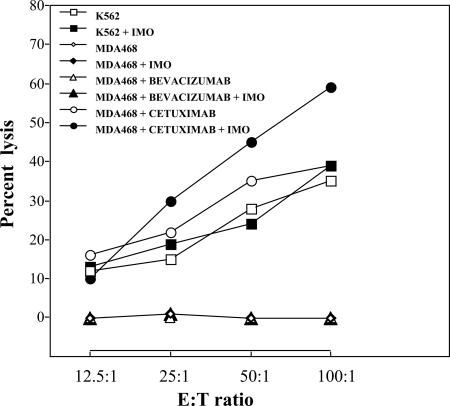
To investigate the capability of bevacizumab to activate an ADCC reaction in cancer cells and the influence of IMO in a combination treatment, we performed a cytotoxic assay using the conventional target MDA-468, a human breast cancer line that expresses both EGFR and VEGF receptors. As expected, in the absence of antibodies, freshly isolated nonadherent human peripheral blood lymphocytes (PBLs) were able to kill the standard NK-target K562 cells but did not induce any detectable lysis of MDA468 cells (Fig. 1). The same result was obtained when PBLs were incubated with IMO. Conversely, the anti-EGFR mAb cetuximab, which possesses a formerly described ADCC mechanism (25), caused a 40% induction of MDA-468 lysis. Preincubation of PBLs with IMO potentiated up to 60% of the MDA-468 killing induced by cetuximab (Fig. 1). On the contrary, preincubation of PBLs with IMO did not affect the inability of bevacizumab to produce the lysis of MDA-468 cells. Therefore, bevacizumab, as well as the combination of IMO and bevacizumab, has no ADCC.
Fig. 1.
ADCC assay. The effector cells (human PBLs) were incubated in the presence or absence of IMO and then mixed with the target cells (K562 and MDA468), which were incubated in the presence or absence of the antibodies cetuximab and bevacizumab. Doses and time of administration are indicated in Materials and Methods.
Combination of Bevacizumab with IMO Synergistically Inhibits GEO and LS174T Colon Cancer Xenografts.
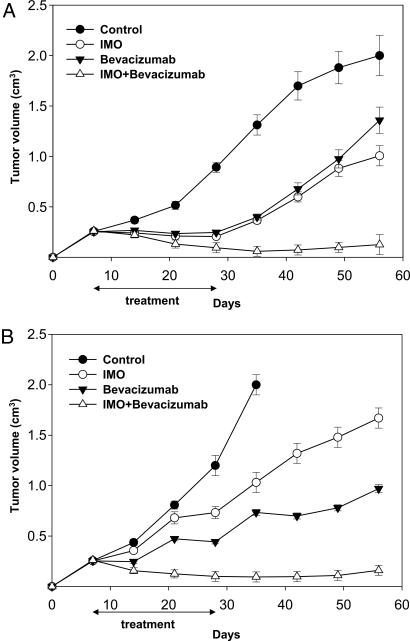
BALB/c nude mice xenografted with GEO and LS174T tumors were treated with IMO or bevacizumab, alone and in combination (Fig. 2). On day 56, 8 weeks after tumor injection, all untreated mice xenografted with GEO cells reached the maximum allowed tumor size of ≈2 cm3, whereas mice treated with IMO alone exhibited 50% growth inhibition, having a size of ≈1 cm3. Treatment with bevacizumab produced a 35% inhibition, because tumors measured 1.3 cm3 at the same time point. The combination of IMO plus bevacizumab caused a potent cooperative antitumor activity, with >95% growth inhibition (tumor size of only 0.12 cm3) (Fig. 2A).
Fig. 2.
Cooperative effect of IMO and bevacizumab on tumor growth of mice bearing human colon cancer xenografts. Seven days after tumor cell injection, mice were randomized (10 per group) to receive the following treatments: i.p. IMO, 1 mg/kg three times per week for 4 weeks; i.p. bevacizumab, 5 mg/kg, twice per week for 3 weeks, or the combination of these agents on days 7–11, 14–18, and 21–25, continuing only IMO on days 28–32. Two mice were killed at day 25 for biochemical and histochemical analyses. Student's t test was used to compare tumor sizes among different treatment groups at day 56 after GEO (A) and LS174T (B) cell injection. The results were statistically significant for IMO plus bevacizumab vs. control (two-sided P < 0.0001), vs. IMO alone (two-sided P < 0.0001), and vs. bevacizumab alone (two-sided P < 0.0001) in both experiments. Error bars indicate SD.
Similar effects were observed in LS174T xenografts. The maximum allowed size of ≈2 cm3 was reached on day 35 in the untreated mice. On day 56, at the end of the experiment, mice treated with IMO or bevacizumab alone measured ≈1.6 and 1 cm3, respectively, whereas those treated with the two agents in combination showed a potent cooperative tumor growth inhibition of ≈95% compared with untreated animals, resulting in a tumor size of 0.16 cm3 (Fig. 2B). No treatment-related side effects were observed in either tumor model studied. Comparison of tumor sizes among different treatment groups, evaluated by Student's t test, was statistically significant both in GEO and LS174T tumors (Fig. 2).
Combination of Bevacizumab with IMO Inhibits the Expression of Signaling Proteins and Angiogenesis in GEO and LS174T Xenografts and Reduces the Levels of Human VEGF (hVEGF), but Not of Murine VEGF (mVEGF), in Mice Serum.
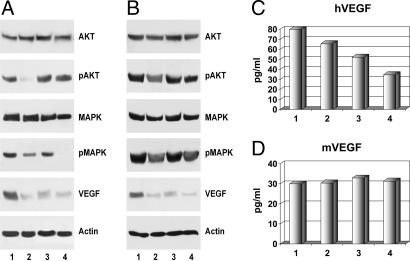
We analyzed the effect of treatment on the expression of a variety of proteins playing a critical role in cancer cell proliferation and angiogenesis. Western blot analysis was performed on cell lysates from tumors removed at the end of the third week of treatment, on day 25. As shown in Fig. 3 A and B, IMO did not affect the total amount of MAPK and Akt but inhibited their activated forms pMAPK and pAkt and VEGF expression. Bevacizumab inhibited the same signaling proteins, although to a lesser degree compared with IMO. When the two agents were used in combination, a more potent inhibition was observed on protein expression. To confirm the effect of a combination of bevacizumab with IMO on hVEGF levels, we performed ELISA on the serum obtained from LS174T-bearing mice (Fig. 3 C and D). Because bevacizumab recognizes only hVEGF, as expected, it caused a reduction in the levels of circulating hVEGF in the serum. The treatment with IMO alone reduced the secreted hVEGF levels, and the combination with bevacizumab caused a more potent inhibition of circulating hVEGF levels compared with treatment with single agents (Fig. 3C). On the contrary, neither single agent nor the combination affected mVEGF compared with untreated mice (Fig. 3D).
Fig. 3.
Western blot analysis of GEO (A) and LS174T (B) tumors and ELISA on mice serum (C and D). (A and B) Western blot analysis was performed on total lysates from tumor specimens of two mice killed on day 25 and treated as in Fig. 1. (C and D) ELISA for hVEGF and mVEGF were performed on the serum of two mice killed on day 25 and treated as in Fig. 2. Doses and time of administration are indicated in Materials and Methods. Lane 1, untreated control; lane 2, IMO; lane 3, bevacizumab; and lane 4, IMO plus bevacizumab.
Immunohistochemical analysis of LS174T tumor specimens performed during the third week of treatment revealed a moderate induction of necrosis caused by IMO treatment and, at a higher degree, by bevacizumab. The combination of the two agents caused a massive hemorrhagic necrosis (in >90% of the tumor). Furthermore, analysis of microvessels demonstrated ≈25% inhibition of CD34-stained host vessels in the animals treated with IMO and ≈40% inhibition after bevacizumab treatment compared with untreated mice. The specimen from mice treated with the two agents in combination showed an inhibition of vessels formation up to 75%.
Combination of Bevacizumab with IMO Causes Potent Antitumor Activity in Cetuximab-Resistant GEO-CR Xenografts.
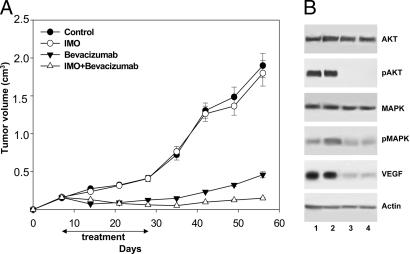
We evaluated whether a cooperative effect could be obtained in cetuximab-resistant GEO-CR tumors, in the absence of an ADCC effect. We have shown that IMO alone is ineffective, whereas bevacizumab markedly inhibited GEO-CR growth (Fig. 4A). When IMO was used in combination with bevacizumab, a cooperative inhibitory effect was observed, because at the end of the experiment, tumors were still ≈0.15 cm3 (Fig. 4A). With the exception of the mice treated with IMO alone, Student's t test demonstrated that the growth inhibition caused by each treatment, in comparison with untreated mice as well as the tumor size among different treatment groups, was statistically different (Fig. 4).
Fig. 4.
Effect of the combination of IMO with bevacizumab in mice bearing cetuximab-resistant GEO-CR tumor xenografts. (A) Seven days after GEO-CR tumor injection, mice were randomized (10 per group) to receive i.p. IMO, 1 mg/kg three times per week for 4 weeks; i.p. bevacizumab, 5 mg/kg, twice per week for 3 weeks, or the combination of these agents, on days 7–11, 14–18, and 21–25, continuing only IMO on days 28–32. Inhibition of growth was significantly different in the IMO plus bevacizumab-treated group vs. the control group, the IMO alone group, and the bevacizumab alone group (two-sided P < 0.0001 for each comparison). (B) Western blot analysis was performed on total lysates from tumor specimens of two mice killed on day 25 and treated as in Fig. 2. Lane 1, untreated control; lane 2, IMO; lane 3, bevacizumab; and lane 4, IMO plus bevacizumab. Doses and time of administration are indicated in Materials and Methods. Error bars indicate SD.
Western Blot Analysis of GEO-CR Tumors.
Western blot analysis of protein extracts from GEO-CR tumors did not reveal any substantial changes in the expression of pAkt, pMAPK, and VEGF in tumor specimens treated with IMO alone, whereas a marked inhibition was seen in those treated with bevacizumab or with the combination IMO plus bevacizumab (Fig. 4B).
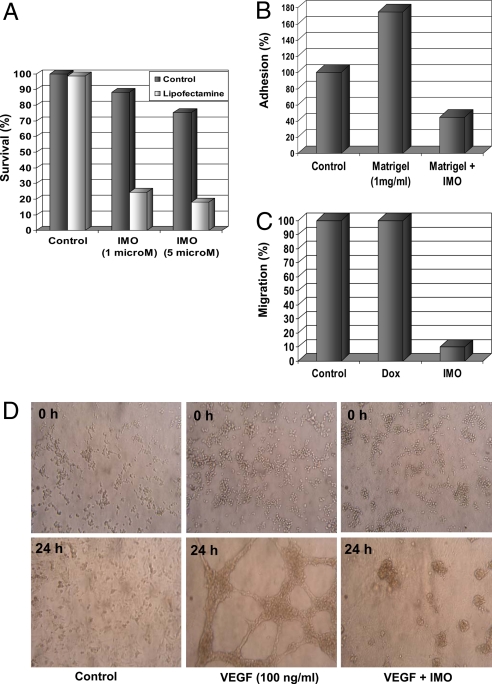
IMO Inhibits Survival, Adhesion to Matrix, Migration, and Capillary Formation Capability in Human Endothelial Cells.
To evaluate whether antiangiogenic activity of IMO could be linked also to a direct effect on human endothelial cells, we performed a cell survival assay on human umbilical vein endothelial cells (HUVEC). At doses of 1 and 5 μM, IMO caused a moderated inhibition of HUVEC survival, which was increased by the addition of lipofectamine, an agent that facilitates cellular uptake of IMO (Fig. 5A). We then analyzed the effects of IMO on HUVEC adhesion and migration by using a cell adhesion assay to matrigel and a wound-healing assay. At 1 μM concentration, IMO showed a potent inhibitory activity on adhesion and migration of endothelial cells (Fig. 5 B and C). Finally, we examined the effects of IMO on VEGF-stimulated capillary tube and network formation and observed that this process is strongly inhibited by IMO (Fig. 5D).
Fig. 5.
IMO effects on HUVEC survival (A), adhesion to matrix (B), migration (C), and tube formation (D). (A) HUVEC were treated with IMO, 1 and 5 μM, in the presence or absence of Lipofectamine (2 μg/ml). The results are statistically significant for each dose of IMO vs. control (two-sided P < 0.0001). (B) HUVEC plated in the presence or absence of 1 μM IMO in serum-free medium (negative control) or Matrigel. The results are statistically significant for Matrigel vs. the negative control and for IMO vs. Matrigel (two-sided P < 0.0001). (C) HUVEC monolayers were wounded in the absence or presence of 10 ng/ml doxorubicin or 1 μM IMO (0 h). The results are statistically significant for IMO vs. control and vs. doxorubicin (two-sided P < 0.0001). (D) HUVEC were incubated with diluted Matrigel in the presence or absence of 1 μM IMO. Matrigel mixed with 100 ng/ml VEGF was used as positive control. Photographs were taken at 0 and 24 h.
TLR9 Is Expressed in LS174T, GEO, and GEO-CR Cells but Does Not Affect Their Growth in Vitro.
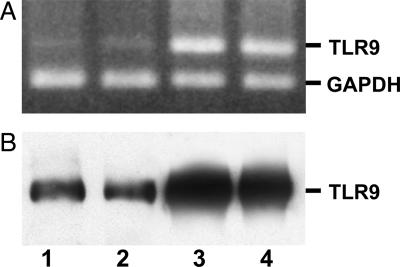
We analyzed TLR9 expression in LS174T, GEO, and GEO-CR cell lines. In an earlier study, we were unable to detect TLR9 on GEO cells by using a direct Western blot assay (12). We have now used a more recent antibody (see Materials and Methods) and assayed each sample by immunoprecipitation followed by Western blot analysis, revealing a different degree of TLR9 protein expression in all cell lines. Further, the RT-PCR assay confirmed TLR9 mRNA expression in all of the cell lines examined (Fig. 6). We then investigated whether IMO could directly affect cell survival. Treatment of these cells with IMO at doses ranging from 0.1 to 1 μM, with or without lipofectamine, did not produce any effect on cell proliferation (data not shown).
Fig. 6.
Evaluation of TLR9 expression in human colon cancer cell lines. (A) TLR9 mRNA expression was evaluated in cultured LS174T, GEO, and GEO-CR colon cancer cell lines. (B) TLR9 expression was confirmed at the protein level by using immunoprecipitation and blotting with the same monoclonal anti-TLR9 antibody. PBMCs were included as positive control as well as RT-PCR targeting human GAPDH. Lane 1, PBMCs; lane 2, LS174T; lane 3, GEO; and lane 4, GEO-CR.
Discussion
Synthetic agonists of TLR9 are a class of compounds with antitumor activity that have entered the clinical evaluation in cancer patients in combination with chemotherapy (3). Although it is well documented that TLR9 agonists induce a potent innate and adaptive immune response and enhance the ADCC of mAbs, the mechanisms by which they affect growth signaling and angiogenesis are yet poorly understood.
We have recently hypothesized and demonstrated that a synthetic agonist of TLR9 (IMO) impairs EGFR and its downstream signaling proteins, including VEGF (12). The combination of IMO with anti-EGFR mAb cetuximab or tyrosine kinase inhibitor gefitinib, synergistically inhibits GEO tumor growth, the expression of several critical proteins and angiogenesis. Conversely, IMO is inactive against cetuximab-resistant GEO-CR tumors, further suggesting its dependence on EGFR signaling (12).
The EGFR pathway has a strong correlation with VEGF. In fact, EGFR can transactivate VEGF production (26, 27) and VEGF overexpression is a major escape pathway used by colon cancer, including GEO-CR tumors, to acquire resistance to EGFR antagonists (16, 21, 28, 29). This notion was recently confirmed in colon cancer patients failing treatment with cetuximab (30). On these bases, recently bevacizumab has been successfully used also in combination with EGFR inhibitors (22). Interestingly, VEGF impairs dendritic cell function, a major target of TLR9 agonists, and bevacizumab can overcome such interference (18, 19). We have previously shown that IMO fails to inhibit the growth as well as VEGF expression in GEO-CR tumors, suggesting that the interference of IMO with VEGF observed in wild-type tumors is actually an EGFR-dependent activity (12).
Based on the above studies, in the present work, we have analyzed the role played by IMO on these signaling pathways and its therapeutic implications. In particular, we have studied whether: (i) the potent inhibitory effect obtained with IMO and cetuximab on VEGF and angiogenesis depends on or is independent of interference in the EGFR pathway; (ii) the dependence of IMO on an integral EGFR pathway may affect its combination with an anti-VEGF agent, such as bevacizumab; and (iii) the ADCC mechanism and the expression of TLR9 are necessary to obtain a cooperative effect with mAbs.
To this aim, we combined IMO with bevacizumab, taking advantage of the fact that bevacizumab targets only the tumor-produced hVEGF and does not interfere with the mVEGF. Because bevacizumab binds the ligand and not the membrane receptor, we verified whether it has any ADCC activity, and whether IMO affects it. We have shown that the basal ADCC activity of cetuximab is enhanced by IMO in an in vitro assay, thus contributing to cetuximab activity with an EGFR-independent mechanism. Conversely, bevacizumab has no ADCC activity, and IMO is unable to affect it. We have then demonstrated that the combination of bevacizumab with IMO causes a synergistic inhibition of tumor growth in human colon cancer xenografts GEO and LS174T and in the cetuximab-resistant GEO-CR, resulting in 90% of mice being tumor-free at pathologic analysis at the end of the experiment, 4 weeks after treatment withdrawal. Therefore, this combination treatment is also very effective in anti-EGFR-resistant tumors in an ADCC-independent fashion, suggesting that other mechanisms, not strictly EGFR- and ADCC-dependent, take place. In support of this notion, the two agents in combination cooperatively inhibit the expression of proteins used by tumors as escape pathways to acquire resistance to targeted therapies, such as pMAPK, pAkt, and VEGF (29) and inhibit neoangiogenesis in all three tumor types.
Analysis of the secreted VEGF in the serum of killed mice confirmed that bevacizumab, as expected, reduces the hVEGF levels, and also that the combination of IMO and bevacizumab cooperates in reducing the levels of hVEGF but not mVEGF. These results suggest that the murine-dependent immune-mediated effects of IMO enhance the activity of bevacizumab only on the human tumor cells. Interestingly, IMO and bevacizumab in combination caused a massive hemorrhagic necrosis, evaluated by pathological and immunohistochemical analysis, as early as the third week of treatment. An important mechanism of antiangiogenic therapy is the blockade of the VEGF-dependent proliferation of endothelial cells in the tumor. In an attempt to provide a clue to explain the non-EGFR-dependent cooperative antiangiogenic effects obtained with IMO and bevacizumab, we measured their activity on several functions of endothelial cells. We demonstrated that IMO inhibits proliferation, adhesion, and migration of HUVEC endothelial cells and, importantly, the VEGF-stimulated capillary tube and network formation. Therefore, it is likely that the well documented inhibitory effect of bevacizumab on vessel formation, due to VEGF inhibition, combined with the interference of IMO on critical functions of tumor endothelial cells, may finally be responsible for the cooperative effect observed. In addition, because it has been reported that colon cancer cell lines, including GEO, express VEGF receptors (31), the combined effect of IMO and bevacizumab may have a direct impact also on tumor cells. Together, these data may also help explain, at least in part, the hemorrhagic necrosis observed in the LS174T tumors.
Finally, we attempted to understand the relevance of TLR9 expression for the antitumor effects observed. Measurement of TLR9 by different techniques demonstrated the presence of mRNA and protein expression, to a different degree, in GEO, LS174T, and GEO-CR cells. Nevertheless, treatment of these cells in vitro with IMO, at different doses, with or without lipofectamine to improve cellular penetration, did not produce any effect on tumor growth or on EGFR expression. In addition, the in vivo effect of IMO with or without bevacizumab was not proportional to the degree of TLR9 expression. Therefore, the expression of TLR9 on these colon tumor cells is not directly responsible for the antitumor effects observed but is more likely related to the systemic immune responses produced by IMO.
Taken together, these studies provide insights into the mechanisms of action of synthetic agonists of TLR9. In fact, they demonstrate that, although dependent on the immune activation, IMO has a much broader range of mechanisms, involving not only the EGFR-dependent pathway but also the neoangiogenesis. These results provide a strong rationale to translate the combination with bevacizumab in the clinical practice, as a potentially powerful and rationally based therapeutic strategy. The possibility of combining IMO with inhibitors of EGFR and of VEGF creates the opportunity to take advantage of multiple chance for cooperativity, involving EGFR- and ADCC-dependent and -independent mechanisms and neoangiogenesis.
Materials and Methods
Compounds.
IMO, 5′-TCTGACRTTCT-X-TCTTRCAGTCT-5′ (X and R are the glycerol linker and 2′-deoxy-7-deazaguanosine, respectively) was synthesized with phosphorothioate backbone, purified, and analyzed as described (6–8, 32). The mAb anti-VEGF bevacizumab was kindly provided by Genentech (South San Francisco, CA).
Cell Cultures.
GEO, LS174T, GEO-CR (16) colon cancer cells, and HUVEC were maintained, respectively, in McCoy's or RPMI medium 1640 supplemented with 10% heat-inactivated FBS/20 mM Hepes, pH 7.4/penicillin (100 units/ml)/streptomycin (100 μg/ml)/4 mM glutamine (ICN, Irvine, CA) in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
ADCC Assay.
A nonadherent fraction of human peripheral blood mononuclear cells (PBMCs) was used as effector cells. Briefly, human PBMCs were isolated by density gradient centrifugation and resuspended in RPMI medium 1640. Before the assay, the PBMCs were cultured for 1 h on plastic dishes to remove adherent cells (monocytes), and then the PBLs were incubated for 24 h in the presence or absence of IMO (5 μM). The target cells (chronic erythroid leukemia K562 and breast cancer MDA468) were loaded with the fluorescence-enhancing ligand (DELFIA BATDA reagent; PerkinElmer, Wellesley, MA) and, after washing, were incubated in the presence or absence of the antibodies bevacizumab or cetuximab (10 μg/ml). Target cells were mixed with effector cells at varying cell concentrations for 2 h at 37°C and centrifuged. Supernatants were added to Europium solution and the signal was measured, as described (33).
Xenografts in Nude Mice.
Five-week-old BALB/cAnNCrlBR athymic (nu+/nu+) mice (Charles River Laboratories, Milan, Italy) were maintained in accordance with the institutional guidelines of the University of Naples Animal Care Committee in accordance with the Declaration of Helsinki. Wild-type GEO, LS174T, or GEO-CR human colon cancer cells (107 cells per mouse) were resuspended in 200 μl of Matrigel (Collaborative Biomedical Products, Bedford, MA) and injected s.c. in mice. After 7 days, tumors were detected, and groups of 10 mice were randomized to receive the following treatments: i.p. IMO, 1 mg/kg, three times per week for 4 weeks; i.p. bevacizumab, 5 mg/kg, twice per week for 3 weeks; or the combination of these agents, on days 7–11, 14–18, and 21–25, continuing only IMO on days 28–32. Tumor volume was measured by using the formula π/6 × larger diameter × (smaller diameter)2, as reported (34). Two mice were killed on day 25 for biochemical analysis.
Immunoprecipitation and Western Blot Analysis.
Total cell lysates were obtained from homogenized tumor specimens removed on day 25. The protein extracts were resolved by 4–15% SDS/PAGE and probed with anti-human polyclonal Akt, monoclonal pAkt (Cell Signaling Technologies, Beverly, MA), monoclonal actin (Sigma-Aldrich, Milan, Italy), monoclonal VEGF, monoclonal pMAPK, and monoclonal MAPK (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive proteins were visualized by enhanced chemiluminescence (Pierce, Rockford, IL), as described (16). TLR9 protein levels were evaluated on total cell lysates by immunoprecipitation by using a monoclonal anti-TLR9 antibody (Calbiochem/EMD Biosciences, La Jolla, CA) and blotting with the same antibody, following the procedures described above.
Immunohistochemical Analysis.
Immunocytochemistry was performed on formalin-fixed paraffin-embedded tissue sections (5 μm) of LS174T xenografts. Sections were processed, reacted with avidin-conjugated horseradish peroxidase H complex, and stained as described (34). New blood vessels were detected by using a mAb against the CD34 antigen (Dako, Milan, Italy) at a dilution of 1:50 and stained with a standard immunoperoxidase method (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). Each slide was scanned at low power (10–100× magnification), and the area with the higher number of new vessels was identified (hot spot). This region was then scanned at 250× magnification (0.37 mm2). The number of microvessels per field was scored by averaging five field counts of two individual tumors for each group.
ELISA.
Anti-hVEGF or anti-mVEGF polyclonal antibody (R&D Systems, Minneapolis, MN), diluted at 1 μg/ml in PBS, pH 7.5, was used to coat a 96-well plate, 100 μl/well, overnight at 4°C. Washings, dilutions of standards (recombinant hVEGF or mVEGF), and samples (serum of killed mice), biotinylation, and mix with preformed avidin and biotinylated HRP macromolecular complex (Vectastain kit) were described (35). The absorbance was measured at 490 nm on a microplate reader (Bio-Rad, Hercules, CA). VEGF concentrations were determined by interpolation of the standard curve by using linear regression analysis.
RT-PCR.
Total RNA was extracted by using the TRIzol reagent from Invitrogen Life Technologies (Grand Island, NY) and was quantified and used to create cDNA. Amplification of TLR9 was accomplished by using primers as published (36), and a portion of the PCR product was visualized by using ethidium bromide on an agarose gel. Human GAPDH was coamplified with TLR9 to verify the quality and expression level of the mRNA.
Cell Survival Assay.
Cells were grown in 24-well plates and exposed to IMO with or without Lipofectamine 2000 (2 μg/ml) from Invitrogen Life Technologies. The percentage of cell survival was determined by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer's instructions.
Adhesion Assay.
Ninety-six-microwell bacterial culture plates were precoated with 50 μl/well of serum-free medium (SFM) containing 0.1% BSA (negative control) or Matrigel (1 mg/ml in water). After 1 h, all coating solutions were removed, and HUVEC (20,000 cells per well) were plated in SFM, in the presence or absence of 1 μM IMO. After incubation, cells were analyzed as described (37).
Wound-Healing Assay.
Monolayers of HUVEC were grown on gridded plastic dishes and scratched as reported (38) with or without 10 ng/ml doxorubicin or 1 μM IMO (0 h), which have the same antiproliferative effect. Because doxorubicin did not interfere with cell migration, it was used as a negative control. The migration distances between the edges of the cells in the wound were photographed (10× magnification) at 0 and 24 h, quantified, and compared by using Adobe Photoshop, Ver. 8.0.1 (38).
Vascular Endothelial Cell Capillary Tube and Network Formation.
Five hundred microliters of diluted Matrigel was added into a 30-mm culture dish and incubated at 37°C for 30 min. After the Matrigel was solidified, HUVEC (4 × 105) in 1 ml of RPMI medium 1640 were added in each dish, in the presence or absence of 1 μM IMO, incubated at 37°C, and photographed (10×) at 0 and 24 h. As a positive control, Matrigel was mixed with 100 ng/ml VEGF (R&D Systems).
Statistical Analysis.
Student's t test was used to evaluate the statistical significance of the results. All reported P values were two-sided. All analyses were performed with the BMDP New System statistical package for Microsoft Windows (Version 1.0; BMDP Statistical Software, Los Angeles, CA).
Acknowledgments
This paper is dedicated to the memory of Dr. Y.-S. Cho-Chung. We acknowledge the excellent technical assistance of Gaetano Borriello. This study was supported by grants from the Associazione Italiana per la Ricerca sul Cancro, the Ministry of Health, and the Regione Campania.
Abbreviations
- TLR9
Toll-like receptor 9
- ADCC
antibody-dependent cell-mediated cytotoxicity
- IMO
immune modulatory oligonucleotide
- EGFR
epidermal growth factor receptor
- PBL
peripheral blood lymphocyte
- hVEGF
human VEGF
- mVEGF
murine VEGF
- HUVEC
human umbilical vein endothelial cells
- GEO-CR
GEO cetuximab-resistant
- PBMC
peripheral blood mononuclear cells.
Footnotes
Conflict of interest statement: G.T. stands in the Advisory Board of, and E.R.K. and S.A. are employees of (and hold stock options in), Idera Pharmaceuticals.
References
- 1.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, et al. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 2.Tokunaga T, Yano O, Kuramoto E, Kimura Y, Yamamoto T, Kataoka T, Yamamoto S. Microbiol Immunol. 1992;36:55–66. doi: 10.1111/j.1348-0421.1992.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 3.Krieg AM. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 4.Kandimalla ER, Bhagat L, Li Y, Yu D, Wang D, Cong YP, Song SS, Tang JX, Sullivan T, et al. Proc Natl Acad Sci USA. 2005;102:6925–6930. doi: 10.1073/pnas.0501729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Ojik HH, Bevaart L, Dahle CE, Bakker A, Jansen MJ, van Vugt MJ, van de Winkel JG, Weiner GJ. Cancer Res. 2003;63:5595–5600. [PubMed] [Google Scholar]
- 6.Kandimalla ER, Bhagat L, Zhu FG, Yu D, Cong YP, Wang D, Tang JX, Tang JY, Knetter CF, et al. Proc Natl Acad Sci USA. 2003;100:14303–14308. doi: 10.1073/pnas.2335947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandimalla ER, Bhagat L, Wang D, Yu D, Zhu FG, Tang J, Wang H, Huang P, Zhang R, et al. Nucleic Acids Res. 2003;31:2393–2400. doi: 10.1093/nar/gkg343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu D, Kandimalla ER, Bhagat L, Tang JY, Cong Y, Tang J, Agrawal S. Nucleic Acids Res. 2002;30:4460–4469. doi: 10.1093/nar/gkf582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandimalla ER, Bhagat L, Yu D, Cong Y, Tang J, Agrawal S. Bioconjug Chem. 2002;13:966–974. doi: 10.1021/bc0200374. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Kandimalla ER, Yu D, Tang JX, Agrawal S. Vaccine. 2005;23:2614–2622. doi: 10.1016/j.vaccine.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Li Y, Yu D, Song SS, Kandimalla ER, Agrawal S. Int J Oncol. 2004;74:901–908. [PubMed] [Google Scholar]
- 12.Damiano V, Caputo R, Bianco R, D'Armiento FP, Leonardi A, De Placido S, Bianco AR, Agrawal S, Ciardiello F, et al. Clin Cancer Res. 2006;12:577–583. doi: 10.1158/1078-0432.CCR-05-1943. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn J, Baselga J. Semin Oncol. 2006;33:369–385. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, et al. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 15.Saltz LB, Meropol NJ, Lochrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 16.Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, Melisi D, De Vita F, De Placido S, et al. Clin Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- 17.Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, Rak J, Kerbel RS. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 18.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 19.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Clin Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- 20.Ciardiello F, Bianco R, Damiano V, Fontanini G, Caputo R, Pomatico G, De Placido S, Bianco AR, Mendelsohn J, et al. Clin Cancer Res. 2000;6:3739–3747. [PubMed] [Google Scholar]
- 21.Ciardiello F, Troiani T, Bianco R, Orditura M, Morgillo F, Martinelli E, Morelli MP, Cascone T, Tortora G. Ann Oncol. 2006;17:109–114. doi: 10.1093/annonc/mdl962. [DOI] [PubMed] [Google Scholar]
- 22.Sandler A, Herbst R. Clin Cancer Res. 2006;12:4421–4425. doi: 10.1158/1078-0432.CCR-06-0796. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz H, Kabbinavar F. Oncology. 2005;69:17–24. doi: 10.1159/000088480. [DOI] [PubMed] [Google Scholar]
- 24.Gasparini G, Longo R, Toi M, Ferrara N. Nat Clin Pract Oncol. 2005;2:562–577. doi: 10.1038/ncponc0342. [DOI] [PubMed] [Google Scholar]
- 25.Fan Z, Masui H, Altas I, Mendelsohn J. Cancer Res. 1993;53:4322–4328. [PubMed] [Google Scholar]
- 26.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 27.Perrotte P, Matsumoto T, Inoue K, Kunivasu H, Eve BY, Hicklin DJ, Radinsky R, Dinney CP. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- 28.Viloria-Petit AM, Kerbel RS. Int J Radiat Oncol Biol Phys. 2004;1:914–926. doi: 10.1016/j.ijrobp.2003.09.091. [DOI] [PubMed] [Google Scholar]
- 29.Bianco R, Troiani T, Tortora G, Ciardiello F. Endoc Relat Cancer. 2005;12:159–171. doi: 10.1677/erc.1.00999. [DOI] [PubMed] [Google Scholar]
- 30.Vallbohmer D, Zhang W, Gordon M, Yang DY, Yun J, Press OA, Rhodes KE, Sherrod AE, Iqbal S, et al. J Clin Oncol. 2005;23:3536–3544. doi: 10.1200/JCO.2005.09.100. [DOI] [PubMed] [Google Scholar]
- 31.Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer T, Somcio RJ, Wu Y, Hooper A, et al. Oncogene. 2005;24:2647–2653. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 32.Yu D, Zhu FG, Bhagat L, Wang H, Kandimalla ER, Zhang R, Agrawal S. Biochem Biophys Res Commun. 2002;297:83–90. doi: 10.1016/s0006-291x(02)02127-7. [DOI] [PubMed] [Google Scholar]
- 33.Blomberg K, Hautala R, Lovgren J, Mukkala VM, Lindgvist C, Akerman K. J Immunol Methods. 1996;193:199–206. doi: 10.1016/0022-1759(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 34.Ciardiello F, Damiano V, Bianco R, Bianco C, Fontanini G, De Laurentiis M, De Placido S, Mendelsohn J, Bianco AR, et al. J Natl Cancer Inst. 1996;88:1770–1776. doi: 10.1093/jnci/88.23.1770. [DOI] [PubMed] [Google Scholar]
- 35.Errico M, Riccioni T, Iyer S, Pisano C, Acharya KR, Persico MG, De Falco S. J Biol Chem. 2004;279:43929–43939. doi: 10.1074/jbc.M401418200. [DOI] [PubMed] [Google Scholar]
- 36.Droemann D, Albrecht D, Gerdes J, Ulmer AJ, Branscheid D, Vollmer E, Dalhoff K, Zabel P, Goldmann T. Respir Res. 2005;6:1. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benelli R, Peissel B, Manenti G, Gariboldi M, Vanzetto C, Albini A, Dragani TA. Oncogene. 2003;22:7711–7715. doi: 10.1038/sj.onc.1207088. [DOI] [PubMed] [Google Scholar]
- 38.Bennett RD, Mauer AS, Strehler EE. J Biol Chem. 2007;282:3205–3212. doi: 10.1074/jbc.M607174200. [DOI] [PubMed] [Google Scholar]