Abstract
Aims: To evaluate the changes in the choroidal vasculature in central serous chorioretinopathy (CSC) after photodynamic therapy (PDT) with verteporfin and to assess its potential role as a treatment option.
Methods: A prospective, non-comparative, interventional study was performed in eyes with persistent CSC or chronic CSC that had fluorescein leakage at the fovea. All eyes received one single session of PDT with verteporfin (6 mg/m2 body surface area) followed by application of 50 J/cm2 laser at 689 nm. The laser spot size was guided by findings in ICG-A.
Results: Six eyes from six patients with a mean follow up of 12.7 months were analysed. Narrowing of the original dilated choroidal vessels and decrease in extravascular leakage could be demonstrated in all (100%) PDT treated eyes. 3 months after PDT, the mean diameter of the dilated choroidal vessel reduced from 546 μm to 371 μm (p = 0.028). Five (83%) patients had improvement in visual symptoms and best corrected visual acuity. Fluorescence leakage stopped at the 1 month follow up in five eyes (83%) and at 3 months in all six eyes (100%). One eye developed choroidal neovascularisation at 3 month follow up. There was no other serious ocular or systemic complication.
Conclusions: PDT is successful in stopping the fluorescein leakage in all six patients without recurrence of CSC. The ICG-A findings of choroidal vascular remodelling and decreased choroidal permeability after PDT are encouraging. As the sample size is small and the mean follow up period is short, further trials of PDT with verteporfin for CSC are required to address the optimal parameters in ensuring longer term safety and efficacy outcome.
Keywords: choroidal vascular remodelling, indocyanine green, photodynamic therapy, central serous chorioretinopathy
Central serous chorioretinopathy (CSC) is a condition commonly seen in young or middle aged adults as a localised detachment of the neurosensory retina.1–3 It may also present with a wide variety of other clinical manifestations.4–10 In acute CSC with focal leakage, the retinal pigment epithelium (RPE) increases its function to absorb the subretinal fluid and the disease is self limiting. However, in those cases with persistent focal leakage or chronic diffuse leakage, RPE may decompensate and thus lead to gradually a less favourable visual prognosis with visual loss.11–15
Over recent years, understanding of the pathology causing CSC has shifted from the RPE level to haemodynamic disturbances at the choroidal level.16–25 The widespread use of indocyanine green angiography (ICG-A) for visualising choroidal abnormalities, such as congested and dilated choroidal vein and capillaries, choroidal staining, and leakage into extracellular space as areas of hyperfluorescence, further provided evidence that the primary pathology of CSC lies at the choroidal level.22,24,25 Endogenous and exogenous corticosteroids with an elevated level of plasma catecholamines are recognised risk factors or contributing factors in causing choroidal hyperpermeability and in developing CSC, especially in susceptible individuals.26–32
Various treatments, including laser photocoagulation and carbonic anhydrase inhibitors, have been proposed for CSC but they are not specific enough in tackling the fundamental choroidal vascular problem.33–38
PDT can have a direct occlusive effect on CNV secondary to various diseases.39,40 It also causes a substantial reduction in the perfusion of physiological choroidal vessels in a dose–response relation, though the response of normal vessels is usually less sensitive than proliferating neovasculature to photochemical damage.41 We postulate that PDT may be beneficial for the treatment of CSC by its effect on the structure of the choroidal vasculature, causing alterations in choroidal permeability. The aim of our prospective pilot study is to evaluate choroidal vascular changes demonstrated by ICG-A in CSC after PDT and to report the visual outcome and changes in fluorescein leakage in CSC patients after PDT.
SUBJECTS AND METHODS
This study was a prospective, non-randomised, interventional series aimed at investigating the effect of PDT on the choroidal vasculature and fluorescein leakage in patients with CSC. The protocol was reviewed and approved by the ethics committee of the Chinese University of Hong Kong. Informed consent was obtained from all eligible participants.
Patient recruitment started in September 2000. The inclusion criteria of the study included: (i) patients with symptomatic CSC with a baseline best corrected visual acuity (BCVA) of 20/30 or worse; (ii) patients younger than 50 years old; (iii) CSC classified to either one of the following characteristics: persistent CSC with focal leakage (group 1) that lasted for 4 months or longer with serous retinal detachment with or without PED shown clinically and in FA, or chronic CSC with diffuse leakage (group 2) that had an RPE transmission defect in early phase and diffuse angiographic leakage in mid to late phases of FA; (iv) angiographic leakage sites involving the geometric centre of the foveal avascular zone (FAZ).
The exclusion criteria included: (i) any systemic contraindications for verteporfin or angiographic dyes; (ii) patients who had received any previous treatment for CSC such as focal laser before enrolment; (iii) presence of CNV on clinical examination or in FA; or (iv) any coexisting maculopathy such as diabetic maculopathy or retinal vascular occlusion.
Before PDT, all participants received comprehensive ocular examinations followed by colour fundus photography, digital camera FA, and ICG-A. The diagnosis of CSC was made based on typical findings in clinical examinations, and compatible features in FA and ICG-A.22,24 Choroidal neovascularisation of various causes and polypoidal choroidal vasculopathy were specific looked for and cases with these features were excluded from the study.42 BCVA was measured by certified optometrists using a standard Snellen chart. To quantify the visual changes, all Snellen BCVA were converted to logarithm of the minimum angle of resolution (logMAR) BCVA for analysis.43
Photodynamic therapy and follow up
Single treatment of PDT with verteporfin (Visudyne, Novartis AG, Bülach, Switzerland) infusion and laser (Coherent Inc, CA, USA) at 689 nm was given following the TAP study.44 The greatest linear diameter (GLD) aimed at covering the area of choroidal abnormalities including the dilated and congested choroidal vessels, and the area with sub-RPE extravascular leakage in the macula according to findings by ICG-A.
Follow up examinations with repeated FA and ICG-A were performed at 1 month, 3 months, and then every 3 months until 1 year after PDT and then every 6 months subsequently. Retreatment with PDT was not considered in this pilot study.
The images of the early phase of ICG-A at around 60 seconds from both the baseline and 3 month post-PDT were enlarged in the computer monitor to 400% magnification. The most congested and dilated choroidal vessel closest to the fovea in the ICG-A would be selected for the serial measurements and calculations. The diameter of the retinal artery at the temporal optic disc margin was used as a reference of 125 μm for measurement. Sites near vascular junctions or arteriovenous crossings were avoided. All measurements were taking the perpendicular distance between the two edges of the vessel.
The primary outcome measurement was the change in the calibre of the choroidal vasculature and in the extravascular leakage seen in ICG-A. Secondary outcome measurements included resolution of leakage in clinical examination and in FA at the first month and at subsequent follow up visits. Any ocular or systemic adverse events due to PDT were also recorded.
RESULTS
The pre-PDT demographics of six eyes of six patients with follow up of at least 6 months are shown in Tables 1 and 2. The mean age of the patients was 44.8 years (range 42–48 years). The mean follow up was 12.7 months (range 9–24 months). Two patients were classified as group 1 CSC with the pattern of persistent focal leakage and four patients were classified as group 2 with the pattern of chronic diffuse leakage. The mean (SD) logMAR BCVA before PDT was 0.24 (0.09). The Snellen BCVA ranged from 20/30 to 20/50 with a mean of 20/35. The mean duration of the diseases before treatment was 9.8 months (range 4–26 months).
Table 1.
Clinical data in patients with CSC treated by PDT
| Patient No | Sex | Age | Eye | Location | Duration of disease (months) | Initial clinical and angiographic staging | Baseline BCVA | BCVA at last follow up | Change in lines | Duration of follow up (months) | Subjective symptoms |
| 1 | F | 48 | R | Subfoveal | 4 | Group 1: persistent focal leakage with SRF and serous PED | 20/30 | 20/20 | +2 | 24 | Improvement |
| 2 | M | 48 | R | Subfoveal | 8 | Group 2: chronic diffuse leakage CSC | 20/30 | 20/40 | −1 | 12 | Same |
| 3 | M | 40 | R | Subfoveal | 5 | Group 1: persistent focal leakage with SRF | 20/35 | 20/20 | +2 | 12 | Improvement |
| 4 | M | 47 | R | Subfoveal | 6 | Group 2: chronic diffuse leakage CSC | 20/40 | 20/30 | +1 | 10 | Improvement |
| 5 | M | 42 | R | Subfoveal | 26 | Group 2: chronic diffuse leakage CSC | 20/30 | 20/20 | +2 | 9 | Improvement |
| 6 | M | 44 | L | Subfoveal | 10 | Group 2: chronic diffuse leakage CSC | 20/50 | 20/30 | +2 | 9 | Improvement |
SRF = subretinal fluid; PED = detachment of the retinal pigment epithelium; RPE = retinal pigment epithelium.
Table 2.
Treatment results in patients with CSC treated by PDT
| Patient No | Fluorescein angiographic features at follow up | ICG-A features at follow up | Size of laser spot applied (ICG-A guided) | Diameter of the largest anterior choroidal vessel before PDT | Diameter of the same choroidal vessel 3 months after PDT | Changes in diameter of the choroidal vessels at 3 months |
| 1 | No leakage at 1 month, resolution of the PED, ring of RPE atrophy | Narrowing of the choroidal vessels, absence of choroidal leakage, PED resolved | 3800 μm | 622 μm | 338 μm | −46% |
| 2 | No leakage at 1 month, development of a juxtafoveal CNV at 3 months | Narrowing of the choroidal vessels, absence of choroidal leakage | 2600 μm | 545 μm | 442 μm | −19% |
| 3 | No leakage at 1 month, focal RPE window defect | Narrowing of the choroidal vessels, absence of choroidal leakage | 3000 μm | 571 μm | 428 μm | −25% |
| 4 | No leakage at 3 months, diffuse RPE atrophy | Narrowing of the choroidal vessels, decreased choroidal leakage | 2800 μm | 605 μm | 382 μm | −37% |
| 5 | No leakage at 1 month, diffuse RPE atrophy | Narrowing of the choroidal vessels, absence of choroidal leakage | 3000 μm | 470 μm | 300 μm | −36% |
| 6 | No leakage at 1 month, diffuse RPE atrophy | Narrowing of the choroidal vessels, decreased choroidal leakage | 3200 μm | 465 μm | 338 μm | −27% |
PED = detachment of the retinal pigment epithelium; RPE = retinal pigment epithelium; CNV = choroidal neovascularisation.
Visual outcome
Four eyes (67%) had visual improvement of two or more lines in BCVA at the last follow up. One eye gained one line of BCVA and one eye (patient 2) suffered a decrease in one line of BCVA due to development of a CNV. At the last follow up visit, the mean logMAR BCVA was 0.11 (SD 0.13). The Snellen BCVA ranged from 20/20 to 20/40 with a mean of 20/26. The mean improvement in visual acuity was 1.4 lines (range −1 to +2 lines).
Angiographic outcome
In all cases, FA showed termination of the initial presenting focal RPE leakage or diffuse RPE late oozing at either the 1 month (83%) or the 3 month (17%) follow up visits. No recurrence of the fluorescein leakage was noticed in subsequent follow up FA except in patient 2, who developed minimal leakage secondary to a newly formed CNV at the 3 month follow up visit. Features of RPE atrophy as local or diffuse transmission window defects were found in all cases after complete resolution of the subretinal fluid. The patterns were compatible with those of retinal pigment epitheliopathy of the CSC rather than the direct effect of the PDT alone.
In the ICG-A, narrowing of the dilated and congested choroidal vessels was observed in all cases (100%) (Table 2). The mean (SD) diameter of the choroidal vessels before and 3 months after PDT was 546 (67) μm and 371 (56) μm respectively. The mean reduction in the diameter of the selected choroidal vessels 3 months after PDT was 32% (range 19–46%). The change in the diameter of the choroidal vessel was statistically significant (Wilcoxon signed ranks test, p = 0.028). Complete resolution in the choroidal vascular hyperpermeability and extravascular leakage in the sub-RPE space seen as late hyperfluorescence in ICG-A was observed in four (67%) eyes with partial resolution observed in two (33%) eyes. In all cases, the choroidal vascular changes and the decrease in extravascular leakage seen in ICG-A at the 1 month follow up remained stable at the 3 month follow up and these changes persisted throughout the follow up period. Case 1 (Fig 1) with chronic combined serous PED and focal RPE leakage and case 6 (Fig 2) with chronic diffuse leakage are shown as illustrations.
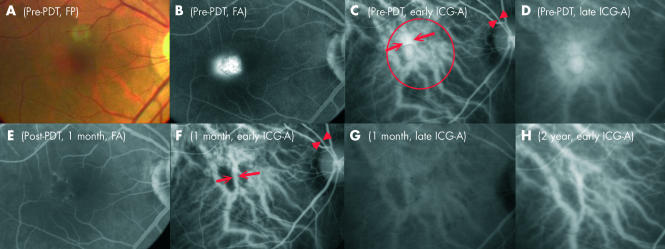
Figure 1.
Right eye of case 1, a 48 year old Chinese woman, with central serous chorioretinopathy (CSC) manifesting as persistent combined focal RPE leakage and serous pigment epithelium detachment (PED). (A) Fundus photography showing with serous PED of 800 μm in diameter and a pocket of subretinal fluid with precipitates. (B) Late phase of FA demonstrating a uniformly filled pocket of hyperfluorescence within the serous pigment epithelium and a focal retinal pigment epithelial leakage adjacent to it. (C) Early phase of the ICG-A showing dilated choriocapillaris in the macular area. The arrowheads mark the diameter of the reference retinal arteriole and the arrows mark the two sides of the most dilated choroidal vessels at fovea. Red circle represents the size and location of the laser spot delivery during PDT. (D) Late phase of the ICG-A revealing a loculated hyperfluorescence caused by the pigment epithelium detachment and a diffuse hyperfluorescence in the macula secondary to extravascular leakage from the congested choroidal vessels. (E) Late phase of FA one month after PDT, showing complete resolution of the subretinal fluid and the serous pigment epithelium detachment. (F) The calibres of the originally dilated choroidal vessels appeared narrower in size as shown by the open arrows in the early phase of the ICG-A at 1 month follow up. (G) No extravascular leakage in the late phase of the ICG-A. (H) The choroidal vasculature remodelling persisted as shown in the early phase of the ICG-A at the 2 year follow up.
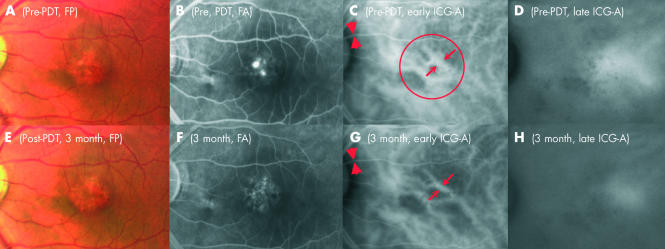
Figure 2.
Left eye of case 6 having CSC with pattern of chronic diffuse leakage. (A) Fundus photograph of the left eye of a 44 year old Chinese man revealing loss of the foveal reflex, unhealthy retinal pigment epithelium (RPE) with mottling and subretinal fibrinous deposition. (B) Mid phase FA demonstrating multiple angiographic leakages on a background of RPE atrophy with transmission defect. (C) Early phase of ICG-A showing congested and dilated choroidal vessels. The diameters of the reference arteriole and the most dilated choroidal vessel are marked with arrows and arrowheads, respectively. The red circle showed the location and the size of the laser spot in PDT. (D) Late phase of ICG-A demonstrating late profuse extravascular leakage. (E) Fundus photograph 3 months after PDT showing a dry macula. (F) No angiographic leakage could be seen in the late phase of the FA. (G and H) Repeated ICG-A at 3 months showed a persistent narrowing of the anterior choroidal vessels and diminished extravascular hyperfluorescence leakage in early and late phases respectively.
Safety and complications
One patient (patient 2) developed a small juxtafoveal CNV as a complication 3 months after PDT. There was minimal angiographic leakage and further intervention was not indicated. The CNV became a fibrotic lesion at the 6 month follow up and there was a decrease by one line of BCVA. Potential ocular complications including choroidal infarct, occlusion of retinal vessels, RPE decompensation, and RPE tear were not observed in our series. No systemic or infusion related adverse events were noticed in our study.
DISCUSSION
The precise pathophysiological event causing the exudative neurosensory detachment in CSC is not known but it is now a common belief that the primary pathology begins with disturbance of the choroidal circulation.17,22–45 Increased choroidal leakage, local hyperperfusion, and elevated hydrostatic pressure may lead to serous detachment of the RPE. A mechanical disruption of the RPE may cause the characteristic focal fluorescein leakage whereas the chronic pressure may induce RPE atrophy.45
CSC is not a totally benign disease, as RPE atrophy and the associated visual loss seen in chronic or persistent CSC is a slow but continuous process.12 Bandello and associates showed that in patients with chronic CSC, 16 (15.7%) of 102 eyes lost at least three lines after a mean follow up of 34.7 months.46 Chappelow and Marmor have also demonstrated diminished multifocal electroretinogram response amplitudes throughout the posterior pole in cases of CSC even after complete absorption of the subretinal fluid.21 In the present series, all the eyes had certain degree of RPE atrophy or epitheliopathy secondary to the persistent or chronic CSC despite a complete resolution of the subretinal fluid.
Different treatments have been previously proposed in managing cases with persistent focal leakage or chronic diffuse leakage in CSC. More rapid resolution of the subretinal fluid may occur after focal or grid laser at the RPE layer; however, these treatments did not affect the final functional outcomes and the recurrent rate of the disease.33–36 These can be easily understood, as zonal hyperperfusion and hyperpermeability of the choriocapillaris that are thought to be the primary alteration in CSC, could not be corrected after the laser photocoagulation therapy.24,33–36,47 Piccolino et al studied 145 patients with CSC by ICG-A and areas of choroidal leakage attributable to hyperpermeability of the choriocapillaris were found in 98.6% of patients in association with active or resolved CSC.24 These changes in the choroidal level persisted even after the resolution of the fluorescein leakage either spontaneously or after laser photocoagulation. Recurrence of the fluorescein leakage point was also more likely to occur over areas of choroidal hyperpermeability in the ICG-A.24
Acetazolamide is another treatment that has been suggested for chronic CSC.37 Pikkel and associates recently showed that acetazolamide shortened the duration of symptoms but did not alter the final visual outcome and the recurrence.38
The incidence of CSC correlates well with the type A personality, high adrenergic activity, endogenous and exogenous corticosteroid level, and elevated plasma catecholamines.1,26–29 However, the intake of oral β blocker did not give more favourable results in the treatment of CSC.48 Newer trends may shift to pharmacological treatment including anti-corticosteroids.32
In our study, PDT resulted in choroidal vascular changes including normalisation in calibres of the dilated and congested choroidal vasculature, and decrease in the extravascular leakage in all cases as early as 1 month after PDT. There was significant reduction in the size of the dilated choroidal vessels as shown in the 3 month follow up ICG-A. The effect of the vascular modulation persisted and was sustained up to the last follow up visits in all cases as shown in subsequent ICG-A. These types of vascular changes could neither be demonstrated nor achieved previously as a result of the natural course of CSC or after pharmacological or laser treatment. With the alterations in blood flow and decreased leakages in the choroidal vessels, the corresponding fluorescence leakages also stopped in 83% of cases at the 1 month FA and in all cases at the 3 month follow up.
The dosage of verteporfin infusion and laser energy parameters used in our study were identical to those used in the TAP study. The laser treatment spot size, however, was selected not based on the angiographic leakage in the FA but on the choroidal abnormality demonstrated in ICG-A. As we believe the pathological level of CSC is at the choroidal level, the treatment area should be guided by ICG-A rather than using a smaller laser spot in treating the manifest leakage sites shown in FA.
One patient (patient 2) developed a juxtafoveal CNV at the 3 month follow up visit despite good response to PDT shown in FA and ICG-A at 1 month. There are a few possibilities including the presence of a tiny CNV before PDT that could not be picked up in the initial examination. CNV may develop in patients with chronic CSC after disruption in the RPE layer as a natural course of the disease or complication.15 The development of CNV might also be the result of the PDT as a treatment. If that is the case, we speculate the localised ischaemia in the choriocapillaris secondary to PDT might contribute to the formation of CNV in chronic CSC patients with unhealthy RPE layers. From the angle of safety, further studies are warranted and a modification in the parameters of laser delivery might lower the risk of CNV development but at the same time without compromising the vascular remodelling ability of PDT since the photochemical response in the choroid is dose-response dependent.
The results of this pilot study in demonstrating the process of choroidal vascular remodelling in CSC after PDT are novel and encouraging. However, the case number is small and the mean follow up period is only 12.7 months. One case developed CNV shortly after the treatment. A randomised controlled clinical trial in the future may provide more definite proof of the therapeutic benefit of PDT in the treatment of chronic or persistent CSC. Optical coherence topography can be adopted in the quantitative measurement of the subretinal fluid.49 Functional visual outcome measurements such as contrast sensitivity, microperimetry, and electrophysiological measurement may also be included in future clinical trials.50,51 The precise treatment parameters in increasing the safety level and efficacy in achieving the remodelling the choroidal vessels also require further evaluation.
Acknowledgments
Financial support: Supported by the Action for Vision Eye Foundation, Hong Kong.
Financial interest: Nil.
REFERENCES
- 1.Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina 1987;7:111–31. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996;103:2070–9. [DOI] [PubMed] [Google Scholar]
- 3.Gass JD, Norton EW, Justice J Jr. Serous detachment of the retinal pigment epithelium. Trans Am Acad Ophthalmol Otolaryngol 1966;70:990–1015. [PubMed] [Google Scholar]
- 4.Yannuzzi LA, Shakin JL, Fisher YL, et al. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology 1984;91:1554–72. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama K, Kawamura M, Ogata T, et al. Retinal vascular loss in idiopathic central serous chorioretinopathy with bullous retinal detachment. Ophthalmology 1987;94:1605–9. [DOI] [PubMed] [Google Scholar]
- 6.Schatz H, Osterloh MD, McDonald HR, et al. Development of retinal vascular leakage and cystoid macular oedema secondary to central serous chorioretinopathy. Br J Ophthalmol 1993;77:744–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ie D, Yannuzzi LA, Spaide RF, et al. Subretinal exudative deposits in central serous chorioretinopathy. Br J Ophthalmol 1993;77:349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma T, Badrinath SS, Gopal L, et al. Subretinal fibrosis and nonrhegmatogenous retinal detachment associated with multifocal central serous chorioretinopathy. Retina 1998;18:23–9. [DOI] [PubMed] [Google Scholar]
- 9.Lip PL, Mowatt-Dixon L, Hope-Ross MW. Central serous retinopathy complicated by massive bilateral subretinal haemorrhage. Br J Ophthalmol 1999;83:990–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laatikainen L, Hoffren M. Long-term follow-up study of nonsenile detachment of the retinal pigment Epithelium. Eur J Ophthalmol 1991;1:79–84. [DOI] [PubMed] [Google Scholar]
- 11.Castro-Correia J, Coutinho MF, Rosas V, et al. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol 1992;81:379–86. [DOI] [PubMed] [Google Scholar]
- 12.Jalkh AE, Jabbour N, Avila MP, et al. Retinal pigment epithelium decompensation. I. Clinical features and natural course. Ophthalmology 1984;91:1544–8. [DOI] [PubMed] [Google Scholar]
- 13.Von Ruckmann A, Fitzke FW, Fan J, et al. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol 2002;133:780–6. [DOI] [PubMed] [Google Scholar]
- 14.Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology 1989;96:854–9. [DOI] [PubMed] [Google Scholar]
- 15.Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 2002;22:19–24. [DOI] [PubMed] [Google Scholar]
- 16.Nadel AJ, Turan MI, Coles RS. Central serous retinopathy. A generalized disease of the pigment epithelium. Mod Probl Ophthalmol 1979;20:76–88. [PubMed] [Google Scholar]
- 17.Marmor MF. New hypotheses on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol 1988;226:548–52. [DOI] [PubMed] [Google Scholar]
- 18.Scheider A, Nasemann JE, Lund OE. Fluorescein and indocyanine green angiographies of central serous choroidopathy by scanning laser ophthalmoscopy. Am J Ophthalmol 1993;115:50–6. [DOI] [PubMed] [Google Scholar]
- 19.Giovannini A, Scassellati-Sforzolini B, D’Altobrando E, et al. Choroidal findings in the course of idiopathic serous pigment epithelial detachment detected by indocyanine green videoangiography. Retina 1997;17:286–93. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyama Y, Mori K, Murayama K, et al. Quantitative analysis of indocyanine green angiographic image in central serous chorioretinopathy. Jpn J Ophthalmol 2001;45:116. [DOI] [PubMed] [Google Scholar]
- 21.Chappelow AV, Marmor MF. Multifocal electroretinogram abnormalities persist following resolution of central serous chorioretinopathy. Arch Ophthalmol 2000;118:1211–5. [DOI] [PubMed] [Google Scholar]
- 22.Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol 1994;112:1057–62. [DOI] [PubMed] [Google Scholar]
- 23.Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 1996;121:26–34. [DOI] [PubMed] [Google Scholar]
- 24.Piccolino FC, Borgia L, Zinicola E, et al. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye 1995;9:324–32. [DOI] [PubMed] [Google Scholar]
- 25.Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina 1996;16:203–13. [DOI] [PubMed] [Google Scholar]
- 26.Wakakura M, Ishikawa S. Central serous chorioretinopathy complicating systemic corticosteroid treatment. Br J Ophthalmol 1984;68:329–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polak BC, Baarsma GS, Snyers B. Diffuse retinal pigment epitheliopathy complicating systemic corticosteroid treatment. Br J Ophthalmol 1995;79:922–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haimovici R, Gragoudas ES, Duker JS, et al. Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology 1997;104:1653–60. [DOI] [PubMed] [Google Scholar]
- 29.Garg SP, Dada T, Talwar D, et al. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol 1997;81:962–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol 1999;128:63–8. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho-Recchia CA, Yannuzzi LA, Negrao S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology 2002;109:1834–7. [DOI] [PubMed] [Google Scholar]
- 32.Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology 2002;109:1765–6. [DOI] [PubMed] [Google Scholar]
- 33.Khosla PK, Rana SS, Tewari HK, et al. Evaluation of visual function following argon laser photocoagulation in central serous retinopathy. Ophthalmic Surg Lasers 1997;28:693–7. [PubMed] [Google Scholar]
- 34.Burumcek E, Mudun A, Karacorlu S, et al. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology 1997;104:616–22. [DOI] [PubMed] [Google Scholar]
- 35.Ficker L, Vafidis G, While A, et al. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 1988;72:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yannuzzi LA, Slakter JS, Kaufman SR, et al. Laser treatment of diffuse retinal pigment epitheliopathy. Eur J Ophthalmol 1992;2:103–14. [DOI] [PubMed] [Google Scholar]
- 37.Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol 1988;106:1190–95. [DOI] [PubMed] [Google Scholar]
- 38.Pikkel J, Beiran I, Ophir A, et al. Acetazolamide for central serous retinopathy. Ophthalmology 2002;109:1723–5. [DOI] [PubMed] [Google Scholar]
- 39.Husain D, Kramer M, Kenny AG, et al. Effects of photodynamic therapy using verteporfin on experimental choroidal neovascularization and normal retina and choroid up to 7 weeks after treatment. Invest Ophthalmol Vis Sci 1999;40:2322–31. [PubMed] [Google Scholar]
- 40.Schmidt-Erfurth U, Hasan T, Gragoudas E, et al. Vascular targeting in photodynamic occlusion of subretinal vessels. Ophthalmology 1994;101:1953–61. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Erfurth U, Michels S, Barbazetto I, et al. Photodynamic effects on choroidal neovascularization and physiological choroid. Invest Ophthalmol Vis Sci. 2002;43:830–41. [PubMed] [Google Scholar]
- 42.Yannuzzi LA, Freund KB, Goldbaum M, et al. Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology 2000;107:767–77. [DOI] [PubMed] [Google Scholar]
- 43.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg 1997;13:388–91. [DOI] [PubMed] [Google Scholar]
- 44.Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group.Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials-TAP report. Arch Ophthalmol 1999;117:1329–45. [PubMed] [Google Scholar]
- 45.Goldstein BG, Pavan PR. ‘Blow-outs’ in the retinal pigment epithelium. Br J Ophthalmol 1987;71:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandello F, Virgili G, Lanzetta P, et al. ICG angiography and retinal pigment epithelial decompensation. J Fr Ophtalmol 2001;24:448–51. [PubMed] [Google Scholar]
- 47.Iida T, Kishi S, Hagimura N, et al. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina 1999;19:508–12. [DOI] [PubMed] [Google Scholar]
- 48.Browning DJ. Nadolol in the treatment of central serous retinopathy. Am J Ophthalmol 1993;116:770–1. [DOI] [PubMed] [Google Scholar]
- 49.Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995;102:217–29. [DOI] [PubMed] [Google Scholar]
- 50.Toonen F, Remky A, Janssen V, et al. Microperimetry in patients with central serous retinopathy. Ger J Ophthalmol 1995;4:311–4. [PubMed] [Google Scholar]
- 51.Maaranen T, Mantyjarvi M. Contrast sensitivity in patients recovered from central serous chorioretinopathy. Int Ophthalmol 1999;23:31–5. [DOI] [PubMed] [Google Scholar]