Abstract
Older, and even some modern, intraocular lenses (IOLs) transmit potentially hazardous ultraviolet radiation (UVR) to the retina. In addition, IOLs transmit more blue and green light to the retina for scotopic vision than the crystalline lenses they replace, light that is also potentially hazardous. The severity of UVR-blue type phototoxicity increases with decreasing wavelength, unlike the action spectrum of blue-green type retinal phototoxicity and the luminous efficiency of scotopic vision which both peak in the blue-green part of the optical spectrum around 500 nm. Theoretically, UVR+blue absorbing IOLs provide better retinal protection but worse scotopic sensitivity than UVR-only absorbing IOLs, but further study is needed to test this analysis. UVR is potentially hazardous and not useful for vision, so it is prudent to protect the retina from it with chromophores in IOLs. Determining authoritatively how much blue light an optimal IOL should block requires definitive studies to determine (1) the action spectrum of the retinal phototoxicity potentially involved in human retinal ageing, and (2) the amount of shorter wavelength blue light required for older adults to perform essential activities in dimly lit environments.
Keywords: intraocular lens, chromophore, scotopic vision, retinal phototoxicity, photic retinopathy, macular degeneration, blue light, ultraviolet radiation
The retina exists in a dangerous environment. Exposure to high concentrations of light and oxygen can damage photoreceptors and retinal pigment epithelial cells.1–3 Intraretinal defences decline as tissues age.3–9 Cells can self destruct when chemical triggers are activated.10–15 Intraocular lenses (IOLs) increase light exposure of an ageing retina as its defences are declining.16–18 Ageing cannot be stopped but the optical radiation that IOLs transmit can be controlled.
Ultraviolet radiation (UVR) and visible light can cause photic retinopathy, also known as retinal photoxicity or foveomacular retinitis.19–23 Solar and operating microscope maculopathy are examples of acute retinal phototoxicity. The cornea and crystalline lens help protect the retina from photic retinopathy by preventing UVR from reaching the retina. The cornea blocks UVR with wavelengths below 300 nm.24–28 The crystalline lens blocks UVR between 300 nm and 400 nm.24–28 The ageing crystalline lens also blocks potentially phototoxic shorter wavelength blue light.24,26,29
Cataract surgery increases the amount of optical radiation that reaches the retina. Intraocular lenses can compromise ocular defences against photic retinopathy, a problem first reported in 1978.16,17 IOLs with UVR blocking chromophores bonded to optic polymers (UVR-only absorbing IOLs) were introduced in the early 1980s, but even some modern IOLs have inadequate UVR protection.18,30,31 IOLs that absorb blue as well as UVR radiation (UVR+blue absorbing IOLs) were introduced in the 1990s.32
UVR is not useful for human vision, so it makes good sense to use IOL chromophores to prevent it from reaching the retina. How much blue light to block is a more difficult decision, however, because the action spectrum of retinal phototoxicity potentially involved in macular ageing is currently unknown, scotopic vision decreases faster than photopic vision in older adults, and blue light is more important in scotopic than photopic vision.33–39 In essence, light transmission through an IOL is a trade off between visual performance and protection against retinal phototoxicity. That balance can be quantified theoretically using standard data on scotopic visual sensitivity40 and retinal phototoxicity.41–44
SCOTOPIC VISION AND AGEING
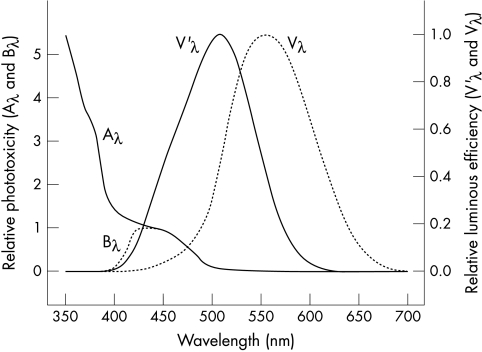
Photopic sensitivity for an eye adapted to bright luminances peaks at 555 nm in the green-yellow part of the spectrum.40 Scotopic sensitivity for an eye adapted to dim luminances peaks at 506 nm in the blue-green part of the spectrum.40 The Commission Internationale de l’Eclairage (CIE) standard spectral luminous efficiency functions for photopic and scotopic vision are Vλ and V’λ, respectively.40 They are illustrated in Figure 1, which shows that scotopic vision is much more dependent on blue light than photopic vision. The similarity between scotopic luminosity V’λ and the absorption spectrum for rhodopsin in human rod photoreceptors (which peaks at 498 nm)45 was a key reason for concluding that rhodopsin mediates scotopic vision.46 Similarly, the resemblance between the absorption spectrum of rhodopsin and the action spectrum of blue-green type retinal photoxicity suggests that rhodopsin may have an important role in this type of photic retinopathy.6,19,47,48
Figure 1.
Aλ and Bλ describe how UVR-blue type phototoxicity varies with wavelength in an aphakic and a phakic eye, respectively.41–43 V’λ and Vλ are the relative spectral luminous scotopic and photopic efficiencies, respectively, of the standard CIE observer.40 They characterise how scotopic and photopic visual sensitivity vary with wavelength in a normal phakic eye.40,170
Visual performance decreases with ageing on most sensory tests, even in individuals with normal high contrast visual acuity.49–53 Ageing has little effect on the number of human foveal cone photoreceptors, but parafoveal rod photoreceptors decrease by 30% with increasing age.38,54 The mechanisms of this loss remain under investigation, but it probably has a significant role in the declining scotopic vision of older adults.55,56 Scotopic visual sensitivity decreases twice as fast as photopic sensitivity with increasing age, a loss that contributes to older adults’ visual difficulties in dim environments and that occurs independent of retinal disease or ocular optical problems.34,36,38 Rod mediated dark adaptation slows progressively with ageing because of delayed rhodopsin regeneration.34 Scotopic contrast sensitivity at low and high spatial frequencies declines with increasing age.35 Ageing related loss of scotopic sensitivity is worst in the blue part of the spectrum.57 Macular degeneration worsens age related decreases in scotopic vision.37,38,58,59
Visual difficulties can limit the activities and reduce the quality of life of older adults.52,60–64 Fear of events such as a hip fracture from a fall can prompt older individuals to limit their activities.63 Impaired dark adaptation is associated with an increased risk of falling.65 Older individuals need to be closer to road signs to read them effectively at night.66 Vision problems may prompt older drivers to curtail their night-time driving activities.39,52,67–69 In general, visual problems in dim environments increase with ageing, and improved scotopic vision is an appropriate goal for cataract surgery and IOL design. The bottom line is that older adults have increasing problems seeing at night or in dim environments even when they don’t have crystalline lens or retinal problems.49–53
PHOTIC RETINOPATHY AND AGEING
Photic retinopathy has been studied as an ocular hazard and used as a technique to investigate retinal degeneration and cell biology. The oxygen rich environment of the neural retina and retinal pigment epithelium (RPE) increases their vulnerability to light damage.48,70–74 Ocular media are the first line of defence against photic retinopathy, but the retina has its own internal defences against phototoxicity, including agents such as superoxide dismutase, catalase, glutathione peroxidase, vitamin E, vitamin C, lutein, and zeaxanthin.6,71,75–77
Photic retinopathy has been studied extensively since it was first reported in 1966.19 It occurs at chorioretinal temperature elevations far too low for retinal photocoagulation.78–80 Retinal photocoagulation is thermal damage caused by radiant heating of the retina and choroid, whereas photic retinopathy is actinic damage caused by photochemical reactions in the neural retina and/or RPE.
Phototoxicity is accelerated by higher body temperature19,78,81 and elevated blood oxygen concentration.1,2 Genetic factors,82–84 time of day,85–88 and diet89–92 all affect the susceptibility of experimental animals to photic retinopathy. Different mechanisms cause phototoxicity in the neural retina and RPE, selective damage at each of these sites being dependent on exposure protocols and animal species.48,93,94 There is reciprocity between retinal irradiance (power/area) and exposure time, so longer exposures produce threshold phototoxicity at lower irradiances.48 Retinal phototoxicity is probably additive so that previous exposure increases the risk of subsequent damage.95
Retinal defences against photic retinopathy decline with ageing.3–9 Environmental light exposure has been postulated to be a potential causative factor in macular degeneration for almost a century,6,17,23,77,96–100 and there are striking similarities in the retinal abnormalities caused by age related macular degeneration and repetitive acute phototoxicity.17,97,101,102 Unfortunately, epidemiological studies correlating macular degeneration with light exposure are problematical because individual susceptibility varies and lifelong photic exposure is difficult to determine accurately in retrospective studies. Some studies have shown a correlation between macular degeneration and lifelong light exposure, whereas others have not found them to be correlated.103–110 Additionally, studies correlating cataract surgery with postoperative progression of macular degeneration also have produced conflicting results.111–119
An action spectrum characterises the relative effectiveness of different wavelengths in producing a photochemical effect. There are at least two classes of action spectra for retinal phototoxicity.
For lengthy exposures typically shorter than 12 hours in aphakic animals, retinal phototoxicity has an action spectrum that increases with decreasing wavelength, as shown by Aλ(for aphakic) in Figure 1.20,41–43,47–48,120–123 This UVR-blue type of retinal photoxicity has been termed blue light, class 2 or Ham type photic retinopathy. It has also been termed “blue light” damage because its action spectrum peaks around 440 nm when a crystalline lens blocks UVR and shorter wavelength blue light, as shown by Bλ(for blue) in Figure 1.41–43
For prolonged exposures typically longer that 12 hours, phototoxicity has an action spectrum that peaks in the blue-green part of the spectrum, similar to the absorption spectrum of rhodopsin or that of scotopic luminous efficiency (V’λ in Fig 1).19,41–43,47–48,121,124 This blue-green type of retinal phototoxicity has been referred to as white light, class 1, or Noell type photic retinopathy. Blue-green type retinal phototoxicity occurs at substantially lower retinal irradiances than UVR-blue type retinal phototoxicity, but very prolonged exposures are required to produce damage in a single irradiation.47–48,121
The photosensitisers responsible for photic retinopathy have not been determined conclusively. Rhodopsin, its photoproducts, or cytochrome-c oxidase in mitochondria may be involved.19,21,93,125–128 A growing body of evidence suggests that lipofuscin fluorophores—for instance, the pyridinium bisretinoid A2E,129 may play significant parts in RPE phototoxicity and macular ageing,15,73,130–137 and that the photoxidative products of A2E are the agents that damage cellular molecules.136,138,139
A2E has an excitation maximum of approximately 430 nm,133,140 a property that may contribute to the susceptibility of RPE to blue light damage in vivo.20,141 Most of the lipofuscin that is amassed by RPE originates from conjugates generated by visual cycle retinoids in photoreceptor cells,138,142–144 this material being deposited in RPE cells subsequent to outer segment disc phagocytosis.145 These retinoid conjugates accumulate because they are not broken down enzymatically. Accordingly, lipofuscin levels in the RPE increase with age,146–148 and the highest levels are present in macular RPE.148–151 The role of RPE melanin as a photosensitiser and/or photoprotective agent in photic retinopathy remains under investigation,135,152–154 but the presence of melanin is not essential for RPE phototoxicity.133
IOL PROTECTION AND PERFORMANCE
IOLs were initially fabricated from poly(methylmethacrylate)(PMMA) without UVR blocking chromophores.16 The dangers of retinal exposure to near-UVR transmitted by clear PMMA IOLs were recognised in 197816,17 and most IOLs had UVR absorbing chromophores by 1986.18 Unfortunately, UVR protection in contemporary IOLs is inconsistent, and some manufacturers still produce IOLs that transmit potentially phototoxic near-UVR to the retina.31
The advantages of UVR-only absorbing IOLs are well documented. UVR-only protective IOLs transmit more blue light than a crystalline lens,155 but they decrease the incidence of erythropsia156–159 and blue cone sensitivity loss in pseudophakes.160 They also decrease blood-retinal barrier disruption in pseudophakes as measured by vitreous fluorophotometry161 and the risk of retinal phototoxicity in experimental animals.162,163 UVR+blue absorbing IOLs increase photopic and mesopic contrast sensitivity at intermediate spatial frequencies.32 UVR-only protective IOLs were reported initially to decrease the risk of angiographically apparent cystoid macular oedema (CMO),164 but a later study found no such effect in individuals with a ultraviolet protective lens in one eye and a non-ultraviolet-absorbing lens in their other eye.165
Blocking UVR with IOL chromophores increases protection from photic retinopathy without decreasing visual sensitivity. It also seemed appropriate in 1986 to use IOL chromophores to decrease the amount of shorter wavelength blue light reaching the retina.18 Now that a growing body of scientific evidence has demonstrated that ageing related decreases in scotopic sensitivity cannot be attributed solely to optical changes but may involve rod and ganglion cell loss as well as central visual pathway alterations,34–36,38,166 how much shorter wavelength blue light should be attenuated by IOL chromophores to reduce the potential risk of retinal phototoxicity?
The scotopic luminous efficiency (V’λ) and aphakic phototoxicity (Aλ) standards shown in Figure 1 can be used to examine how the optical transmittance spectrum of a crystalline or intraocular lens affects scotopic vision and the risk of photic retinopathy. The areas under the V’λ and Aλ curves in Figure 1 represent total scotopic sensitivity and total aphakic UVR-blue retinal phototoxicity, respectively. If V’λ and Aλ are convolved with a transmittance spectrum of a particular lens, the percentage difference between the original and convolved areas under the curve represents the percentage loss in scotopic sensitivity or gain in UVR-blue phototoxicity protection from the lens.
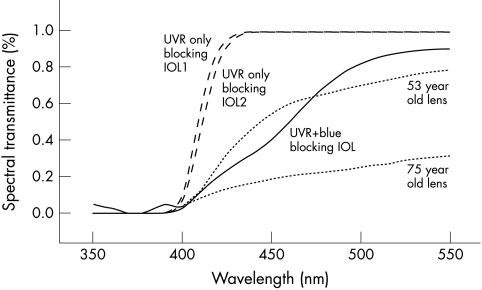
Calculations were performed for the five lenses shown in Figure 2, which included two UVR-only absorbing IOLs, one UVR+blue absorbing IOL, and a 53 year old and 75 year old crystalline lens. The results of this analysis are presented in Table 1. As expected, the calculations predict that increasing retinal protection with an IOL decreases its overall scotopic performance and that the UVR+blue absorbing IOL affords better retinal protection but worse scotopic performance than the conventional UVR-only absorbing IOLs. Only clinical studies can determine the potential significance of these theoretical predictions.
Figure 2.
The percentage spectral transmittance of crystalline and intraocular lenses listed in Table 1. Spectral transmittance data on 20D UVR-only absorbing IOL 1 (Alcon AcrySof MA60BM) and 2 (Pharmacia & Upjohn 720A) 20D lenses are from Lin, et al.31 UVR+blue absorbing IOL data (Alcon AcrySof Natural 20D lens) are from Mr Raphael Chan, Alcon Surgical Division, Forth Worth, TX, USA. The 53 and 75 year old crystalline lens transmittance data are from Boettner and Wolter.24
Table 1.
Theoretical predictions of how several IOLs and human crystalline lenses decrease scotopic visual sensitivity and increase protection from blue-green type photic retinopathy
| Lens | % decrease in scotopic visual sensitivity* | % increase in protection from UVR blue type phototoxicity† |
| 75 year old crystalline lens‡ | 75 | 93 |
| 53 year old crystalline lens‡ | 33 | 86 |
| UVR+blue absorbing IOL§ | 27 | 90 |
| UVR-only blocking IOL, No 1¶ | 1.5 | 75 |
| UVR-only blocking IOL, No 2** | 1.6 | 76 |
*The percentage difference between the total areas under the V’λ curve in Figure 1 and that curve convolved with the spectral transmittance of a particular lens.
†The percentage difference between the total areas under the Aλ curve in Figure 1 and that curve convolved with the spectral transmittance of a particular lens.
‡Data on human crystalline lens transmittance are from Boettner and Wolter.24
§Alcon acrylic AcrySof Natural 20D lens. IOL transmittance data are from Mr Rafael Chan, Alcon Surgical Division, Forth Worth, TX, USA.
¶Alcon acrylic AcrySof MA60BM 20D lens. IOL transmittance data are from Lin et al.31
**Pharmacia & Upjohn poly(methylmethacrylate) 720A 20D lens. IOL transmittance data are from Lin et al.31
The results in Table 1 are subject to numerous limitations:(1) If chronic environmental light exposure does play an important part in macular ageing, it probably affects individuals quite differently depending on unrelated environmental factors such as smoking and on pigmentation and other genetic factors such as the rate at which A2E accumulates in RPE cells, which in turn may be affected by abcr gene mutations.167(2) V’λ does describe overall scotopic performance, but it represents the performance of a phakic “standard CIE observer”40,168 rather than an aphakic older adult. Psychophysical studies are needed to determine:(a) how much shorter wavelength blue light is needed for older adults to perform essential scotopic tasks in dimly illuminated environments, and (b) whether the shorter wavelength blue light attenuated by a UVR+blue absorbing IOL but transmitted by a UVR-only absorbing IOL can compensate in any significant way for ageing related losses in scotopic sensitivity. (3) Aλ does characterise threshold, acute, UVR-blue type photic retinopathy in experimental animals, but it may differ significantly from the action spectrum of the repetitive or chronic retinal phototoxicity potentially involved in but not conclusively proved to have a significant role in human retinal ageing. (4) Recent threshold studies on primate retinal phototoxicity have found that some of the classic data incorporated into the international Aλ standard may significantly overestimate the UVR-blue type phototoxicity of shorter wavelength blue light.169 Thus, international phototoxicity standards may change, and results in Table 1 based on Aλ probably significantly overestimate the protection from phototoxicity provided by UVR-only and UVR+blue absorbing IOLs.
DISCUSSION
Cataract surgery removes the crystalline lens which provides optical protection against retinal phototoxicity in an ageing eye. Light absorbing chromophores in an IOL determine which optical wavelengths are transmitted to the retina, balancing retinal protection with visual performance.
An ideal IOL would adapt to changing illumination, transmitting all visible light in dim environments for optimal scotopic performance, but blocking a variable amount of visible light in bright environments depending on an individual’s visual requirements and chorioretinal condition. Adaptive photochromic IOLs are not available. The two current choices are UVR-only and UVR+blue absorbing IOLs. In both cases, sunglasses and other forms of ocular protection such as a brimmed hat probably should be worn in very bright environments because of the potential risk of blue-green type retinal phototoxicity.
As shown in Table 1, UVR only blocking IOLs theoretically provide less protection from UVR-blue type phototoxicity than UVR+blue absorbing IOLs. If only the spectral region between 400–550 nm is considered, this protection is roughly a third of that of UVR+blue absorbing IOLs. Conversely, UVR-only blocking IOLs theoretically do not significantly diminish scotopic visual sensitivity. These data predict that UVR+blue absorbing IOLs diminish scotopic visual sensitivity by roughly 25%, but the practical significance of that loss is unknown. The preceding analysis addresses only UVR-blue type retinal phototoxicity, not the blue-green type retinal phototoxicity which has an action spectrum similar to the spectral sensitivity of scotopic vision or the absorption spectrum of rhodopsin. Any increase in protection against blue-green type phototoxicity that an IOL provided would be accompanied by an equivalent percentage decrease in scotopic sensitivity.
One might argue that replacing an ageing crystalline lens with a UVR-only blocking IOL increases the amount of potentially hazardous blue light reaching senescent macular RPE with its increased lipofuscin content, that decreasing blue light even in non-brilliant photopic environments could decrease background UVR-blue type phototoxic damage which might have a role in macular ageing, that shorter wavelength blue light has not been proved to be valuable for essential scotopic visual tasks of older adults after IOL implantation, and that blue light absorbing chromophores in an IOL are always there for some optical radiation protection even in individuals who fail to wear sunglasses in appropriate circumstances.
Conversely, one might argue that UVR-blue type of phototoxicity has not been proved to have a significant role in human macular ageing, that improved blue light transmission might help compensate for visual losses as a result of decreased rod photoreceptor density in ageing, that the hypothetical benefit of avoiding fractures from tripping in dim illumination is more significant than the hypothetical benefit of decreasing the risk of age related macular degeneration, and that it’s easier to switch sunglasses than IOLs should future research demonstrate that shorter wavelength blue light is useful for the scotopic vision of older adults.
Neither author has an IOL, but if and when we need one, we would both make sure that it had appropriate UVR blocking chromophores. Based on current information, one of us (MAM) would choose to have a UVR-only blocking IOL that would provide maximal protection against UVR, and wear sunglasses in very bright environments, which could be removed for optimal vision in dim environments. JRS would choose a UVR+blue absorbing IOL that would provide maximal protection against UVR, afford roughly the same protection against phototoxicity and diminution of scotopic sensitivity as a 50 year old crystalline lens, and wear sunglasses in very bright environments, which could be removed for improved vision in dimmer environments. Until photochromic IOLs become available, the decision on which strategy is optimal awaits conclusive data on the role of UVR-blue type retinal phototoxicity in age related macular degeneration and the value of shorter wavelength blue light in essential scotopic activities of older adults.
Supported in part by the Kansas Lions Sight Foundation, Inc (MAM), grant EY-12951 from the National Eye Institute, Bethesda MD (JRS), and a grant from Alcon (JRS).
The authors have no proprietary interests in the development or marketing of any product mentioned in this study.
REFERENCES
- 1.Ruffolo JJ Jr, Ham WT Jr, Mueller HA, et al. Photochemical lesions in the primate retina under conditions of elevated blood oxygen. Invest Ophthalmol Vis Sci 1984;25:893–8. [PubMed] [Google Scholar]
- 2.Crockett RS, Lawwill T. Oxygen dependence of damage by 435 nm light in cultured retinal epithelium. Curr Eye Res 1984;3:209–15. [DOI] [PubMed] [Google Scholar]
- 3.Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000;45:115–34. [DOI] [PubMed] [Google Scholar]
- 4.Robison WG, Kuwabara T, Bieri JG. The roles of vitamin E and unsaturated fatty acids in the visual process. Retina 1982;2:263–81. [PubMed] [Google Scholar]
- 5.Marshall J. The ageing retina: physiology or pathology. Eye 1987;1(Pt 2):282–95. [DOI] [PubMed] [Google Scholar]
- 6.Mainster MA. Light and macular degeneration: a biophysical and clinical perspective. Eye 1987;1(Pt 2):304–10. [DOI] [PubMed] [Google Scholar]
- 7.Hunyor AB. Solar retinopathy: its significance for the ageing eye and the younger pseudophakic patient. Aust N Z J Ophthalmol 1987;15:371–5. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JE. Ocular phototoxicity. J Photochem Photobiol B 2001;64:136–43. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein PS, Zhao DY, Wintch SW, et al. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology 2002;109:1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abler AS, Chang CJ, Ful J, et al. Photic injury triggers apoptosis of photoreceptor cells. Res Commun Mol Pathol Pharmacol 1996;92:177–89. [PubMed] [Google Scholar]
- 11.Wu J, Seregard S, Spangberg B, et al. Blue light induced apoptosis in rat retina. Eye 1999;13(Pt 4):577–83. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel A, Grimm C, Marti A, et al. c-fos controls the “private pathway” of light-induced apoptosis of retinal photoreceptors. J Neurosci 2000;20:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanos S, Heiduschka P, Romann I. Exposure to a solar eclipse causes neuronal death in the retina. Graefes Arch Clin Exp Ophthalmol 2001;239:794–800. [DOI] [PubMed] [Google Scholar]
- 14.Dunaief JL, Dentchev T, Ying GS, et al. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol 2002;120:1435–42. [DOI] [PubMed] [Google Scholar]
- 15.Sparrow JR, Zhou J, Ben-Shabat S, et al. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci 2002;43:1222–7. [PubMed] [Google Scholar]
- 16.Mainster MA. Spectral transmittance of intraocular lenses and retinal damage from intense light sources. Am J Ophthalmol 1978;85:167–70. [DOI] [PubMed] [Google Scholar]
- 17.Mainster MA. Solar retinitis, photic maculopathy and the pseudophakic eye. J Am Intraocul Implant Soc 1978;4:84–6. [DOI] [PubMed] [Google Scholar]
- 18.Mainster MA. The spectra, classification, and rationale of ultraviolet-protective intraocular lenses. Am J Ophthalmol 1986;102:727–32. [DOI] [PubMed] [Google Scholar]
- 19.Noell WK, Walker VS, Kang BS, et al. Retinal damage by light in rats. Invest Ophthalmol 1966;5:450–73. [PubMed] [Google Scholar]
- 20.Ham WT Jr, Mueller HA, Sliney DH. Retinal sensitivity to damage from short wavelength light. Nature 1976;260:153–5. [DOI] [PubMed] [Google Scholar]
- 21.Lawwill T. Three major pathologic processes caused by light in the primate retina: a search for mechanisms. Trans Am Ophthalmol Soc 1982;80:517–79. [PMC free article] [PubMed] [Google Scholar]
- 22.Mainster MA, Ham WT Jr, Delori FC. Potential retinal hazards. Instrument and environmental light sources. Ophthalmology 1983;90:927–32. [DOI] [PubMed] [Google Scholar]
- 23.Mainster MA, Turner PL. Photic retinal injury and safety. In: Ryan SJ, Ogden TE, Hinton DR, Schachat AP, eds. Retina. 3rd ed. St Louis: Mosby, 2001:1797–809.
- 24.Boettner EA, Wolter JR. Transmission of the ocular media. Invest Ophthalmol 1962;1:776–83. [Google Scholar]
- 25.Van Norren D, Vos JJ. Spectral transmission of the human ocular media. Vis Res 1974;14:1237–44. [DOI] [PubMed] [Google Scholar]
- 26.Mellerio J. Yellowing of the human lens: nuclear and cortical contributions. Vis Res 1987;27:1581–7. [DOI] [PubMed] [Google Scholar]
- 27.Weale RA. Age and the transmittance of the human crystalline lens. J Physiol 1988;395:577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griswold MS, Stark WS. Scotopic spectral sensitivity of phakic and aphakic observers extending into the near ultraviolet. Vis Res 1992;32:1739–43. [DOI] [PubMed] [Google Scholar]
- 29.Bron AJ, Vrensen GF, Koretz J, et al. The ageing lens. Ophthalmologica 2000;214:86–104. [DOI] [PubMed] [Google Scholar]
- 30.Lindstrom RL, Doddi N. Ultraviolet light absorption in intraocular lenses. J Cataract Refract Surg 1986;12:285–9. [DOI] [PubMed] [Google Scholar]
- 31.Lin K, Lin Y, Lee J, et al. Spectral transmission characteristics of spectacle, contact, and intraocular lenses. Ann Ophthalmol 2002;34:206–15. [Google Scholar]
- 32.Niwa K, Yoshino Y, Okuyama F, et al. Effects of tinted intraocular lens on contrast sensitivity. Ophthalmic Physiol Opt 1996;16:297–302. [PubMed] [Google Scholar]
- 33.Jackson GR, Owsley C, Cordle EP, et al. Aging and scotopic sensitivity. Vis Res 1998;38:3655–62. [DOI] [PubMed] [Google Scholar]
- 34.Jackson GR, Owsley C, McGwin G, Jr. Aging and dark adaptation. Vis Res 1999;39:3975–82. [DOI] [PubMed] [Google Scholar]
- 35.Schefrin BE, Tregear SJ, Harvey LO Jr, et al. Senescent changes in scotopic contrast sensitivity. Vis Res 1999;39:3728–36. [DOI] [PubMed] [Google Scholar]
- 36.Jackson GR, Owsley C. Scotopic sensitivity during adulthood. Vis Res 2000;40:2467–73. [DOI] [PubMed] [Google Scholar]
- 37.Owsley C, Jackson GR, White M, et al. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology 2001;108:1196–202. [DOI] [PubMed] [Google Scholar]
- 38.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev 2002;1:381–96. [DOI] [PubMed] [Google Scholar]
- 39.Mainster MA, Timberlake G. Why HID headlights bother older drivers. Br J Ophthalmol 2003;87:113–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyszecki G, Stiles WS. Color science. New York: John Wiley & Sons, 1967.
- 41.American Conference of Governmental Industrial Hygienists. Threshold limit values for chemical substances physical agents: biological exposure indices. Cincinnati: ACGIH, 1997.
- 42.International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to broad-band incoherent optical radiation (0.38 to 3 microM). Health Phys 1997;73:539–54. [PubMed] [Google Scholar]
- 43.Sliney DH, Bitran M. The ACGIH action spectra for hazard assessment: The TLV’s. In: Matthes R, Sliney DH, eds. Measurements of optical radiation hazards. Oberschleissheim, Germany: International Commission on Non-Ionizing Radiation Protection (ICNIRP 6/98) and CIE (x016-1998), 1998:241–29.
- 44.American Conference of Governmental Industrial Hygienists. Threshold limit values and biological exposure indices. Cincinnati: ACGIH, 2000.
- 45.Crescitelli F, Dartnall HJ A. Human visual purple. Nature 1953;172:195–200. [DOI] [PubMed] [Google Scholar]
- 46.Hsia Y. Photochemistry of vision. In: Graham CH, Bartlett NR, Brown JL, Hsia Y, Mueller CG, Riggs LA, eds. Vision and visual perception. New York: John Wiley and Sons, 1966.
- 47.Kremers JJ, van Norren D. Two classes of photochemical damage of the retina. Lasers Light Ophthalmol 1988;2:41–52. [Google Scholar]
- 48.Mellerio J. Light effects on the retina. In: Albert DM, Jakobiec FA, eds. Principles and practice of ophthalmology. Philadelphia: WB Saunders, 1994:1326–45.
- 49.Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Seeing into old age: vision function beyond acuity. Optom Vis Sci 1999;76:141–58. [DOI] [PubMed] [Google Scholar]
- 50.Ivers RQ, Mitchell P, Cumming RG. Visual function tests, eye disease and symptoms of visual disability: a population-based assessment. Clin Exp Ophthalmol 2000;28:41–7. [DOI] [PubMed] [Google Scholar]
- 51.Rubin GS, West SK, Munoz B, et al. A comprehensive assessment of visual impairment in a population of older Americans. The SEE Study. Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci 1997;38:557–68. [PubMed] [Google Scholar]
- 52.Klein BE, Klein R, Lee KE, et al. Associations of performance-based and self-reported measures of visual function. The Beaver Dam Eye Study. Ophthalmic Epidemiol 1999;6:49–60. [DOI] [PubMed] [Google Scholar]
- 53.Johnson CA, Keltner JL. Incidence of visual field loss in 20,000 eyes and its relationship to driving performance. Arch Ophthalmol 1983;101:371–5. [DOI] [PubMed] [Google Scholar]
- 54.Curcio CA, Millican CL, Allen KA, et al. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci 1993;34:3278–96. [PubMed] [Google Scholar]
- 55.Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci 2000;41:2015–8. [PubMed] [Google Scholar]
- 56.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye 2001;15(Pt 3):376–83. [DOI] [PubMed] [Google Scholar]
- 57.Gunkel RD, Gouras P. Changes in scotopic visibility thresholds with age. Arch Ophthalmol 1963;69:38–43. [DOI] [PubMed] [Google Scholar]
- 58.Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology 1997;104:1677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci 2000;41:267–73. [PubMed] [Google Scholar]
- 60.Kosnik W, Winslow L, Kline D, et al. Visual changes in daily life throughout adulthood. J Gerontol 1988;43:63–70. [DOI] [PubMed] [Google Scholar]
- 61.Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol 1998;116:227–33. [DOI] [PubMed] [Google Scholar]
- 62.Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc 1999;97:473–511. [DOI] [PubMed] [Google Scholar]
- 63.Klein BE, Moss SE, Klein R, et al. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the Beaver Dam eye study. Ophthalmology 2003;110:644–50. [DOI] [PubMed] [Google Scholar]
- 64.Brown MM, Brown GC, Sharma S, et al. Quality of life associated with visual loss: a time tradeoff utility analysis comparison with medical health states. Ophthalmology 2003;110:1076–81. [DOI] [PubMed] [Google Scholar]
- 65.McMurdo ME, Gaskell A. Dark adaptation and falls in the elderly. Gerontology 1991;37:221–4. [DOI] [PubMed] [Google Scholar]
- 66.Sivak M, Olson PL, Pastalan LA. Effect of driver’s age on nighttime legibility of highway signs. Hum Factors 1981;23:59–64. [DOI] [PubMed] [Google Scholar]
- 67.Kline DW. Light, ageing and visual performance. In: Marshall J, ed. The susceptible visual apparatus. London: Macmillan Press, 1991:150–61.
- 68.Owsley C, McGwin G, Jr. Vision impairment and driving. Surv Ophthalmol 1999;43:535–50. [DOI] [PubMed] [Google Scholar]
- 69.Owsley C, Stalvey BT, Phillips JM. The efficacy of an educational intervention in promoting self-regulation among high-risk older drivers. Accid Anal Prev 2003;35:393–400. [DOI] [PubMed] [Google Scholar]
- 70.Feeney-Burns L, Ellersieck MR. Age-related changes in the ultrastructure of Bruch’s membrane. Am J Ophthalmol 1985;100:686–97. [DOI] [PubMed] [Google Scholar]
- 71.Feeney L, Berman ER. Oxygen toxicity: membrane damage by free radicals. Invest Ophthalmol 1976;15:789–92. [PubMed] [Google Scholar]
- 72.Ham WT Jr , Mueller HA, Ruffolo JJ, Jr, et al. Basic mechanisms underlying the production of photochemical lesions in the mammalian retina. Curr Eye Res 1984;3:165–74. [DOI] [PubMed] [Google Scholar]
- 73.Boulton M, Rozanowska M, Rozanowski B. Retinal photodamage. J Photochem Photobiol B 2001;64:144–61. [DOI] [PubMed] [Google Scholar]
- 74.Ben-Shabat S, Parish CA, Hashimoto M, et al. Fluorescent pigments of the retinal pigment epithelium and age-related macular degeneration. Bioorg Med Chem Lett 2001;11:1533–40. [DOI] [PubMed] [Google Scholar]
- 75.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr 1995;62(Suppl):1448–61. [DOI] [PubMed] [Google Scholar]
- 76.Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye 1995;9(Pt 6):763–71. [DOI] [PubMed] [Google Scholar]
- 77.Reme C, Reinboth J, Clausen M, et al. Light damage revisited: converging evidence, diverging views? Graefes Arch Clin Exp Ophthalmol 1996;234:2–11. [DOI] [PubMed] [Google Scholar]
- 78.Friedman E, Kuwabara T. The retinal pigment epithelium. IV. The damaging effects of radiant energy. Arch Ophthalmol 1968;80:265–79. [DOI] [PubMed] [Google Scholar]
- 79.Mainster MA. Destructive light adaptation. Ann Ophthalmol 1970;2:44–8. [Google Scholar]
- 80.White TJ, Mainster MA, Wilson PW, et al. Chorioretinal temperature increases from solar observation. Bull Math Biophys 1971;33:1–17. [DOI] [PubMed] [Google Scholar]
- 81.Gorgels TG, van Beek L, van Norren D. Effect of body temperature on retinal damage by 488 nm light in rat. Microsc Res Tech 1997;36:89–95. [DOI] [PubMed] [Google Scholar]
- 82.Grimm C, Wenzel A, Hafezi F, et al. Gene expression in the mouse retina: the effect of damaging light. Mol Vis 2000;6:252–60. [PubMed] [Google Scholar]
- 83.Grimm C, Wenzel A, Hafezi F, et al. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat Genet 2000;25:63–6. [DOI] [PubMed] [Google Scholar]
- 84.Wenzel A, Grimm C, Samardzija M, et al. The genetic modifier Rpe65Leu(450): effect on light damage susceptibility in c-Fos-deficient mice. Invest Ophthalmol Vis Sci 2003;44:2798–802. [DOI] [PubMed] [Google Scholar]
- 85.Organisciak DT, Darrow RM, Barsalou L, et al. Light history and age-related changes in retinal light damage. Invest Ophthalmol Vis Sci 1998;39:1107–16. [PubMed] [Google Scholar]
- 86.Organisciak DT, Darrow RM, Barsalou L, et al. Circadian-dependent retinal light damage in rats. Invest Ophthalmol Vis Sci 2000;41:3694–701. [PubMed] [Google Scholar]
- 87.Vaughan DK, Nemke JL, Fliesler SJ, et al. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem Photobiol 2002;75:547–53. [DOI] [PubMed] [Google Scholar]
- 88.Beatrice J, Wenzel A, Reme CE, et al. Increased light damage susceptibility at night does not correlate with RPE65 levels and rhodopsin regeneration in rats. Exp Eye Res 2003;76:695–700. [DOI] [PubMed] [Google Scholar]
- 89.Tso MO. Retinal photic injury in normal and scorbutic monkeys. Trans Am Ophthalmol Soc 1987;85:498–556. [PMC free article] [PubMed] [Google Scholar]
- 90.Wiegand RD, Koutz CA, Chen H, et al. Effect of dietary fat and environmental lighting on the phospholipid molecular species of rat photoreceptor membranes. Exp Eye Res 1995;60:291–306. [DOI] [PubMed] [Google Scholar]
- 91.Koutz CA, Wiegand RD, Rapp LM, et al. Effect of dietary fat on the response of the rat retina to chronic and acute light stress. Exp Eye Res 1995;60:307–16. [DOI] [PubMed] [Google Scholar]
- 92.Aonuma H, Koide K, Masuda K, et al. Retinal light damage: protective effect of alpha-tocopherol. Jpn J Ophthalmol 1997;41:160–7. [DOI] [PubMed] [Google Scholar]
- 93.Gorgels TG, van Norren D. Ultraviolet and green light cause different types of damage in rat retina. Invest Ophthalmol Vis Sci 1995;36:851–63. [PubMed] [Google Scholar]
- 94.Reme CE, Hafezi F, Marti A, et al. Light damage to the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, eds. The retinal pigment epithelium: function and disease. New York: Oxford University Press, 1998.
- 95.Griess GA, Blankenstein MF. Additivity and repair of actinic retinal lesions. Invest Ophthalmol Vis Sci 1981;20:803–7. [PubMed] [Google Scholar]
- 96.van der Hoeve J. Eye lesions produced by light rich in ultraviolet rays: senile cataract, senile degeneration of the macula. Am J Ophthalmol 1920;3:178–94. [Google Scholar]
- 97.Ts’o MO, La Piana FG, Appleton B. The human fovea after sungazing. Trans Am Acad Ophthalmol Otolaryngol 1974;78:OP677. [PubMed] [Google Scholar]
- 98.Marshall J. Radiation and the ageing eye. Ophthalmic Physiol Opt 1985;5:241–63. [PubMed] [Google Scholar]
- 99.Young RW. Solar radiation and age-related macular degeneration. Surv Ophthalmol 1988;32:252–69. [DOI] [PubMed] [Google Scholar]
- 100.Weale RA. Do years or quanta age the retina? Photochem Photobiol 1989;50:429–38. [DOI] [PubMed] [Google Scholar]
- 101.Marshall J. Ageing changes in human cones. Acta XXIII Concilium Ophthalmologicum (Kyoto) 1978;1:375–8. [Google Scholar]
- 102.Borges J, Li ZY, Tso MO. Effects of repeated photic exposures on the monkey macula. Arch Ophthalmol 1990;108:727–33. [DOI] [PubMed] [Google Scholar]
- 103.Liu IY, White L, LaCroix AZ. The association of age-related macular degeneration and lens opacities in the aged. Am J Public Health 1989;79:765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taylor HR, Munoz B, West S, et al. Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc 1990;88:163–73 discussion 173–8. [PMC free article] [PubMed] [Google Scholar]
- 105.Taylor HR, West S, Munoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol 1992;110:99–104. [DOI] [PubMed] [Google Scholar]
- 106.Cruickshanks KJ, Klein R, Klein BE. Sunlight and age-related macular degeneration. The Beaver Dam Eye Study. Arch Ophthalmol 1993;111:514–8. [DOI] [PubMed] [Google Scholar]
- 107.Darzins P, Mitchell P, Heller RF. Sun exposure and age-related macular degeneration. An Australian case-control study. Ophthalmology 1997;104:770–6. [DOI] [PubMed] [Google Scholar]
- 108.Cruickshanks KJ, Klein R, Klein BE, et al. Sunlight and the 5-year incidence of early age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol 2001;119:246–50. [PubMed] [Google Scholar]
- 109.Delcourt C, Carriere I, Ponton-Sanchez A, et al. Light exposure and the risk of age-related macular degeneration: the Pathologies Oculaires Liees a l’Age (POLA) study. Arch Ophthalmol 2001;119:1463–8. [DOI] [PubMed] [Google Scholar]
- 110.McCarty CA, Mukesh BN, Fu CL, et al. Risk factors for age-related maculopathy: the Visual Impairment Project. Arch Ophthalmol 2001;119:1455–62. [DOI] [PubMed] [Google Scholar]
- 111.Oliver M. Posterior pole changes after cataract extraction in elderly subjects. Am J Ophthalmol 1966;62:1145–8. [DOI] [PubMed] [Google Scholar]
- 112.Pollack A, Marcovich A, Bukelman A, et al. Age-related macular degeneration after extracapsular cataract extraction with intraocular lens implantation. Ophthalmology 1996;103:1546–54. [DOI] [PubMed] [Google Scholar]
- 113.Van der Schaft TL, Mooy CM, de Bruijn WC, et al. Increased prevalence of disciform macular degeneration after cataract extraction with implantation of an intraocular lens. Br J Ophthalmol 1994;78:441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pollack A, Marcovich A, Bukelman A, et al. Development of exudative age-related macular degeneration after cataract surgery. Eye 1997;11(Pt 4):523–30. [DOI] [PubMed] [Google Scholar]
- 115.Armbrecht AM, Findlay C, Kaushal S, et al. Is cataract surgery justified in patients with age related macular degeneration? A visual function and quality of life assessment. Br J Ophthalmol 2000;84:1343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Armbrecht AM, Findlay C, Aspinall PA, et al. Cataract surgery in patients with age-related macular degeneration: one-year outcomes. J Cataract Refract Surg 2003;29:686–93. [DOI] [PubMed] [Google Scholar]
- 117.Klein R, Klein BE, Wong TY, et al. The association of cataract and cataract surgery with the long-term incidence of age-related maculopathy: the Beaver Dam eye study. Arch Ophthalmol 2002;120:1551–8. [DOI] [PubMed] [Google Scholar]
- 118.Wong TY. Cataract surgery in patients with cataract and age related macular degeneration: do the benefits outweigh the risks? Br J Ophthalmol 2000;84:1337–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Freeman EE, Munoz B, West SK, et al. Is there an association between cataract surgery and age-related macular degeneration? Data from three population-based studies. Am J Ophthalmol 2003;135:849–56. [DOI] [PubMed] [Google Scholar]
- 120.Sliney DH, Wolbarsht ML. Safety with lasers and other optical sources: a comprehensive handbook. New York: Plenum Press, 1980.
- 121.Kremers JJ, van Norren D. Retinal damage in macaque after white light exposures lasting ten minutes to twelve hours. Invest Ophthalmol Vis Sci 1989;30:1032–40. [PubMed] [Google Scholar]
- 122.Van Norren D, Schellekens P. Blue light hazard in rat. Vis Res 1990;30:1517–20. [DOI] [PubMed] [Google Scholar]
- 123.Rapp LM, Smith SC. Morphologic comparisons between rhodopsin-mediated and short-wavelength classes of retinal light damage. Invest Ophthalmol Vis Sci 1992;33:3367–77. [PubMed] [Google Scholar]
- 124.Williams TP, Howell WL. Action spectrum of retinal light-damage in albino rats. Invest Ophthalmol Vis Sci 1983;24:285–7. [PubMed] [Google Scholar]
- 125.Pautler EL, Morita M, Beezley D. Hemoprotein(s) mediate blue light damage in the retinal pigment epithelium. Photochem Photobiol 1990;51:599–605. [DOI] [PubMed] [Google Scholar]
- 126.Saari JC, Garwin GG, Van Hooser JP, et al. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vis Res 1998;38:1325–33. [DOI] [PubMed] [Google Scholar]
- 127.Grimm C, Reme CE, Rol PO, et al. Blue Light’s effects on rhodopsin: photoreversal of bleaching in living rat eyes. Invest Ophthalmol Vis Sci 2000;41:3984–90. [PubMed] [Google Scholar]
- 128.Grimm C, Wenzel A, Williams T, et al. Rhodopsin-mediated blue-light damage to the rat retina: effect of photoreversal of bleaching. Invest Ophthalmol Vis Sci 2001;42:497–505. [PubMed] [Google Scholar]
- 129.Parish CA, Hashimoto M, Nakanishi K, et al. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci USA 1998;95:14609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rozanowska M, Jarvis-Evans J, Korytowski W, et al. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem 1995;270:18825–30. [DOI] [PubMed] [Google Scholar]
- 131.Schutt F, Davies S, Kopitz J, et al. Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci 2000;41:2303–8. [PubMed] [Google Scholar]
- 132.Suter M, Reme C, Grimm C, et al. Age-related macular degeneration. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. J Biol Chem 2000;275:39625–30. [DOI] [PubMed] [Google Scholar]
- 133.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci 2000;41:1981–9. [PubMed] [Google Scholar]
- 134.Sparrow JR, Cai B. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Invest Ophthalmol Vis Sci 2001;42:1356–62. [PubMed] [Google Scholar]
- 135.Rozanowska M, Korytowski W, Rozanowski B, et al. Photoreactivity of aged human RPE melanosomes: a comparison with lipofuscin. Invest Ophthalmol Vis Sci 2002;43:2088–96. [PubMed] [Google Scholar]
- 136.Sparrow JR, Zhou J, Cai B. DNA is a target of the photodynamic effects elicited in A2E-laden RPE by blue-light illumination. Invest Ophthalmol Vis Sci 2003;44:2245–51. [DOI] [PubMed] [Google Scholar]
- 137.Sparrow JR. Therapy for macular degeneration: insights from acne. Proc Natl Acad Sci USA 2003;100:4353–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ben-Shabat S, Parish CA, Vollmer HR, et al. Biosynthetic studies of A2E, a major fluorophore of retinal pigment epithelial lipofuscin. J Biol Chem 2002;277:7183–90. [DOI] [PubMed] [Google Scholar]
- 139.Sparrow JR, Vollmer-Snarr HR, Zhou J, et al. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J Biol Chem 2003;278:18207–13. [DOI] [PubMed] [Google Scholar]
- 140.Sparrow JR, Parish CA, Hashimoto M, et al. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci 1999;40:2988–95. [PubMed] [Google Scholar]
- 141.Ham WT Jr, Ruffolo JJ Jr, Mueller HA, et al. The nature of retinal radiation damage: dependence on wavelength, power level and exposure time. Vis Res 1980;20:1105–11. [DOI] [PubMed] [Google Scholar]
- 142.Katz ML, Eldred GE, Robison WG Jr. Lipofuscin autofluorescence: evidence for vitamin A involvement in the retina. Mech Ageing Dev 1987;39:81–90. [DOI] [PubMed] [Google Scholar]
- 143.Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 2001;42:3023–30. [PubMed] [Google Scholar]
- 144.Liu J, Itagaki Y, Ben-Shabat S, et al. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem 2000;275:29354–60. [DOI] [PubMed] [Google Scholar]
- 145.Katz ML, Drea CM, Eldred GE, et al. Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp Eye Res 1986;43:561–73. [DOI] [PubMed] [Google Scholar]
- 146.Feeney-Burns L, Berman ER, Rothman H. Lipofuscin of human retinal pigment epithelium. Am J Ophthalmol 1980;90:783–91. [DOI] [PubMed] [Google Scholar]
- 147.Weiter JJ, Delori FC, Wing GL, et al. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci 1986;27:145–52. [PubMed] [Google Scholar]
- 148.Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci 1984;25:195–200. [PubMed] [Google Scholar]
- 149.Dorey CK, Wu G, Ebenstein D, et al. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci 1989;30:1691–9. [PubMed] [Google Scholar]
- 150.von Ruckmann A, Fitzke FW, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci 1997;38:478–86. [PubMed] [Google Scholar]
- 151.Delori FC, Fleckner MR, Goger DG, et al. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci 2000;41:496–504. [PubMed] [Google Scholar]
- 152.Lawwill T. Effects of prolonged exposure of rabbit retina to low-intensity light. Invest Ophthalmol 1973;12:45–51. [PubMed] [Google Scholar]
- 153.Hoppeler T, Hendrickson P, Dietrich C, et al. Morphology and time-course of defined photochemical lesions in the rabbit retina. Curr Eye Res 1988;7:849–60. [DOI] [PubMed] [Google Scholar]
- 154.Gorgels TG, Van Norren D. Two spectral types of retinal light damage occur in albino as well as in pigmented rat: no essential role for melanin. Exp Eye Res 1998;66:155–62. [DOI] [PubMed] [Google Scholar]
- 155.Mantyjarvi M, Syrjakoski J, Tuppurainen K, et al. Colour vision through intraocular lens. Acta Ophthalmol Scand 1997;75:166–9. [DOI] [PubMed] [Google Scholar]
- 156.Kamel ID, Parker JA. Protection from ultraviolet exposure in aphakic erythropsia. Can J Ophthalmol 1973;8:563–5. [PubMed] [Google Scholar]
- 157.Saraux H, Manent JP, Laroche L. Erythropsia in a patient with lens implant. Physiologic and electrophysiologic study. J Fr Ophtalmol 1984;7:557–62. [PubMed] [Google Scholar]
- 158.Jordan DR, Valberg JD. Dyschromatopsia following cataract surgery. Can J Ophthalmol 1986;21:140–3. [PubMed] [Google Scholar]
- 159.Bennett LW. Pseudophakic erythropsia. J Am Optom Assoc 1994;65:273–6. [PubMed] [Google Scholar]
- 160.Werner JS, Steele VG, Pfoff DS. Loss of human photoreceptor sensitivity associated with chronic exposure to ultraviolet radiation. Ophthalmology 1989;96:1552–8. [DOI] [PubMed] [Google Scholar]
- 161.Miyake K, Ichihashi S, Shibuya Y, et al. Blood-retinal barrier and autofluorescence of the posterior polar retina in long-standing pseudophakia. J Cataract Refract Surg 1999;25:891–7. [DOI] [PubMed] [Google Scholar]
- 162.Peyman GA, Zak R, Sloane H. Ultraviolet-absorbing pseudophakos: an efficacy study. J Am Intraocul Implant Soc 1983;9:161–70. [DOI] [PubMed] [Google Scholar]
- 163.Nilsson SE, Textorius O, Andersson BE, et al. Clear PMMA versus yellow intraocular lens material. An electrophysiologic study on pigmented rabbits regarding “the blue light hazard”. Prog Clin Biol Res 1989;314:539–53. [PubMed] [Google Scholar]
- 164.Kraff MC, Sanders DR, Jampol LM, et al. Effect of an ultraviolet-filtering intraocular lens on cystoid macular edema. Ophthalmology 1985;92:366–9. [DOI] [PubMed] [Google Scholar]
- 165.Komatsu M, Kanagami S, Shimizu K. Ultraviolet-absorbing intraocular lens versus non-UV-absorbing intraocular lens: comparison of angiographic cystoid macular edema. J Cataract Refract Surg 1989;15:654–7. [DOI] [PubMed] [Google Scholar]
- 166.Schefrin BE, Bieber ML, McLean R, et al. The area of complete scotopic spatial summation enlarges with age. J Opt Soc Am A Opt Image Sci Vis 1998;15:340–8. [DOI] [PubMed] [Google Scholar]
- 167.Allikmets R. Simple and complex ABCR: genetic predisposition to retinal disease. Am J Hum Genet 2000;67:793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boynton RM. Frederic Ives Medal paper. History and current status of a physiologically based system of photometry and colorimetry. J Opt Soc Am A 1996;13:1609–21. [DOI] [PubMed] [Google Scholar]
- 169.Lund DJ, Stuck BE. Study rewrites blue light hazard: the retina’s damage threshold to blue laser light may be much higher than previously believed. International Laser Safety Conference, 2003. (www.optics.org/articles/news/9/4/1/1): Optics.org, 2 April 2003, 2003.
- 170.Le Grand Y. Light, color and vision. 2nd ed. London: Chapman and Hall, 1968.