Abstract
Heart valve structures derived from mesenchymal cells of the endocardial cushions (EC) are composed of highly organized cell lineages and extracellular matrix. Sox9 is a transcription factor required for both early and late stages of cartilage formation that is also expressed in the developing valves of the heart. The requirements for Sox9 function during valvulogenesis and adult valve homeostasis in mice were examined by conditional inactivation of Sox9 using Tie2-cre and Col2a1-cre transgenes. Sox9flox/flox;Tie2-cre mice die before E14.5 with hypoplastic ECs, reduced cell proliferation and altered extracellular matrix protein (ECM) deposition. Sox9flox/flox;Col2a1-cre mice die at birth with thickened heart valve leaflets, reduced expression of cartilage-associated proteins, and abnormal ECM patterning. Thickened valve leaflets and calcium deposits, characteristic of valve disease, are observed in heterozygous adult Sox9flox/+;Col2a1-cre mice. Therefore, Sox9 is required early in valve development for expansion of the precursor cell population and later is required for normal expression and distribution of valvular ECM proteins. These data indicate that Sox9 is required for early and late stages of valvulogenesis and identify a potential role for Sox9 in valve disease mechanisms.
Keywords: Heart valves, transcription factor, extracellular matrix, cartilage, Sox9
Introduction
Congenital heart defects are among the most common developmental anomalies, and often include malformations of valve structures; however, the origins of these abnormalities are not well known (Hoffman and Kaplan, 2002). During embryonic development, heart valve formation begins in the atrioventricular (AV) canal and outflow tract (OFT) with endothelial to mesenchymal cell transformation (EMT) and subsequent formation of endocardial cushions (ECs) (Armstrong and Bischoff, 2004; Person et al., 2005). Newly transformed mesenchymal cells continue to proliferate and diversify into distinct cell lineages that form mature heart valve leaflets and supporting chordae tendineae (Armstrong and Bischoff, 2004; Lincoln et al., 2004; Lincoln et al., 2006c; Person et al., 2005). The mature heart valve structures are highly organized and express specific extracellular matrix (ECM) proteins characteristic of cartilage in the valve leaflets and tendon in the chordae tendineae (Lincoln et al., 2004; Rabkin-Aikawa et al., 2005). These diversified valve structures arise from a common pool of precursor cells in the ECs, and there is increasing evidence to suggest that mechanisms required for valve precursor cell diversification and differentiation are common with regulatory hierarchies occurring in other connective tissues (de Lange et al., 2004; Edom-Vovard and Duprez, 2004; Goldring et al., 2006; Lange and Yutzey, 2006; Lincoln et al., 2004; Lincoln et al., 2006c).
Sox9 is a SRY-related transcription factor required for cartilage formation and male sex determination (Bi et al., 1999; Chaboissier et al., 2004; Goldring et al., 2006). Mutations of human SOX9 cause Campomelic Dysplasia (CD), a disease characterized by sex reversal, and skeletal defects including bowing and thickening of long bones, and cleft palate (Foster et al., 1994; Wagner et al., 1994). Genetically manipulated mice with reduced Sox9 function have been used to determine requirements for Sox9 during early and late stages of cartilage formation (Akiyama et al., 2002; Bi et al., 1999; Bi et al., 2001; Kist et al., 2002). During skeletal development, cartilage cell lineage diversification is initiated in a population of mesenchymal precursor cells that condense and form pre-cartilaginous aggregates (Goldring et al., 2006). Inactivation of Sox9 in early stage cartilage-precursor cells leads to a complete absence of cartilage formation, indicating a requirement during early stages of chondrogenesis (Akiyama et al., 2002). Later during chondrogenic differentiation, Sox9 directly binds regulatory elements of cartilage-specific genes including type II collagen and Cartilage Link Protein (CLP) (Akiyama et al., 2002; Kou and Ikegawa, 2004; Lefebvre and Smits, 2005; Ng et al., 1997). Together, these studies demonstrate that Sox9 is required during early cartilage cell lineage condensation and later for differentiation of chondrogenic cell types.
In the developing heart, a common pool of progenitor cells within the EC develops into the heart valve leaflets and supporting structures (Lincoln et al., 2004). There is initial evidence from avian embryos that this progenitor pool diversifies into distinct cell lineages that are subject to regulatory pathways that also control cartilage and tendon lineage development (Lincoln et al., 2006c). Sox9, primarily associated with cartilage lineages, is expressed early in valve precursor cells of the EC, and expression is maintained in the mature valve leaflets during later stages of development (Akiyama et al., 2004; Lincoln et al., 2006a; Montero et al., 2002; Rahkonen et al., 2003). The role of Sox9 during valvulogenesis is not fully understood. Mice with germline inactivation of Sox9 die between E11.5 and E12.5 and display hypoplastic ECs that fail to complete EMT, suggesting a role during initial stages of EC formation (Akiyama et al., 2004). Sox9 is predominantly expressed throughout all stages of valvulogenesis, however the functions of Sox9 during post-EMT valve precursor cell maturation have not yet been defined. Recently it has been shown that BMP signaling activates Sox9 expression and up-regulates cartilage-associated genes in cultured avian mesenchyme valve precursor cells, consistent with regulatory pathways reported in developing cartilage (Lincoln et al., 2006a; Yoon and Lyons, 2004). However, the in vivo function of Sox9 in valve precursor cell lineage development, as well as formation and patterning of later stage valvular structures, has not yet been determined.
In this study, we use conditional inactivation of Sox9 during early and late stages of valvulogenesis to examine the requirements for Sox9 during expansion and diversification of valve precursor cells, as well in expression and organization of ECM proteins characteristic of cartilage-like cells types in the mature valves. The Sox9 floxed allele was inactivated in endothelial cell derivatives that make up the EC using Tie2-cre, or in a subset of differentiating valve cells using Type II collagen a1-cre (Col2a1-cre) (Kist et al., 2002; Le and Sauer, 2001; Nagy, 2000). Loss of Sox9 function in endothelial-derived cells shows that it is required for expansion and diversification of the valve precursor cell pool following EMT. Later inactivation of Sox9 with Col2a1-cre results in reduced expression of cartilage matrix-associated markers and abnormal ECM patterning in remodeling valve leaflets. Heart valve calcification and increased ECM production was observed in adult mice with Col2a1-cre mediated heterozygous loss of Sox9. These data support an early role for Sox9 in EC cell proliferation and later roles in differentiation, patterning and homeostasis of mature valve structures.
Materials and Methods
Generation of mice
Sox9flox/flox female mice (Kist et al., 2002) were bred with Tie2-cre (Tek-cre) or Col2a1-cre males (Jackson Laboratories) (Kisanuki et al., 2001; Ovchinnikov et al., 2000) to generate heterozygous offspring that were obtained at expected Mendelian ratios. Heterozygous Sox9flox/+;Tie2-cre or Sox9flox/+;Col2a1-cre males were mated with Sox9flox/flox females to generate homozygous offspring (Sox9flox/flox;Tie2-cre or Sox9flox/flox;Col2a1-cre). Alternatively, Sox9flox/+;Col2a1-cre male mice were sacrificed at 1 or 3 months of age and the hearts collected in 1x phosphate buffered saline (PBS). Timed embryonic (E) staged litters of mice were collected at E11.5-E18.5, counting day E0.5 by evidence of a copulation plug for Sox9flox/flox;Tie2-cre (E11.5-E14.5 n=11 litters), Sox9flox/flox;Col2a1-cre (E15.5-E18.5, n=14 litters) and non-transgenic (NTG) FVBN mice (E13.0, E13.5, E18.5). Tie2-cre or Col2a1-cre transgenic mice were crossed with Rosa26R-lacz (Rosa26R) reporter mice to analyze β-galactosidase expression at embryonic stages E13.5 (Tie2-cre) and E15.5-E18.5 (Col2a1-cre) (n=7 total litters) as previously reported (Lincoln et al., 2004; Soriano, 1999). Genotyping for the Cre transgene and Sox9 floxed alleles was performed by RT-PCR as previously described for embryonic yolk sac or adult genomic DNA (Kist et al., 2002; Lincoln et al., 2004).
Histological Analysis
E11.5-E14.5 whole embryos, or hearts from E18.5 embryos and adult mice, were collected in 1xPBS and fixed in 4% paraformaldehyde (PFA) overnight at 4oC. Tissues were subsequently processed for either frozen or paraffin embedding as previously described (Lincoln et al., 2006a). Frozen sections were cut at 14 μm, mounted on Permafrost slides (Fisher) and in situ hybridization was performed as previously reported (Lincoln et al., 2006a). The Sox9 riboprobe was a kind gift from Dr. Ravi Elluru, and antisense probe was generated as previously described (Elluru and Whitsett, 2004). In situ probes for Msx-1 and Has-2 were kind gifts from Dr. James Martin (Ma et al., 2005). Frozen 14 μm sections from E13.5 Tie2-cre mice bred with Rosa26R were used for X-gal staining as previously reported (Lincoln et al., 2004; Stenman et al., 2003).
Paraffin-embedded tissue was sectioned at 5 μm and, following deparaffinization and hydration through a graded ethanol series (100%, 95%, 75%, 50%), tissue was stained with Movat’s Pentachrome, Von Kossa, Alcian blue, or subject to immunohistochemistry (IHC). Procedures for Pentachrome and Von Kossa staining have been previously described (Jones, 2001). Sections subject to Alcian blue (0.1%/0.5% acetic acid, pH 3.1) were stained for 15 minutes, rinsed in water, counterstained in 15% nuclear fast red (Vectastain), dehydrated and mounted in Cytoseal (Richard Allen Scientific). IHC for β-galactosidase (Abcam, 1:200), Sox9 (Santa Cruz, 1:200), phosphohistone H3 (pHH3) (Upstate, 1:100), cleaved caspase 3 (Cell Signaling, 1:200) and β-catenin (Signal Transduction, 1:200) was performed using diaminobenzidine (DAB) colormetric analysis following the manufacturer’s instructions (Santa Cruz). In addition, boiling tissue sections for 10 minutes in 0.1 M sodium citrate was required for antigen retrieval and detection of β-catenin. To determine cell proliferation, the number of pHH3 positive nuclei was calculated as a percentage of the total number of nuclei in 15 sections from 3 independent embryos for each group. The value of the total number of cells in E11.5 and E12.5 in these sections was averaged and statistical significance of observed differences was determined by Student’s t-test (P<0.05).
Elastin (Sigma, 1:1000), Cartilage Link Protein (CLP) (Hybridoma Bank, Iowa, 1:5), tenascin (Chemicon, 1:400), β-galactosidase (Abcam, 1:200) and type II collagen (Abcam, 1:200) were detected using immunofluorescence (IF). Following deparaffinization, tissue sections for IF were incubated with blocking solution (1% bovine serum albumin, 0.1% cold water fish skin gelatin, 0.1% Tween-20, 0.05% NaN3/PBS) for 1 hour at room temperature. Tissues were incubated overnight at 4oC with primary antibodies in 1:1 block/PBS using Coverwell chamber slides (Grace Biolabs). Following incubation, sections were washed in 1xPBS and incubated with corresponding Alexa fluorescent-conjugated secondary antibodies (Molecular probes, 1:100/PBS) and TOPRO-3 (Molecular probes, 1:1000) for 1 hour at room temperature. Sections were washed thoroughly in 1xPBS and mounted in hard set Vectashield (Vectorlabs). For double staining, tissue sections were incubated overnight with both primary antibodies, while fluorescent secondary antibodies were applied separately as described. Fluorescent images were analyzed using the Nikon PCM2000 confocal microscope and Simple PCI software. Immuno- and cyto- chemical stained sections were visualized with an Olympus BX60 microscope and captured using Advanced SPOT image software.
Quantitative analysis of histological changes
Immunoreactivity of CLP and tenascin in IF double stained tissue sections was measured using Metamorph software (version 6.3, Universal Imaging, Media, PA) and recorded as a percentage of the calculated total EC area. This analysis was performed on hearts from 3 independent Sox9flox/+;Tie2-cre and 3 Sox9flox/flox;Tie2-cre E12.5 embryos. Based on the position of the ventricles and great vessels, 6 comparable sections selected from throughout the EC of control and mutant mice were analyzed, and immunoreactivity values were averaged. Statistical significance of observed differences was determined using Student’s t-test (P<0.05).
Changes in valve leaflet area were determined in hematoxylin and eosin stained sections from 2 Sox9flox/flox;Col2a1-cre and 2 control Sox9flox/f+;Col2a1-cre mice at 3 months of age. Using NIH Image J (version 1.32), the area of each valve leaflet in the AV canal and OFT was measured in 12 sections taken from comparable regions throughout the respective hearts. The total valve leaflet area was measured for each valve within the AV canal and OFT, and includes the area from where the leaflet begins to protrude from the mural wall, to where the leaflet rejoins (indicated in Figure 8C and E). The average size of the AV and OFT leaflets on each tissue section was calculated and reported as a fold change over control (Sox9flox/f+;Col2a1-cre) measurements. Based upon multiple sections (12) from 2 control and 2 Sox9flox/flox;Col2a1-cre mice, statistical significance was calculated using Student’s t-test (P<0.01).
Results
Sox9 and Cartilage Link Protein are expressed during early and late stages of murine valvulogenesis
Immunohistochemistry was used to determine the expression patterns of Sox9 and the Cartilage Link Protein (CLP) during cell lineage diversification (E13.5) and differentiation stages (E18.5) of valvulogenesis in the mouse. Nuclear-specific Sox9 protein expression is observed in the majority of valve precursor cells within the mural (arrowheads) and septal (arrows) ECs at E13.5 (arrows, Figure 1A), but not in the developing myocardium (*, Figures 1A, C). This is in contrast to avian embryos, which exhibited more localized expression of sox9 in AV EC at comparable stages (Lincoln et al., 2006a). However, there are some nuclei that are not positive for Sox9 in the E13.5 mouse valve primordia which is suggestive of a diversity of cell types at this stage. As detected by IF, CLP protein is expressed predominantly in on the right and left sides of the central EC (arrows) and also within the mural ECs (arrowheads), where Sox9 expression is also apparent. In the core of the central EC that overlies the interventricular septum (IVS), there is less CLP expression than in the more lateral regions that form the valve primodia (arrows, Figure 1B). At E18.5 in the tricuspid valve, Sox9 expression is apparent on both the atrial (arrows) and ventricular (arrowheads) surfaces of the elongating mural and septal heart valve leaflets (Figure 1C). Similarly at E18.5, expression levels are undetected in the myocardium (*, Figure 1C). At this stage, CLP expression is also observed on the atrial surface (arrows, Figure 1D) and ventricular aspect, including the distal tips, of the valves (arrowheads, Figure 1D). Similar to Sox9, CLP expression is highly expressed along the atrial surface of the valve leaflets (arrows, Figures 1C, D), but expression of both also is apparent on the ventricular surfaces especially at the distal tips. These expression studies show that Sox9 and CLP are expressed in overlapping regions of the EC and developing heart valve leaflets during valvulogenesis.
Figure 1. Sox9 and Cartilage Link Protein are expressed in endocardial cushions and primitive heart valve leaflet structures during mouse valvulogenesis.
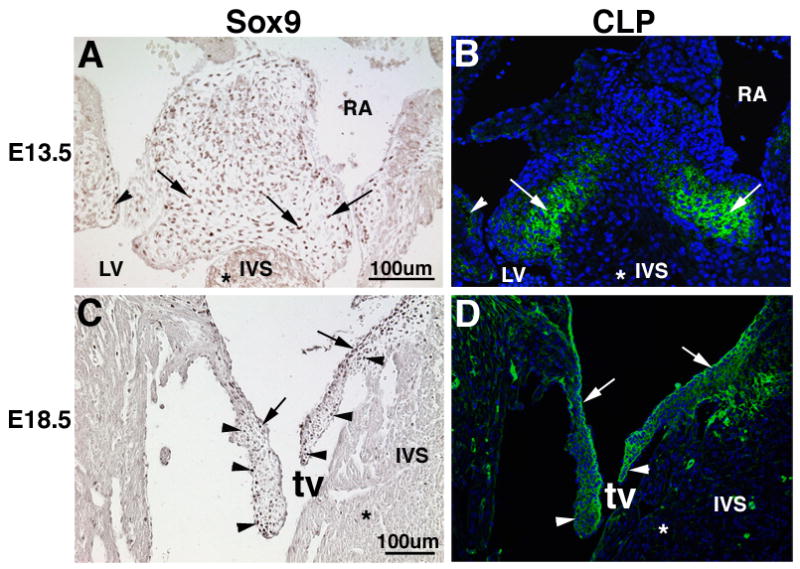
Immunohistochemistry was used to examine expression patterns of Sox9 (A, C) and CLP (B, D) in the endocardial cushions at E13.5 (A, B) and developing heart valve leaflets at E18.5 (C, D). (A) Nuclear Sox9 expression is detected throughout valve precursor cells of the central (arrows) and mural (arrowhead) ECs. (B) CLP expression is also detected in the matrix associated with Sox-9 positive cells within the mural EC (arrowhead) and the more lateral ventricular region of the central EC (arrows). At E18.5, Sox9 (C) and CLP (D) expression are detected in the maturing septal and mural tricuspid valve leaflets (arrows), with predominant expression observed on the atrial surface (arrow) and ventricular surface with enhanced expression at the distal tips (arrowheads). Notably, Sox9 and CLP expression is not detected in the myocardium (*). tv, tricuspid valve; IVS, interventricular septum; RA, right atrium; LV, left ventricle; CLP, Cartilage Link Protein.
Sox9 is required during early stages of heart valve development for formation of valve primordia
Tie2-cre and Col2a1-cre transgenes were used for targeted conditional inactivation of Sox9 in Tie2- and Col2a1-expressing cell types during early and late stages of valve development, respectively. The Tie2-cre transgene is expressed in endothelial cells prior to EMT and drives recombination throughout derived mesenchyme cells that contribute to the mature valves (de Lange et al., 2004; Lincoln et al., 2006a). Consequently, Sox9flox/flox;Tie2-cre mice die between E11.5-E14.5 displaying pericardial edema (arrow, Figure 2B) and increased blood pooling (arrowhead, Figure 2B) compared to controls (arrow and arrowhead, Figure 2A). Upon histological examination Sox9flox/flox;Tie2-cre mice also show obvious defects in valve maturation. At E13.5 in Tie2-cre;ROSA26R mice, recombination occurs in the majority of valve precursor cells that populate the valve primordia, as detected by X-gal staining (arrows, Figure 2C). At this stage in wild-type embryos, Sox9 also is expressed broadly in the valve primordia (arrows, Figure 2D) consistent with previous report (Montero et al., 2002). Loss of Sox9 protein expression in Sox9flox/flox;Tie2-cre mice (arrow, Figure 2F) compared to Sox9flox/+;Tie2-cre controls (arrows, Figure 2E) indicates efficient recombination and targeting of the Sox9 locus with Tie2-cre in valve precursor cells of the ECs. Histologically, Alcian blue staining indicates hypoplastic ECs in Sox9flox/flox;Tie2-cre mice that fail to elongate and form AV valve primordia at E13.5 (arrows, Figure 2G, H). EC within the OFT of Sox9flox/flox;Tie2-cre mice also appear grossly reduced in size (data not shown). In the AV canal there is malalignment of the atrial septum (as) (arrowhead, Figure 2G, H), although the septum does meet the ECs in more distal tissue sections (data not shown). These data support a role for Sox9 in formation of distinct mitral and tricuspid valve primordia in the AV canal.
Figure 2. Sox9flox/flox;Tie2-cre mice have embryonic heart failure and display hypoplastic endocardial cushions.
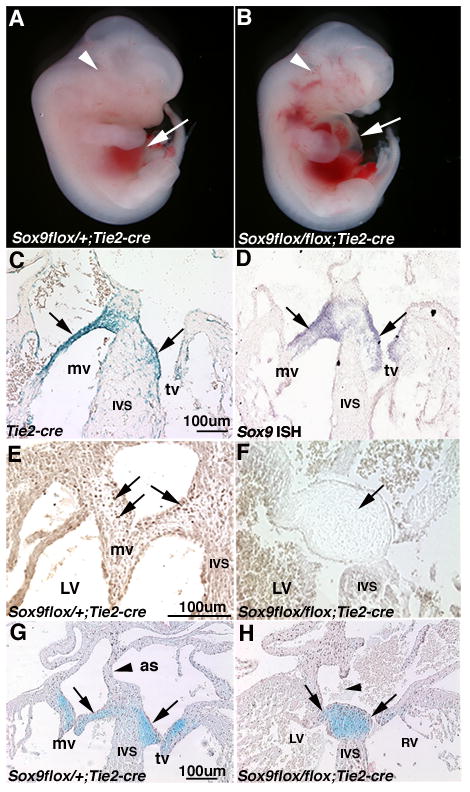
(B) Grossly, Sox9flox/flox;Tie2-cre mice display pericardial edema (arrow) and increased blood pooling (arrowhead), compared to controls (A) at E12.5. At the cellular level, X-gal staining and in situ hybridization were performed on tissue sections of heart valve structures at E13.5 from Tie2-cre mice bred with ROSA26R to determine Tie2-cre-mediated recombination with Sox9 in the developing heart valves. (C) Tie2-cre recombination is detected throughout the valve primordia at E13.5, consistent with Sox9 expression (arrows, D). (E) Compared to control Sox9flox/+;Tie2-cre control mice, Sox9 protein expression (arrows) is diminished in ECs of Sox9flox/flox;Tie2-cre mice (F) indicative of recombination. Alcian blue staining was used to indicate proteoglycans within the heart valve structures in control Sox9flox/+;Tie2-cre (G) and mutant Sox9flox/flox;Tie2-cre (H) mice. Arrows indicate hypoplastic EC in Sox9flox/flox;Tie2-cre mice (D) compared to controls (C). Arrowheads indicate incomplete formation of the atrial septum (as). IVS, interventricular septum; mv, mitral valve; tv, tricuspid valve; LV, left ventricle; RV, right ventricle.
Sox9 is required during later stages of valvulogenesis for heart valve leaflet maturation
Inactivation of Sox9 during later stages of valve development was achieved using the Col2a1-cre transgene, shown previously to recombine in chondrogenic-derived cells (Ovchinnikov et al., 2000). Col2a1-cre recombination was detected in embryos of Col2a1-cre;ROSA26R mice using X-gal staining. Cre recombination is first observed at E15.5 and is shown at E18.5 along the atrial and more predominantly on the ventricular surfaces of the primitive heart valve leaflets (Figure 3A). At this time, Sox9 expression is observed on both atrial and ventricular surfaces of the mural and septal tricuspid valve leaflets (Figure 1C, 3B, C). Following recombination, Sox9flox/flox;Col2a1-cre mice show reduced nuclear Sox9 protein expression (arrows, Figure 3D) predominantly on the ventricular surface of the valve leaflets where recombination has occurred (arrows, Figure 3D). Sox9 protein expression is still apparent on the atrial surface where there is less activity of Col2a1-cre (arrowhead, Figure 3D). Sox9flox/flox;Col2a1-cre mice die at birth due to presumed respiratory defects, and therefore heart valves were examined in embryos harvested at E18.5 (Akiyama et al., 2002). At E18.5, Sox9flox/flox;Col2a1-cre mice display thickened heart valve leaflets with apparently reduced Alcian blue staining (arrows, Figure 3F), compared to the long, thin, glycosaminoglycan-rich valve leaflets of Sox9flox/+;Col2a1-cre control mice (arrows, Figure 3E). In addition, the width of the interventricular septum (IVS) of Sox9flox/flox;Col2a1-cre mice is increased, which may be related to compromised cardiac function (Figures 3E, F) (Baudino et al., 2006). The morphological valve defects observed using Col2a1-cre suggest a role for Sox9 in maturation and function of the valve leaflets during later stages of valve development. The differential phenotypes resulting from loss of Sox9 in valve precursor cells (Tie2-cre), or a subset of remodeling valve cells (Col2a1-cre), indicates that Sox9 may have distinct functions during early and late stages of valvulogenesis.
Figure 3. Heart valve leaflets in Sox9flox/flox;Col2a1-cre mice are abnormal with reduced Alcian blue staining.
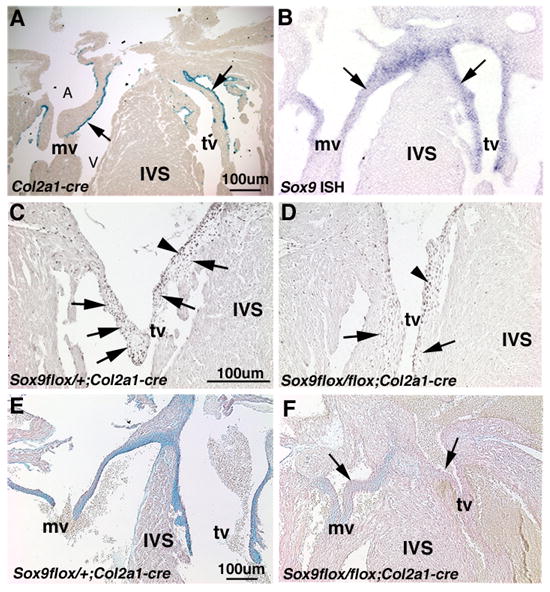
Col2a1-cre recombination (A) is observed in a subset of cells on the atrial (A) and ventricular (V) surfaces of the valve leaflets at E18.5 in Col2a1-cre mice bred with Rosa26R. (B) At this time, Sox9 expression is detected by in situ hybridization throughout the valve leaflet structures (arrows). (C) Compared to Sox9flox/+;Col2a1-cre control mice, nuclear Sox9 protein expression is diminished on the ventricular aspect of valve leaflets of Sox9flox/flox;Col2a1-cre mice (arrow) (D), although expression remains on the atrial surface where recombination has not taken place (arrowhead). Heart valve leaflets of Sox9flox/flox;Col2a1-cre mice (F) are abnormal at E18.5 and Alcian blue staining is reduced compared to Sox9flox/+;Col2a1-cre control mice (E). IVS, interventricular septum; mv, mitral valve; tv, tricuspid valve.
Sox9flox/flox;Tie2-cre mice have hypoplastic endocardial cushions associated with decreased cell proliferation
To determine the developmental mechanisms underlying hypoplastic ECs observed in E13.5 Sox9flox/flox;Tie2-cre hearts, cell proliferation and apoptosis were examined at E11.5 and E12.5 during stages of EC maturation (Figures 4A, B). EMT has occured in Sox9flox/flox;Tie2-cre mice at E11.5 as indicated by the presence of mesenchymal cells in the EC (arrows, Figure 4A, B). However the number of cells populating the cushion area is significantly reduced (Figure 4A-C) and the cushion morphology is abnormal as indicated by Alcian blue staining. At E12.5, cell proliferation within ECs of Sox9flox/flox;Tie2-cre mice is reduced by 11% , as indicated by quantitative analyses of IHC of phosphohistone H3 (pHH3) in valve precursor cells compared to controls (Figure 4D-F). Notably, proliferation in the myocardium, where recombination does not occur, is unchanged (arrowhead, Figure 4D, E). Changes in cleaved caspase-3 activity, an indicator of apoptotic cells, was not observed in EC of Sox9flox/flox;Tie2-cre mice (data not shown). These data provide evidence for a requirement for Sox9 in cell proliferation of mesenchyme cells after initiation of EMT to expand the heart valve progenitor cell pool.
Figure 4. Valve precursor cells of Sox9flox/flox;Tie2-cre mice have decreased proliferation and perturbed cell lineage diversification.
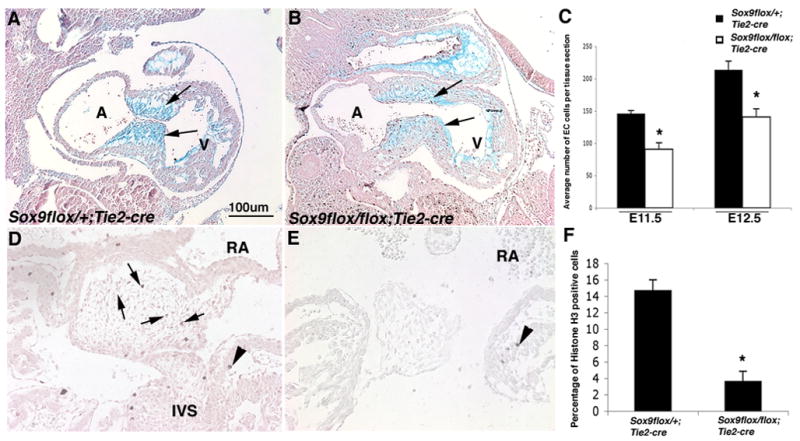
Alcian blue staining of histological sections of E11.5 Sox9flox/+;Tie2-cre (A) and Sox9flox/flox;Tie2-cre (B) embryos shows abnormal morphology and reduced size of EC lacking Sox9 expression indicated by arrows. Phospho-histone H3 (pHH3) immunohistochemistry was used to detect proliferating cells in EC tissue sections from Sox9flox/+;Tie2-cre (D) and Sox9flox/flox;Tie2-cre (E) mice at E12.5. (C) Quantitative analyses were performed to detect significant changes in the average number of mesenchymal cells in tissue sections of the EC from Sox9flox/flox;Tie2-cre mice compared to Sox9flox/+;Tie2-cre controls at E11.5 and E12.5. (F) The number of nuclei stained positive for pHH3 in each tissue section of EC from E12.5 Sox9flox/+;Tie2-cre and Sox9flox/flox;Tie2-cre mice was used to calculate a proliferative index represented as a percentage of the total number of nuclei. Note proliferation is reduced in Sox9flox/flox;Tie2-cre mice. (F). A, atrium; V, ventricle; RA, right atrium; IVS, interventricular septum.
The endocardial cushions of Sox9flox/flox;Tie2-cre mice have altered ECM protein expression and distribution
Previous work has shown that avian AV valve progenitor cells have the potential to diversify into cartilage- and tendon-like cell types (Lincoln et al., 2006a). CLP and tenascin immunoreactivity was examined as indicators of valve cell differentiation in ECs from Sox9flox/flox;Tie2-cre mice. Although not exclusive to cartilaginous and tendinous cell types, these markers are differentially expressed in the developing valves and are therefore indicators of valve differentiation and ECM organization (Lincoln et al., 2006a). In control embryos, tenascin expression (arrows, Figure 5A) is localized in the distal regions and ventricular aspect of the cushion (highlighted in white). Normally, CLP is more widely expressed in lateral aspects of the central EC (arrowheads, Figure 5C) and is not detected at the central atrial aspect of the cushion (*). In the Sox9flox/flox;Tie2-cre embryos, the ECs are strikingly much smaller (Figure 5B, D, F). Associated with the change in EC size and morphology, the relative distribution of tenascin and CLP in Sox9flox/flox;Tie2-cre ECs is altered compared to controls. Tenascin expression is no longer predominant in ventricular regions, but is observed in a more atrial location (arrows, Figure 5B, F). In contrast, CLP expression continues to be widespread throughout the core of the central cushion (arrowheads, Figure 5D, F). The immunopositive areas for tenascin or CLP were quantified as a percentage of the outlined EC area of control and Sox9flox/flox;Tie2-cre embryos to determine changes in the relative distribution of these extracellular matrix proteins (Figure 5G). The percent of the total EC area that was positive for tenascin immunoreactivity is increased by approximately 2 fold in the EC of Sox9flox/flox;Tie2-cre mice compared to controls, but the percentage of EC area positive for CLP expression was unchanged. The altered proportions of EC area that express each of these matrix proteins likely reflects a significant disruption in ECM distribution and patterning in the Sox9flox/flox;Tie2-cre mice. By gross examination, markers of undifferentiated EC mesenchyme cells including Msx-2, Has-2 and β-catenin were apparently unaffected by the loss of Sox9 (data not shown). Thus, loss of Sox9 in EC mesenchyme specifically affects differentiation and patterning of the valve primordia.
Figure 5. Tenascin and CLP expression is altered in Sox9flox/flox;Tie2-cre mice.
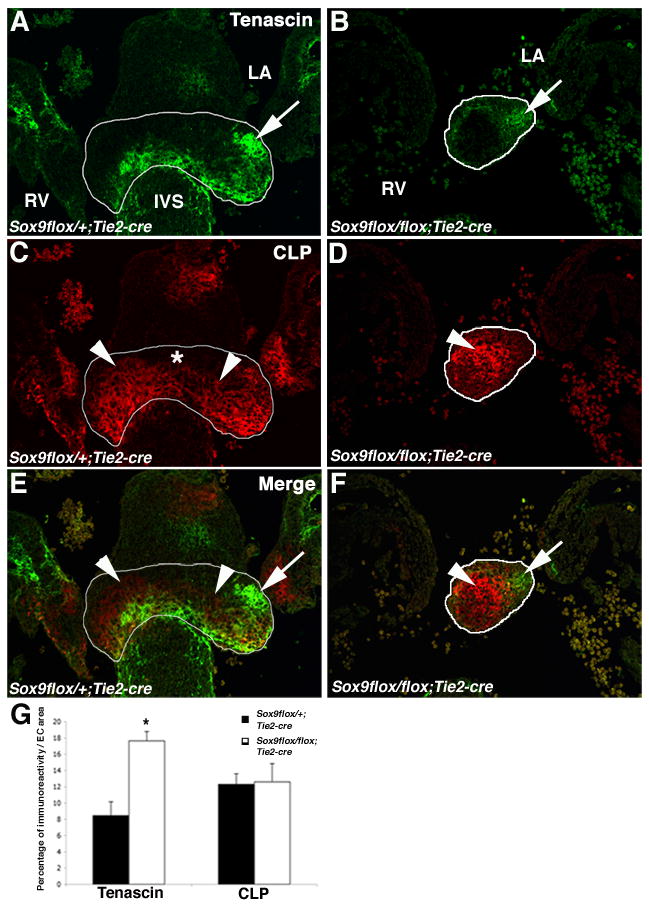
(A, B) Immunofluorescence staining of tenascin (A, B) and CLP (C, D) were used to show ECM protein expression in tissue sections of ECs (highlighted by white lines) from Sox9flox/+;Tie2-cre (A, C, E) and Sox9flox/flox;Tie2-cre (B, D, F) mice at E12.5. Tenascin-positive regions are indicated by arrows and CLP-positive regions are indicated by arrowheads. (D) The atrial aspect of the central EC where CLP is not expressed is indicated by (*). Merged images from (A-D) are shown in (E, F). The percent area of the immunoreactivity of CLP and tenascin within the central EC is reported in (G). Error bars represent standard error of the mean, and statistical significance (*) is calculated by Student’s t-test compared to Sox9flox/+;Tie2-cre mice (P<0.05; n=3). IVS, interventricular septum; LA, left atrium; RV, right ventricle.
Sox9flox/flox;Col2a1-cre mice have reduced expression of cartilage-associated proteins, CLP and type II collagen, and exhibit altered ECM patterning and organization
In differentiating cartilage, Sox9 regulates expression of structural genes including type II collagen and CLP (Bell et al., 1997; Kou and Ikegawa, 2004; Ng et al., 1997). Immunofluorescence was used to determine the expression patterns of type II collagen (Figure 6A, B), CLP (Figure 6C, D), and elastin (Figure 6E, F) in Sox9flox/flox;Col2a1-cre valves (Figure 6B, D, F) compared to controls at E18.5 (Figure 6, A, C, E). In control mice, type II collagen is expressed predominantly on the ventricular surfaces of heart AV valve leaflets (arrows, Figure 6A). In contrast, type II collagen expression in the AV heart valves is undetected in Sox9flox/flox;Col2a1-cre mice (arrows, Figure 6B). CLP expression is observed more broadly in the valve leaflets of control mice (arrowhead, Figure 6C). In Sox9flox/flox;Col2a1-cre mice, CLP expression is diminished, especially on the valve leaflet ventricular surfaces and distal tips where Col2a1-cre is active (arrow, Figure 6D). However, expression is not completely absent (arrowhead, Figure 6D), likely due to remaining Sox9 protein in the more atrial surfaces where Col2a1-cre and Sox9 do not overlap (Figure 3D). Expression of CLP was also dramatically reduced in the OFT valves (data not shown). The expression pattern of elastin, an ECM protein not associated with Sox9 or cartilage lineage development, was examined in control and Sox9flox/flox;Col2a1-cre mice (Figure 6E, F). Normally, elastin is expressed primarily along the atrial surface of the valve leaflet (arrows, Figure 6E). In addition to the normal elastin expression observed on the atrial surface of the valve leaflets in Sox9flox/flox;Col2a1-cre mice (arrows, Figure 6F), ectopic expression is also noted on the ventricular surface of the enlarged valve leaflets (arrowhead, Figure 6F). However, it is noted that the overall expression of elastin is lower in mouse valves compared to previous reports of avian valves, and is likely due to the differential functional demands between species (Hinton et al., 2006; Hurle et al., 1994). These observations of the Sox9flox/flox;Col2a1-cre mice indicate that the overall organization of the stratified ECM layers within the valve leaflets of Sox9flox/flox;Col2a1-cre mice is affected by loss of Sox9 in a subpopulation of cells. Collectively, these data show that ECM proteins regulated by Sox9 in cartilage are decreased in the remodeling heart valves of Sox9flox/flox;Col2a1-cre mice and support the hypothesis that Sox9 is required for differentiation of valve cell lineages and organization of the ECM.
Figure 6. Type II collagen and CLP expression are reduced in Sox9flox/+;Col2a1-cre mice.
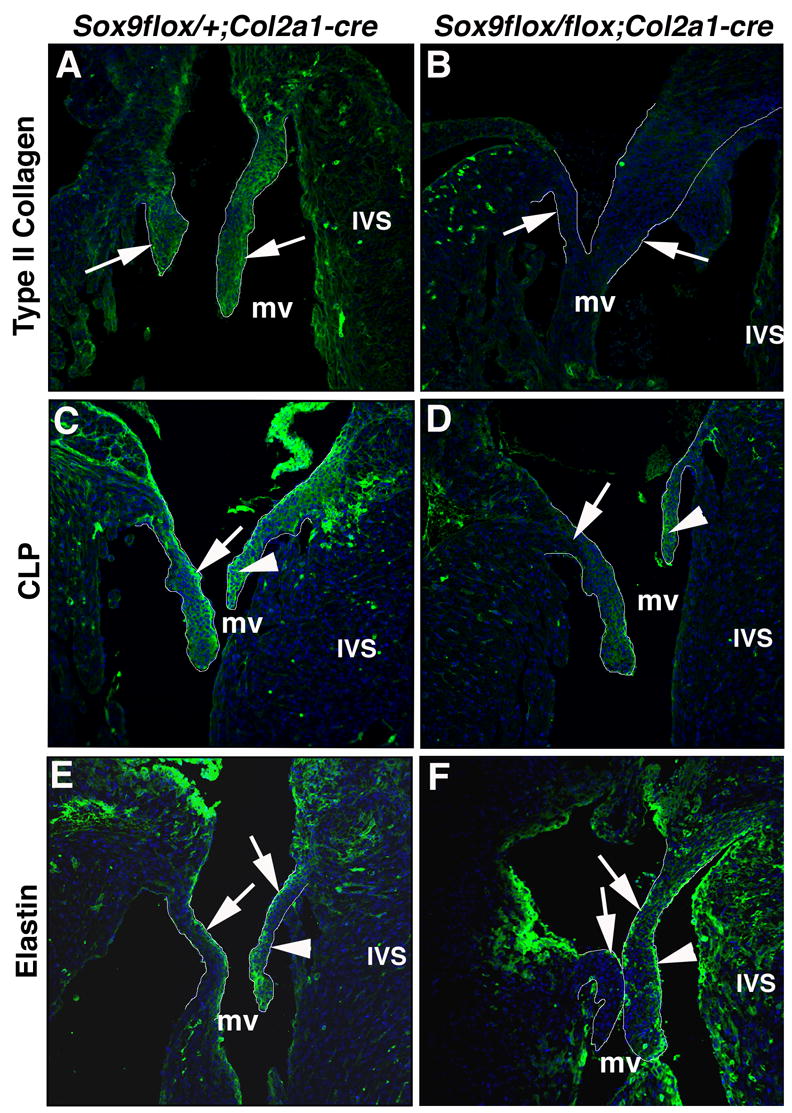
Immunostaining for type II collagen (A, B), CLP (C, D) and elastin (E, F) was used to determine ECM expression and organization in Sox9flox/+;Col2a1-cre (A, C, E) and Sox9flox/flox;Col2a1-cre (B, D, F) mitral valve mural and septal leaflets at E18.5 (outlined in white). The expression of type II collagen (A) and CLP (C) in the mural and septal valve leaflets (arrows) of Sox9flox/+;Col2a1-cre mice is diminished in Sox9flox/flox;Col2a1-cre mice (B, D). (E) Normal elastin expression is detected on the atrial surface of mitral valve mural and septal leaflets of Sox9flox/+;Col2a1-cre embryos. (F) Elastin expression is also observed on the atrial surface of valve leaflets from Sox9flox/flox;Col2a1-cre embryos, but ectopic expression is additionally noted on the ventricular surface of the septal leaflet (arrowhead, F). mv, mitral valve; IVS, interventricular septum.
Adult heart valve leaflets from Sox9flox/+;Col2a1-cre mice are thickened and show calcium deposits
Changes in Sox9 expression have been implicated in forms of adult degenerative heart valve disease associated with leaflet calcification (Caira et al., 2006). ECM distribution and calcification of heart valve structures was examined in Sox9flox/+;Col2a1-cre adult mice using Movat’s pentachrome and Von Kossa staining, respectively. During embryogenesis and early stages of adult life, the heart valves of Sox9flox/+;Col2a1-cre mice appear structurally normal without signs of calcification as indicated by an absence of Von Kossa stain (data not shown). However, from 3 months of age, increased Von Kossa staining, indicative of mineral deposits, was apparent in AV and OFT heart valve leaflets in 75% of the Sox9flox/+;Col2a1-cre mice (arrows, Figure 7B) compared to lower frequency in Sox9flox/+;Col2a1-cre-/- control mice (37.5%) (Figure 6A). In control mice that exhibited Von Kossa staining, the extent of mineralization is less and encompasses a smaller fraction of the valve surface area by gross analysis compared to mutant mice. Notably Von Kossa staining was not observed in cardiac structures that do not express Sox9, including the aortic root of Sox9flox/+;Col2a1-cre mice (data not shown). ECM expression and distribution was assessed by Movat’s Pentachrome stain in mitral valve (MV) and outflow tract (OFT) valve leaflets of Sox9flox/flox;Col2a1-cre and control mice (Figures 7C-F). The AV and OFT valve leaflet area of Sox9flox/flox;Col2a1-cre mice is significantly greater than control valves (Figures 7G, H). This is associated with an increased abundance of proteoglycans at the tip of the valve leaflets, as indicated by Alcian blue staining (arrows, Figures 7C-H). These cytochemical analyses demonstrate that Sox9flox/+;Col2a1-cre mice show characteristics of heart valve disease associated with leaflet thickening, calcium deposition, and altered ECM production.
Figure 7. Heart valves from adult Sox9flox/+;Col2a1-cre mice are thickened and calcified.

Von Kossa stain was used to examine calcium deposition in mitral valve (MV) leaflets of control Sox9flox/+;Col2a1-cre−/− (A) and Sox9flox/+;Col2a1-cre+/− (B) mice at 4 months of age. Von Kossa stains calcium deposits black, as indicated in Sox9flox/+;Col2a1-cre+/− mice (arrows, B). Movat’s Pentachrome staining was used to show ECM distribution and organization in the MV (C, D) and semilunar (OFT) (E, F) valve leaflets of control and Sox9flox/+;Col2a1-cre+/− mice. Morphometric analyses show thickened valve leaflets in Sox9flox/+;Col2a1-cre+/− (D, F) mice compared to controls (C, E). Quantitative analysis of the fold change in the AV (G) and OFT (H) leaflet area (outlined in black) of Sox9flox/+;Col2a1-cre mice over controls is shown. Statistical significance (*) was performed by Student’s t-test (P < 0.01). MV, mitral valve; IVS, interventricular spetum; OFT, outflow tract.
Discussion
Recent studies have shown conservation of cartilage, tendon and bone regulatory hierarchies with heart valve development (Lincoln et al., 2006a; Lincoln et al., 2006c). During cartilage formation, Sox9 is required early in precursor cells during mesenchyme cell condensation and later during chondrogenic lineage differentiation (Lefebvre and Smits, 2005). In the developing heart, Sox9 is expressed during early and late stages of valve development and, in avian embryos, Sox9 expression is coordinately induced with aggrecan in response to BMP2 signaling in multipotential valve progenitor cells (Lincoln et al., 2006a). The requirements for Sox9 in mouse heart valve development in vivo was examined using the Cre/loxP system to target Sox9 inactivation early in endothelial-derived cells of the EC (Tie2-cre) (Kisanuki et al., 2001) and later in a subset of cells within the mature valve leaflets using Col2a1-cre, which also is expressed in differentiating cartilage cell lineages (Ovchinnikov et al., 2000). In Sox9flox/flox;Tie2-cre embryos, initial EMT and formation of the EC mesenchyme occurs, however, mice die between E11.5-E14.5 with hypoplastic ECs, and consequently a decrease in the number of valve precursor cells. ECM expression and organization also is disrupted in Sox9flox/flox;Tie2-cre mice, as indicated by an abnormal relative distribution of tenascin expression. Together these data support roles for Sox9 during expansion and differentiation of heart valve precursor cells. Targeted inactivation of Sox9 in a subpopulation of valve cells during later stages of remodeling in Sox9flox/flox;Col2a1-cre mice leads to thickened valve leaflets, with reduced expression of type II collagen and CLP, which are regulated by Sox9 in cartilage. In addition, overall ECM distribution and organization of the valve leaflets is disrupted in Sox9flox/flox;Col2a1-cre mice, as indicated by ectopic elastin expression on the ventricular surface of the valve leaflets. These data provide evidence for a later role for Sox9 during differentiation and remodeling of heart valve structures. Together these studies demonstrate early functions for Sox9 in valve progenitor cell proliferation and later functions in valve differentiation, ECM organization and homeostasis.
Sox9 is required for proliferation and diversification of valve progenitor cells
ECs of Sox9flox/flox;Tie2-cre mice form initially, but are hypoplastic with abnormal distribution of ECM protein expression. Previously, Akiyama and colleagues have shown that germline inactivation of Sox9 leads to a reduced number of valve precursor cells due to insufficient EMT (Akiyama et al., 2002). The Tie2-cre transgene used in this study has enabled us to examine the effects of loss of Sox9 on later events of cushion cell proliferation and differentiation (Lincoln et al., 2004). In Sox9flox/flox;Tie2-cre embryos, endothelial cells are able to undergo EMT, as indicated by the presence of mesenchymal cells in the EC at E11.5. However, proliferation of EC cells after EMT is significantly reduced, with no apparent change in cell death, leading to a diminished progenitor pool of valve precursor cells. These findings are in contrast to the requirement of Sox9 in EMT reported by Akiyama, and are likely due to kinetics of the Cre-mediated recombination and Sox9 protein turnover in Sox9flox/flox;Tie2-cre mice. Therefore the targeted loss of Sox9 in EC mesenchymal cells after EMT demonstrates an additional function of Sox9 in cell proliferation and expansion of the heart valve progenitor pool (Akiyama et al., 2004; Goldring et al., 2006; Lefebvre and Smits, 2005).
Valve precursors isolated from avian embryos differentially express ECM markers characteristic of cartilage and tendon cells within diversified subsets of differentiating cells (Lincoln et al., 2004). The localization of these cell lineages remains distinct as cartilage-like cells occupy the valve leaflet and tendon-like cells are observed in the supporting chordae (Lincoln et al., 2004). Previous work indicated that a balance of cartilage and tendon-like cell lineages originates from this cell population depending on exposure to BMP and FGF signaling (Lincoln et al., 2006c). In cultured avian valve progenitor cells, BMP2 treatment induces expression of Sox9 and chondrogenic-associated gene markers and promotes diversification of cartilage-like cell lineages (Lincoln et al., 2006a). In mice with targeted loss of Sox9 at different stages of valve development, expression and localization of tenascin, type II collagen and CLP is altered. Further studies are necessary to determine definitively if these alterations in ECM gene expression are the result of aberrant valve cell lineage diversification. However, these data support a role for Sox9 in valve cell precursor differentiation and patterning in mouse embryos.
Sox9 is required for normal valve patterning and expression of type II collagen and CLP
Reduced expression of Sox9 target genes type II collagen and CLP was observed in heart valves of Sox9flox/flox;Col2a1-cre mice. The Col2a1-cre transgene targets Sox9 inactivation in a subset of cells at the edges of the valve leaflets from E15.5. The reduced expression of type II collagen and CLP observed in Sox9flox/flox;Col2a1-cre embryos by E18.5 suggests that Sox9 is required during this later developmental stage for expression of these genes, which have previously been demonstrated to be direct targets of Sox9 in cartilage progenitor cells (Kou and Ikegawa, 2004; Ng et al., 1997). In cartilage of the developing limbs, Sox9 is required early for lineage determination and later for differentiation and expression of type II collagen (Akiyama et al., 2002; Bi et al., 1999). In the differentiating valves, Sox9 is similarly required for type II collagen expression, but it has not yet been determined if this is a direct interaction. The loss of type II collagen and CLP expression in differentiating heart valves is consistent with a requirement for Sox9 in the differentiation of a valve cell population with characteristics of cartilage. The observation that loss of Sox9 during early stages of valvulogenesis does not result in reduced CLP expression may be indicative of distinct regulatory mechanisms acting in the primitive valve progenitor cells. These studies provide further evidence for conserved regulatory relationships between signaling pathways, transcription factors and its downstream target genes in developing cartilage and differentiating heart valve cell lineages.
Mature valve leaflets of vertebrates are composed of fibrosa (collagen), spongiosa (proteoglycan) and atrialis (elastin) layers which are not fully apparent until after birth (Hinton et al., 2006). In general the ECM layers of avian and human valves are relatively thickened with increased matrix deposition relative to mouse valves, but the overall organization and structure is conserved (Hinton et al., 2006). It has not been yet been demonstrated that these different matrix cell layers are produced by distinct valve cell lineages, but differential expression patterns of regulatory genes and their downstream targets during chicken and mouse valve development supports this idea. In the Sox9flox/flox;Col2a1-cre mice, CLP expression is reduced throughout the heart valve leaflets and elastin expression is abnormally expressed on the ventricular surface of AV valves at E18.5 which likely reflects an overall disruption in ECM distribution and patterning of the valves. It is possible that these alterations in valve patterning are secondary to compromised function, since they occur in cells that do not express the Co2a1-cre transgene. Similarly the thickened IVS observed in Sox9flox/flox;Col2a1-cre mice is a likely secondary effect of cardiac dysfunction and was also observed in mice with altered collagen expression in the developing valve (Lincoln et al., 2006b). These changes in valve structure and chamber morphology with loss of Sox9 in a subset of valve cells underscores the importance of the distribution of specific types of ECM proteins that form matrices with distinct biochemical and mechanical characteristics for development and maintenance of normal heart valve structure and function.
Sox9 and heart valve disease
Defects in heart valve structures represent 20–30% of all congenital cardiovascular malformations (Hoffman and Kaplan, 2002; Supino et al., 2004). These anomalies can lead to valve disease apparent as heart valve leaflet thickening (or myxomatous valves), degeneration and calcification in adults (Lincoln et al., 2006c; Rabkin-Aikawa et al., 2005; Schoen and Levy, 2005). These defects are associated with changes in ECM organization and increased mineralization with detrimental effects on valve function (Garg et al., 2005; Lincoln et al., 2006c; Schoen and Levy, 2005). However, the underlying causes of disorganized and ectopic ECM or mineralization that occur during valve pathogenesis are poorly understood. There is increasing evidence to support developmental origins of heart valve disease manifested later in life (Garg et al., 2005; Hinton et al., 2006; Lincoln et al., 2006c). This study has shown a requirement for Sox9 during heart valve development. In addition, reduced Sox9 function in Sox9flox/+;Col2a1-cre mice is associated with histological changes characteristic of diseased valves, including an increase in valve leaflet area and calcium deposition. The heart valves of Sox9flox/+;Col2a1-cre mice with localized reduction of Sox9 function are apparently normal at birth, but undergo histological changes over several months during post-natal stages. It is possible that the progressive degeneration of the valves in these animals is exacerbated by compromised heart function and hemodynamics, as is often the case in human heart disease. Therefore these mice are a potentially useful model for studies of the pathogenesis of valve disease.
The mechanisms of the altered ECM homeostasis associated with reduced Sox9 function are not clear, but in human patients, elevated levels of Sox9 have been reported in degenerative and calcified human mitral valves (Caira et al., 2006). This observation is evidence that precise regulation of Sox9 is required for normal valve homeostasis and that altered Sox9 function may be related to valve disease associated with connective tissue dysfunction. In developing cartilage, loss of Sox9 function results in premature ectopic mineralization of bone and changes in ECM organization and distribution. It is possible that increased calcium deposition observed in valves from mice with reduced Sox9 may be related to similar pathogenic mechanisms and that aberrations in Sox9 function may underlie certain types of calcified valve disease. Interestingly, cardiac phenotypes, including septal defects, are reported in 22% of CD patients, supporting a role for Sox9 in normal heart development (Houston et al., 1983; Mansour et al., 1995). Likewise, this study has identified critical roles for Sox9 in mouse heart valve development in vivo. Therefore, histological analysis of heart valve ECM homeostasis in CD patients may be informative and unveil previously unappreciated forms of valve defects. Collectively, Sox9 is required for early and late stages of heart valve formation through mechanisms similar to those previously described in cartilage formation. In addition, there is evidence to suggest that Sox9 may contribute to mechanisms of congenital heart valve disease.
Acknowledgments
We thank Dr. Hanna Osinska and Christina M. Alfieri for technical support, and Dr. Robert Hinton Jr. for scientific advice. JL was funded by AHA Ohio Valley Postdoctoral Fellow Award (0426366B) with additional funding to KEY from SCCOR Pediatric Heart Development and Disease Award (P50 HL074728).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Chaboissier M-C, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Nat Acad Sci. 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–28. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: Endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudino T, Carver W, Giles WR, Borg TK. Cardiac Fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–26. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–8. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficieny of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Nat Acad Sci. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–12. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gierde Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95:645–54. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Duprez D. Signals regulating tendon formation during chick embryonic development. Dev Dyn. 2004;229:449–457. doi: 10.1002/dvdy.10481. [DOI] [PubMed] [Google Scholar]
- Elluru RG, Whitsett JA. Potential role of Sox9 in patterning tracheal cartilage ring formation in an embryonic mouse model. Arch Otolaryngol Head Neck Surg. 2004;130:732–6. doi: 10.1001/archotol.130.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Shafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- Hinton RBJ, Lincoln J, Deutsch GH, Osinaka H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–8. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Houston CS, Opitz JM, Spranger JW, Macpherson RI, Reed MH, Gilbert EF, Herrmann J, Schinzel A. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am J Med Genet. 1983;15:3–28. doi: 10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Kitten GT, Sakai LY, Volpin D, Solursh M. Elastic extracellular matrix of the embryonic chick heart: an immunohistological study using laser confocal microscopy. Dev Dyn. 1994;200:321–32. doi: 10.1002/aja.1002000407. [DOI] [PubMed] [Google Scholar]
- Jones ML. Connective tissues and stains. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. Churchill Livingstone; London: 2001. [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–42. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kist R, Schrewe H, Balling R, Scherer G. Conditional inactivation of Sox9: a mouse model for campomelic dysplasia. Genesis. 2002;32:121–3. doi: 10.1002/gene.10050. [DOI] [PubMed] [Google Scholar]
- Kou I, Ikegawa S. SOX9-dependent and -independent transcriptional regulation of human cartilage link protein. J Biol Chem. 2004;279:50942–8. doi: 10.1074/jbc.M406786200. [DOI] [PubMed] [Google Scholar]
- Lange AW, Yutzey KE. NFATc1 expression in the developing valves is responsive to the RANKL pathways and is requires for endocardial expression of cathepsin K. Dev Biol. 2006;292:407–417. doi: 10.1016/j.ydbio.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Le Y, Sauer B. Conditional gene knockout using Cre recombinase. Mol Biotechnol. 2001;17:269–75. doi: 10.1385/MB:17:3:269. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–12. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230:239–50. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006a;292:292–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Florer JB, Deutsch GH, Wenstrup RJ, Yutzey KE. ColVa1 and ColXIa1 are required for myocardial morphogenesis and heart valve development. Dev Dyn. 2006b;235:3295–305. doi: 10.1002/dvdy.20980. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006c;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Mansour S, Hall CM, Pembrey ME, Young ID. A clinical and genetic study of campomelic dysplasia. J Med Genet. 1995;32:415–20. doi: 10.1136/jmg.32.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero JA, Giron B, Arrechedera H, Cheng YC, Scotting P, Chimal-Monroy J, Garcia-Porrero JA, Hurle JM. Expression of Sox8, Sox9 and Sox10 in the developing valves and autonomic nerves of the embryonic heart. Mech Dev. 2002;118:199–202. doi: 10.1016/s0925-4773(02)00249-6. [DOI] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles JE, Bell DM, Tam PP, Cheah KS, Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997:183. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–6. [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cyt. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Rabkin-Aikawa E, Mayer JE, Schoen FJ. Heart valve regeneration. Adv Biochem Engin/Biotechnol. 2005;94:141–179. doi: 10.1007/b100003. [DOI] [PubMed] [Google Scholar]
- Rahkonen O, Savontaus M, Abdelwahid E, Vuorio E, Jokinen E. Expression patterns of cartilage collagens and Sox9 during mouse heart development. Histochem Cell Biol. 2003;120:103–110. doi: 10.1007/s00418-003-0549-9. [DOI] [PubMed] [Google Scholar]
- Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: Progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–1080. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–74. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supino PG, Borer JS, Yin A, Dillingham E, McClymont W. The epidemiology of valvular heart diseases: the problem is growing. Adv Cardiol. 2004;41:9–15. doi: 10.1159/000079779. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–20. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Yoon SB, Lyons K. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]


