Abstract
Aims: To measure characteristics of the retinal blood vessels close to the optic disc in full term and preterm infants, with and without retinopathy of prematurity (ROP), using digital imaging. To determine whether these measures are indicative of the presence or severity of ROP in the retinal periphery.
Methods: 52 digital fundus images from 42 babies were analysed with a semiautomated analysis program developed at Imperial College London. Analysis was limited to the principal temporal vessels close to the optic disc: recording venular diameter and arteriolar diameter and tortuosity.
Results: Each result was categorised by the gestational age of the infant (“very premature” 24–27 weeks, “moderately premature” 28–31 weeks, and “near term” ⩾32 weeks) and by the highest stage of ROP present (“no ROP,” “mild ROP” stage 1 or 2, and “severe ROP” stage 3). Arteriolar tortuosity was found to vary significantly (Kruskal-Wallis p = 0.002) with ROP severity. Although venular and arteriolar diameters increased monotonically with ROP severity the differences were not significant. Venular diameter, arteriolar diameter, and arterial tortuosity did not vary significantly between gestational age groups.
Conclusions: This study confirms it is possible to quantify the size and tortuosity of retinal blood vessels in term and preterm babies using digital image analysis software. This method detected significant increases in arteriolar tortuosity with increasing ROP severity.
Keywords: computer analysis, vessel growth, infants, retinopathy of prematurity
Plus disease is a portent that retinopathy of prematurity (ROP) is likely to become severe and require treatment.1 It comprises a constellation of signs which are superimposed on disease staging2 that consists of venous engorgement, arteriolar tortuosity, iris vessel engorgement, pupil rigidity, and vitreous haze.
In 1949 Owens and Owens3 reported that retinal venous dilatation and arteriolar tortuosity were indicators of ROP. Two decades later Baum4 reported that “the earliest detectable ophthalmoscopic signs of oxygen toxicity to the retina are considered to be tortuosity and dilatation of the retinal vessels.” Such vascular changes were included in several of the earlier classification schemes of acute phase ROP.5–7 Saunders et al8 observed that if the posterior pole vasculature was normal (that is, no venous congestion or increased arteriolar tortuosity) then there was less than 3% probability of there being ROP stage 3 or above.9 In 1995 Capowski et al10 reported arterial tortuosity to be a useful measure of ROP disease state, while Wallace et al1 and Freedman et al11 demonstrated that vessel diameter and tortuosity could be used to indicate the risk that ROP would progress to requiring treatment. There is a need to identify and quantify signs of plus disease as early as possible before ROP has progressed to the point where outcome is compromised. The earliest signs of plus disease are venous engorgement and increased arteriolar tortuosity around the optic disc.
Three methods have been employed to quantify these retinal vessel characteristics. Firstly, simple grading from the indirect ophthalmoscopic appearance or from retinal photographs.1,4,12 Secondly, by the analysis of digitised fundus photographs upon which the blood vessels were traced by hand.10,11,13–15 Thirdly, from images captured directly by digital cameras. While this has been undertaken in the adult,16 there have been few attempts to apply this to the study of the preterm retinal vessels.17,18
The growing use of digital imaging in ophthalmology has led to substantial developments in the field of computer assisted analysis of retinal vessel morphology. Computer algorithms have now the potential to achieve high levels of accuracy and objectivity in the quantitative measurement of retinal vascular parameters such as diameter, branching patterns, and tortuosity. Although human intervention and guidance are still features of such systems, the future holds the promise of fully automated computer analysis of retinal vasculature for the investigation of diseases such as hypertension and diabetes.16,19
In this study, our aim was to measure characteristics of the retinal blood vessels close to the optic disc—diameter and tortuosity—in full term and preterm infants, with and without ROP, using digital imaging and to determine whether these measures are indicative of the presence or severity of ROP in the retinal periphery.
METHODS
Image acquisition
Images were captured from babies undergoing routine ROP screening examinations using the RetCam 120 contact digital fundus camera (Massie Labs, Dublin, CA, USA), at St Mary’s Hospital NHS Trust, London. Images were 640×480 pixels and 24 bit RGB (red-green-blue) colour.
Screening examinations were scheduled according to UK guidelines9 that recommend all infants of less than 1500 g birth weight and less than 32 weeks gestational age (GA) be examined. Images from a few larger babies were acquired if they were thought to be at risk of ocular disease (for example, from infection or maternal drug misuse). Babies’ pupils were dilated with cyclopentolate 0.5% and phenylephrine 2.5% eye drops instilled at least 30 minutes before the examination. Topical anaesthetic eye drops (oxybuprocaine (proxymetacaine) 0.4%) were instilled immediately before examination. The eyelids were held open by an eyelid speculum and, on occasion, a scleral indenter was used to position the eye. A contact lens gel (Viscotears, Novartis) was instilled onto the cornea onto which the camera probe was gently placed. ROP was documented according to the International Classification of ROP.2
Subjects
Fifty two images from the left eyes of 42 preterm babies with GA ranging from 24–40 weeks were studied within a postmenstrual age (PMA) time frame of 34–49 weeks.
Image selection, quality control, and analysis
Images were preprocessed, using Paint-Shop Pro (Jasc Software). The images were cropped to a circle of diameter 200 pixels, equivalent to 600 retinal μm) centred on the optic nerve head, limiting analysis to the vessels around the optic disc. For convenience the images were then reorientated with the temporal vessels uppermost.
Image analysis was undertaken using RISA (retinal image scale-space analysis), a semiautomated digital computer analysis program, full details of which have been published elsewhere.20–22 The program provides various measurements of vessel morphology through the use of a segmentation process that divides each vessel into individual segments, each segment being analysed independently of the rest of the vascular tree. A segment was defined by the length of vessel between branches or from the origin to the first branch. All the principal vessels, venules, and arterioles, including their branches, within the 200 pixel-wide field of view were analysed.
Arterioles present a greater challenge to the analysis software since they are of a smaller calibre and are closer to—or, sometimes, in babies less than 30 weeks PMA, beyond—the 90 μm resolution limit of RISA. Differences in image quality (blur, colour, and brightness) introduce variability in measurements. Vessel visibility is also affected by diffraction shadows caused by a suboptimal pupil size during image capture. Identification of the optic disc margin (presence of double rings) and the vessel origin requires manual input. To remove variation associated with defining the optic disc margin, a “standardised” optic disc of 40 pixels diameter was placed over the original optic nerve head. Prominent choroidal vasculature also interferes in the segmentation process of the RISA analysis.
Tortuosity measurements were also found to be occasionally inconsistent when compared to the vessel’s actual bowing. This occurred as a result of RISA’s insensitivity to the frequency at which a vessel bows, from the beginning of its path to the end. This measurement variability has been discussed by Capowski et al.10
RISA determines the average diameter of a vessel by dividing the total number of pixels in the vessel segment by its length. The program calculates arteriole tortuosity by dividing the straight-line distance between the beginning and end of a vessel segment by its true length.
Statistical analysis of measured variables grouped independently by prematurity and ROP status was undertaken using the non-parametric Kruskall-Wallis one way ANOVA with post hoc comparisons conducted using Dunn’s control test.
RESULTS
Forty two babies contributed images from 52 examinations. Subjects were categorised into three GA groups: “very premature” (24–27 weeks; 28 examinations), “moderately premature” (28–31 weeks; 16 examinations), and “near term” (⩾32 weeks; eight examinations). Nominal categorisation of ROP based on the highest stage seen during the examination period was as follows: “no ROP,” “mild” (stages 1 and 2), and “severe” (stage 3). A total of 99 venular diameters and 65 arteriolar diameter and tortuosity measurements were obtained from both the superior and inferior temporal vessels. Table 1 shows the medians and interquartile ranges for these venular and arteriolar diameters and arteriolar tortuosities grouped by prematurity and by ROP category.
Table 1.
Medians and interquartile ranges (IQR) for each measure for data grouped by ROP category (top) and prematurity (bottom)
| No ROP | Mild ROP | Severe ROP | ||||
| Median | IQR | Median | IQR | Median | IQR | |
| DV (μm) | 82.5 | 18.4 | 85.4 | 16.2 | 89.9 | 21.9 |
| DA (μm) | 59.1 | 9.9 | 60.2 | 17.2 | 65.6 | 7.0 |
| TA | 1.080 | 0.014 | 1.090 | 0.044 | 1.172 | 0.163 |
| Very premature | Premature | Near term | ||||
| Median | IQR | Median | IQR | Median | IQR | |
| DV = venule diameter, DA = arteriolar diameter, TA = arteriolar tortuosity. | ||||||
| DV (μm) | 87.4 | 17.4 | 78.2 | 16.4 | 88.2 | 20. 5 |
| DA (μm) | 62.4 | 9.6 | 58.4 | 10.4 | 65.7 | 8.9 |
| TA | 1.090 | 0.022 | 1.083 | 0.040 | 1.080 | 0.022 |
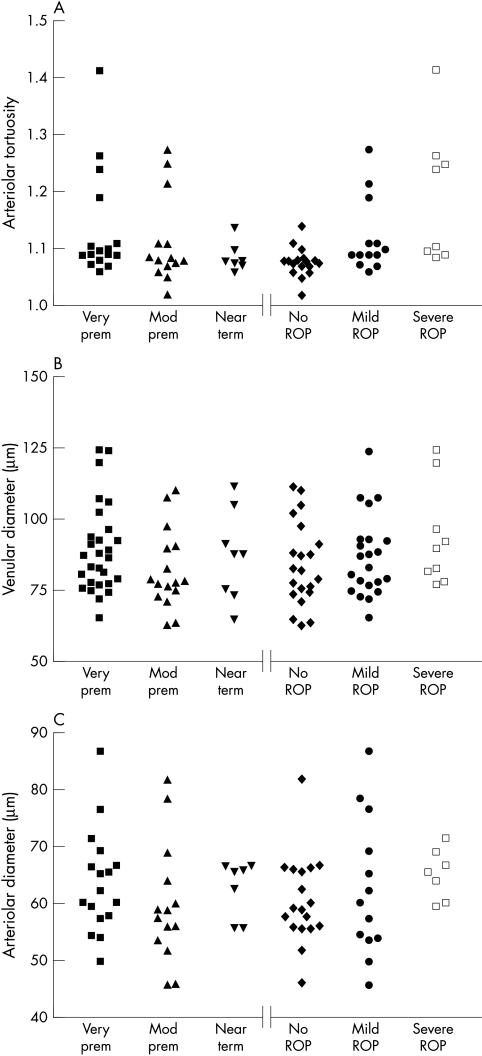
Arteriolar tortuosity (AT) was found to vary significantly (Kruskal-Wallis p = 0.002) as a function of ROP category (Fig 1A). Post hoc comparisons (Dunn’s) revealed there to be significantly increased tortuosity in babies with both mild and severe ROP compared to those with no ROP (p<0.05). Venular and arteriolar diameters did not differ significantly as a function of ROP category (Fig 1B, C) although the median diameters increased monotonically with severity of ROP. Venular diameter, arteriolar diameter, and arterial tortuosity did not vary significantly between gestational age groups. The design of this study does not allow us to assess the interaction between gestational age and severity of ROP on vessel measurements. Further investigation is required in order to elucidate these potentially complex relations.
Figure 1.
Scatter diagrams of vessel parameters measured. (A) Arteriolar tortuosity, (B) venular diameter, (C) arteriolar diameter, each parameter is grouped by prematurity and by ROP category.
DISCUSSION
This study has confirmed that it is possible to measure, in a semiautomated fashion, the size (diameter) and tortuosity of the retinal blood vessels of term and preterm babies using image analysis software. Heneghan et al18 found that vessel width increased with increasing severity of ROP, while in our study only arteriolar tortuosity increased with severity.
Several factors contribute to measurement variability: pupil dilatation, image illumination and focus, the subjective identification of a precise optic disc margin, and the problem of differentiation of the retinal and choroidal vasculature. The reliability of measuring vessel diameters decreases with the narrower retinal vessels as they approach the measurement resolution of RISA, of 3 pixels (90 μm). Geometric factors dictate that diameter measurements are more greatly affected by defocus and illumination than tortuosity. It is a longstanding clinical impression that in the first few weeks after birth the retinal blood vessels are relatively thin and straight, and later become broader and more tortuous as ROP becomes severe. Some of these very fine vessels fall below RISA’s resolution limit, although higher magnification lenses, now available, hold promise.
Tortuosity of the principal temporal posterior pole arterioles was found to reflect the presence and severity of ROP in the peripheral retina. This objective finding agrees in part with those of Wallace et al1 who observed that mild vessel dilation and tortuosity were indicative of future severity of ROP, progression to plus disease, and the need for intervention. There was a clear trend for both arteriolar and venule diameters to increase with increasing severity of ROP. This observed trend does accord with that of Saunders et al,8 who reported that eyes with severe peripheral ROP had both arteriolar and venular dilation at the posterior pole. That the trend observed in this study fails to attain statistical significance is most probably attributable to data overlap consequent to sample size limitations.
The finding that objectively measured posterior pole vessel morphology predicts severe ROP in the periphery has several clinical implications. The number of premature babies who require screening is increasing because of increased survival in developed countries.23 Detailed examination of the peripheral retina is difficult and not always possible, and a more efficient, simpler, and quicker procedure for screening for ROP is needed. A method based on visualising the posterior pole alone with the aim of diagnosing plus disease rather than the ROP lesion itself would considerably reduce the duration and trauma of examination and hence the stress experienced by babies. This approach would also allow healthcare professionals other than ophthalmologists to undertake screening, as Saunders et al8 found that non-ophthalmologists are capable of detecting posterior pole vessel abnormalities in preterm babies and Flynn et al24 also suggested that non-ophthalmologists would be capable of screening for ROP in babies over 1500 g.
The use of digital imaging combined with “store and forward” telemedicine may increase the cost effectiveness of ROP screening. This study used digital imaging to screen for ROP and other studies have already been undertaken to detect, evaluate, and diagnose ROP at remote sites.25–27 The use of computer algorithms paves the way for objective retinal vasculature quantification as demonstrated here. It seems likely that in the future digital cameras, such as the RetCam will be used by non-ophthalmologists to capture images of the posterior pole that will then be subject to automated analysis to inform diagnosis and treatment.
REFERENCES
- 1.Wallace DK, Kylstra JA, Chesnutt DA. Prognostic significance of vascular dilation and tortuosity insufficient for plus disease in retinopathy of prematurity. J AAPOS 2000;4:224–9. [DOI] [PubMed] [Google Scholar]
- 2.The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol 1984;102:1130–4. [DOI] [PubMed] [Google Scholar]
- 3.Owens WC, Owens EU. Retrolental fibroplasia in premature infants. Am J Ophthalmol 1949;32:1–18 (dicussion 18–21). [DOI] [PubMed] [Google Scholar]
- 4.Baum JD.Retinal photography in premature infants: forme fruste retrolental fibroplasia. Proc Roy Soc Med 1971;64:777–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingham JD. Acute retrolental fibroplasia. Arch Ophthalmol 1977;95:39–47. [DOI] [PubMed] [Google Scholar]
- 6.Uemura Y. Current status of retrolental fibroplasia: report of the joint committee for the study of retrolental fibroplasia in Japan. Jpn J Ophthalmol 1977;21:366–78. [Google Scholar]
- 7.Schaffer DB, Johnson L, Quin GE, et al. A classification of retrolental fibroplasia to evaluate vitamin E therapy.</org-name> Am J Ophthalmol 1979;86:1749–60. [DOI] [PubMed] [Google Scholar]
- 8.Saunders RA, Bluestein EC, Sinatra RB, et al. The predictive value of posterior pole vessels in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 1995;32:302–4. [DOI] [PubMed] [Google Scholar]
- 9.Joint Working Party of the Royal College of Ophthalmologists and British Association of Perinatal Medicine. Retinopathy of Prematurity: guidelines for Screening and Treatment. Early Hum Dev 1996;46:239–58. [PubMed] [Google Scholar]
- 10.Capowski JJ, Klystra JA, Freedman SF. A numerical index based on spatial frequency for the tortuosity of retinal vessels and its application to plus disease in retinopathy of prematurity. Retina 1995;15:490–500. [DOI] [PubMed] [Google Scholar]
- 11.Freedman SF, Klystra JA, Capowski MS, et al. Observer sensitivity to retinal vessel diameter and tortuosity in retinopathy of prematurity: a model system. J Pediatr Ophthalmol Strabismus 1996;33:248–54. [DOI] [PubMed] [Google Scholar]
- 12.Fielder AR, Shaw DE, Robinson J, et al. Natural history of retinopathy of prematurity: a prospective study. Eye 1992;6:233–42. [DOI] [PubMed] [Google Scholar]
- 13.Strömland K, Hellström A, Gustavsson T. Morphometry of the optic nerve and retinal vessels in children by computer-assisted image analysis of fundus photographs. Graefes Arch Clin Exp Ophthalmol 1995;233:150–3. [DOI] [PubMed] [Google Scholar]
- 14.Hellström A, Hard AL, Chen Y, et al. Ocular fundus morphology in preterm children. Invest Ophthalmol Vis Sci 1997;38:1184–92. [PubMed] [Google Scholar]
- 15.Hellström A. Optic nerve morphology may reveal adverse events during prenatal and perinatal life—digital image analysis. Surv Ophthalmol 1999;44:S63–73. [DOI] [PubMed] [Google Scholar]
- 16.Chapman N, Witt N, Gao X, et al. Computer algorithms for the automated measurement of retinal arteriolar diameters. Br J Ophthalmol 2001;85:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanson CR, Cocker KD, Parker KH, et al. Semi-automated computer analysis of vessel growth in preterm infants with ROP [abstract]. Annual Meeting Abstract and Program Planner 2002; [on CD-ROM or accessible at www.arvo.org]. Association for Research in Vision and Ophthalmology. Abstract 1250.
- 18.Heneghan C, Flynn J, O’Keefe M, et al. Characterization of changes in blood vessel width and tortuosity in retinopathy of prematurity using image analysis. Med Image Anal 2002;6:407–29. [DOI] [PubMed] [Google Scholar]
- 19.Sinthanayothin C, Boyce JF, Williamson TH, et al. Automated detection of diabetic retinopathy on digital fundus images. Diabet Med 2002;19:105–12. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Perez ME, Hughes AD, Stanton AV, et al. Geometrical and morphological analysis of vascular branches from fundus retinal images. 2000:756–65.
- 21.Martinez-Perez ME. Computer analysis of the geometry of the retinal vasculature. PhD Thesis. London: Imperial College, 2001.
- 22.Martinez-Perez M, Hughes AD, Stanton AV, et al. Retinal vascular tree morphology: a semi-automatic quantification. IEEE Trans Biomed Eng 2002;49:912–17. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert C, Rahi J, Eckstein M, et al. Retinopathy of prematurity in middle-income countries. Lancet 1997;50:12–14. [DOI] [PubMed] [Google Scholar]
- 24.Flynn JT, Sola A, Good WV, et al. Screening for retinopathy of prematurity—a problem solved? Pediatrics 1995;95:755–7. [PubMed] [Google Scholar]
- 25.Schwartz SD, Harrison SA, Ferrone PJ, et al. Telemedical evaluation and management of retinopathy of prematurity using a digital fundus camera. Ophthalmology 2000;107:25–8. [DOI] [PubMed] [Google Scholar]
- 26.Elflein HM, Lorenz B, Preising MN, et al. Telemedicine in acute ROP: a multicenter study using a digital retinal imaging system [abstract]. Annual Meeting Abstract and Program Planner. 2002; [on CD-ROM or accessible at www.arvo.org]. Association for Research in Vision and Ophthalmology. Abstract 1243.
- 27.Roth DB, Morales D, Feuer WJ, et al. Screening for retinopathy of prematurity employing the Retcam 120: sensitivity and specificity. Arch Ophthalmol 2001;119:268–71. [PubMed] [Google Scholar]