Abstract
HERG (human ether-à-go-go-related gene) encodes channels responsible for the cardiac rapid delayed rectifier potassium current, IKr. This study investigated the effects on HERG channels of doxepin, a tricyclic antidepressant linked to QT interval prolongation and cardiac arrhythmia. Whole-cell patch-clamp recordings were made at 37 °C of recombinant HERG channel current (IHERG), and of native IKr ‘tails’ from rabbit ventricular myocytes. Doxepin inhibited IHERG with an IC50 value of 6.5 ± 1.4 μM and native IKr with an IC50 of 4.4 ± 0.6 μM. The inhibitory effect on IHERG developed rapidly upon membrane depolarization, but with no significant dependence on voltage and with little alteration to the voltage-dependent kinetics of IHERG. Neither the S631A nor N588K inactivation-attenuating mutations (of residues located in the channel pore and external S5-Pore linker, respectively) significantly reduced the potency of inhibition. The S6 point mutation Y652A increased the IC50 for IHERG blockade by ∼4.2-fold; the F656A mutant also attenuated doxepin's action at some concentrations. HERG channel blockade is likely to underpin reported cases of QT interval prolongation with doxepin. Notably, this study also establishes doxepin as an effective inhibitor of mutant (N588K) HERG channels responsible for variant 1 of the short QT syndrome.
Keywords: Antidepressant, Arrhythmia, Doxepin, HERG, IKr, Long QT syndrome, Potassium channel, QT interval, QT-prolongation, Rapid delayed rectifier, Short QT syndrome, Torsade de pointes
1. Introduction
Diverse cardiac and non-cardiac drugs are associated with prolongation of the rate-corrected QT (QTc) interval of the electrocardiogram and with a risk of the potentially fatal arrhythmia Torsade de Pointes (TdP) [1–4]. The majority of such agents exert a common action of inhibiting the cardiac rapidly activating delayed rectifier K+ current (IKr). IKr is a major determinant of ventricular action potential repolarization and, thereby, of the QTc interval [2,4,5]. The pore-forming subunit of IKr channels is encoded by HERG (human ether-à-go-go-related gene [6,7]). HERG channels appear to have a larger pore cavity than other (Kv) six transmembrane domain K+ channels and possess particular aromatic amino-acid residues in the S6 region of the channel [5,8–10]. These features combine to confer a high susceptibility to pharmacological blockade upon the HERG channel. Indeed, the association between drug-induced QTc interval prolongation and pharmacological blockade of HERG channels is sufficiently strong that drug-screening against recombinant HERG channels is now an important component of cardiac safety-pharmacology during drug development [11–13].
Doxepin is a tricyclic antidepressant (TCA) structurally related to amitriptyline and imipramine that combines antidepressant and sedative actions [14]. Initially doxepin was suggested to have better cardiac safety than other TCA drugs [15]. However, a subsequent review of the clinical and animal data [16] and a study in depressed patients [17] found no evidence that doxepin had fewer cardiovascular effects than other TCAs. Moreover, similar to other TCAs, doxepin can be cardiotoxic in overdose [14]. Adverse cardiac effects associated with doxepin include premature ventricular complexes (PVCs) and wide QRS complexes on the electrocardiogram. Furthermore, there are a number of documented cases of QT interval prolongation with doxepin [18–20]. For example, in overdose doxepin has been associated with a greatly prolonged QTc interval (reaching 580 ms) and TdP [19], whilst QT interval prolongation and syncope have been reported for doxepin in combination with methadone and β blocker use [20]. At present there is no information on the basis for QTc prolongation with doxepin. However, other TCAs including imipramine and amitryptiline have been demonstrated to inhibit recombinant HERG channels [21–23] and we hypothesised that doxepin is also likely to act as an inhibitor of HERG channel current (IHERG). The present study was conducted to test this hypothesis and to characterise the nature of any observed IHERG blockade.
2. Methods
2.1. Maintenance of mammalian cell lines stably expressing wild-type and mutant HERG channels
Experiments on wild-type HERG were performed on a cell line (Human Embryonic Kidney; HEK 293) stably expressing HERG (donated by Dr Craig January, University of Wisconsin [24]), except for those in Fig. 8; these utilised a cell line stably expressing lower levels of HERG developed in this laboratory, for use with high external [K+] (for comparison with the F656A mutant, see Section 2.2 below). Cell lines stably expressing HERG and its mutants, F656A and Y652A were maintained as described previously [25]. Cell lines stably expressing the S631A [26] and N588K [27] mutants were made from appropriately mutated HERG sequences using previously described methods [25]. Cells were passed using a non-enzymatic dissociating agent (Splitase, AutogenBioclear) and plated out onto small sterilised glass coverslips in 30 mm petri dishes containing a modification of Dulbecco's modified Eagle's medium with Glutamax-1 (DMEM; Gibco, Gibco/Invitrogen, Paisley, UK), supplemented with 10% fetal bovine serum (Gibco), 400 μg ml−1 gentamycin (Gibco) and 400 μg ml−1 geneticin (G418; Gibco). Treatment of the mutant cell lines was identical to treatment of the wild-type cell line except that cultures were maintained with 800 μg ml−1 of hygromycin. The cells were incubated at 37 °C for a minimum of two days prior to any electrophysiological study.
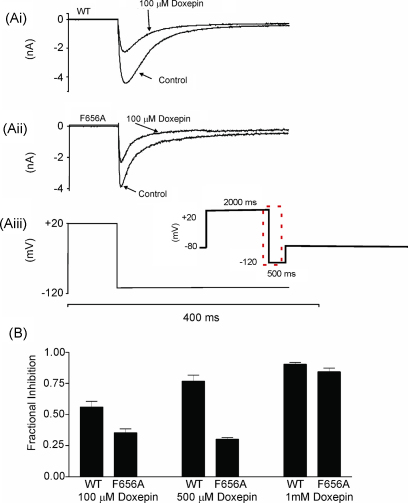
Fig. 8.
(A) Effect of the F656A mutation on IHERG inhibition by doxepin. (Ai) Representative WT–HERG and (Aii) F656A-HERG currents in the absence and presence of 100 μM doxepin, elicited by voltage protocol (Aiii) on an expanded time-scale (full protocol shown as an inset). (B) Bar chart showing the mean fractional block levels produced for WT–HERG and F656A–HERG tails measured at −120 mV after addition of 100, 500 μM or 1 mM doxepin (n = 5 for each). The F656A mutation significantly attenuated the level of blockade produced by both 100 μM doxepin (p < 0.01) and 500 μM doxepin (p < < 0.001), but not that by 1 mM doxepin (p > 0.05).
2.2. Experimental solutions
Whole-cell patch-clamp measurements of wild-type (WT) and mutant IHERG were made at 37 ± 1 °C. Once in the experimental chamber cells were superfused with a standard extracellular Tyrode's solution containing (in mM): 140 NaCl, 4 KCl, 2.5 CaCl2, 1 MgCl2, 10 glucose, 5 HEPES, (titrated to pH 7.45 with NaOH). Similar to other studies from our laboratory (e.g. [28,29]), for experiments employing the S6 mutant F656A (which shows comparatively low levels of channel expression [8,25]) and its WT control, the external solution contained 94 mM KCl (the NaCl concentration was correspondingly reduced). Experimental solutions were applied using a home-built, warmed, solution delivery system that exchanged the solution surrounding a cell in <1 s. Doxepin powder (Sequoia Research Products and Sigma-Aldrich) was dissolved in Tyrode's solution to produce initial stock solutions of either 10 or 50 mM, which were serially diluted to produce working solutions ranging from 0.1 μM to 1 mM.
The pipette dialysis solution for IHERG measurement contained (in mM): 130 KCl, 1 MgCl2, 5 EGTA, 5 MgATP, 10 HEPES (titrated to pH 7.2 with KOH) [28,29]. Patch-pipettes were heat-polished to 2.5–4 MΩ. No correction was made for the ‘pipette-to-bath’ liquid junction potential, which was measured to be −3.2 mV.
2.3. Experiments on rabbit isolated ventricular myocytes
One series of experiments was performed to investigate blockade by doxepin of native IKr from adult ventricular myocytes (Results, Fig. 2). For these, male New Zealand white rabbits (2–3 kg) were killed humanely in accordance with UK Home Office legislation. Ventricular myocytes were then isolated by a combination of mechanical and enzymatic dispersion, using previously described methods [30,31]. Pipette and external solutions for IKr measurement were identical to those described above for IHERG measurement.
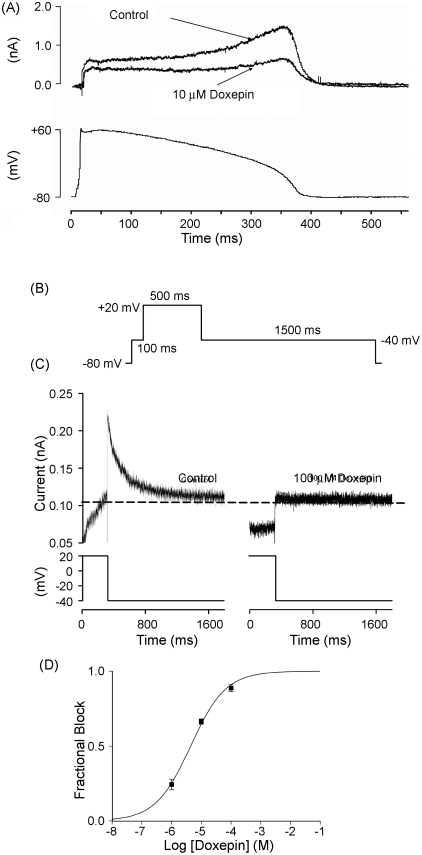
Fig. 2.
Doxepin inhibition of IHERG during action potential (AP) voltage clamp and of native IKr tails. (A) The upper traces show (leak corrected) representative currents of heterologously expressed IHERG in the absence and presence of 10 μM doxepin elicited by an AP clamp protocol (lower trace; voltage command applied at 4 s intervals). (B) Schematic representation of protocol used to assess blockade of native IKr tails by doxepin. (C) Representative tail currents from a rabbit ventricular myocyte upon repolarization from +20 to −40 mV in the absence (left panel) and in the presence of 100 μM doxepin (right panel). Upper traces show current records and lower traces show corresponding portion of the voltage protocol. The horizontal dashed line is drawn at the level of the current at −40 mV at the end of the initial (100 ms) step to −40 mV, against which peak IKr amplitude on repolarization from +20 to +40 mV was measured. (D) Mean fractional tail current block produced by three different concentrations of doxepin fitted with Eq. (2), which yielded an IC50 value of 4.4 (± 0.6 μM) with a Hill coefficient of 0.7 (± 0.1). Each drug concentration was applied to a minimum of twelve cells.
2.4. Electrophysiological recording and analysis
Whole-cell patch-clamp recordings were made using Axopatch 200 or 200B amplifiers (Axon Instruments) and a CV201 head-stage. Between 75 and 80% of the pipette series resistance was compensated. Voltage-clamp commands were generated using ‘WinWCP’ (John Dempster, Strathclyde University), Clampex 8 (Axon Instruments), or ‘Pulse’ software (HEKA Electronik). Data were recorded either via a Digidata 1200B interface (Axon Instruments) or an Instrutech VR-10B interface and stored on the hard-disk of a personal computer. The voltage-protocols employed for specific experiments are described either in the relevant ‘results’ text, or are shown diagrammatically on the relevant figures; unless otherwise stated in the text, the holding membrane potential between experimental sweeps was −80 mV. Data are presented as mean ± S.E.M. Statistical comparisons were made using, as appropriate, paired and unpaired t-tests or one-way analysis of variance (Anova) (Prism 3 or Instat, Graphpad Inc.). P values of less than 0.05 were taken as significant; ns = no statistically significant difference.
The following equations were used for numerical analysis and graphical fits to data:
The extent of IHERG inhibition by differing concentrations of doxepin was determined using the equation:
| (1) |
where ‘Fractional block’ refers to the degree of inhibition of IHERG by a given concentration of doxepin; IHERG–DOXEPIN and IHERG–CONTROL represent current amplitudes in the presence and absence of doxepin.
Concentration–response data were fitted by a standard Hill equation of the form:
| (2) |
where IC50 is [DOXEPIN] producing half-maximal inhibition of the IHERG tail and h is the Hill coefficient for the fit.
Half-maximal voltage values for IHERG activation were obtained by fitting IHERG tail-voltage (I–V) relations with a Boltzmann distribution equation of the form:
| (3) |
where I is the IHERG tail amplitude following test potential Vm, Imax the maximal IHERG tail observed during the protocol, V0.5 the potential at which IHERG was maximally activated, and k is the slope factor describing IHERG activation. Data from each individual experiment were fitted by this equation to derive V0.5 and k values in ‘control’ and with doxepin. The resultant mean V0.5 and k values obtained from pooling values from each experiment were then used to calculate the mean activation relations plotted in Fig. 3.
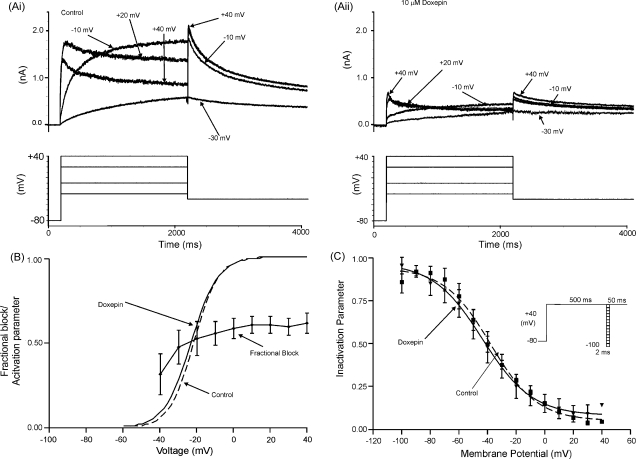
Fig. 3.
Voltage dependence of IHERG inhibition by doxepin. (A) Representative currents (upper traces) at selected voltages in: (Ai) the absence and (Aii) presence of 10 μM doxepin, elicited by the voltage-protocol shown in the lower traces (only some steps shown, for purposes of clarity of display). (B) Mean fractional block (n = 5) of peak IHERG tails produced by 10 μM doxepin, between test potentials of −40 and +40 mV. Superimposed are the continuous plots of activation curves for IHERG in the absence (dashed line) and presence (continuous line) of doxepin, calculated as described in Section 2. The mean activation V0.5 and k values obtained from IHERG tail-voltage relations using Eq. (3) are given in Section 3. (C) Voltage-dependence of IHERG availability/inactivation (protocol shown in inset). The mean (n = 5) normalised data (I/Imax) were plotted against the test potential and fitted with Eq. (4). The derived half-maximal inactivation values were: control V0.5 = −37.9 ± 2.4 mV and doxepin V0.5 = − 43.5 ± 4.1 mV in the absence (filled squares, dashed line) and presence (filled triangles, continuous line) of 10 μM doxepin, respectively (ns paired t-test p = 0.79), with corresponding k values of −16.2 ± 2.4 and −17.3 ± 8.8 mV (ns paired t-test p = 0.59).
Parameters describing voltage-dependent inactivation of IHERG were derived from fits to voltage-dependent availability plots with the equation:
| (4) |
where ‘inactivation parameter’ at any test potential, Vm, occurs within the range 1–0, V0.5 is the voltage at which IHERG was half-maximally inactivated and k describes the slope factor for the relationship.
3. Results
3.1. Doxepin produces concentration-dependent inhibition of WT IHERG
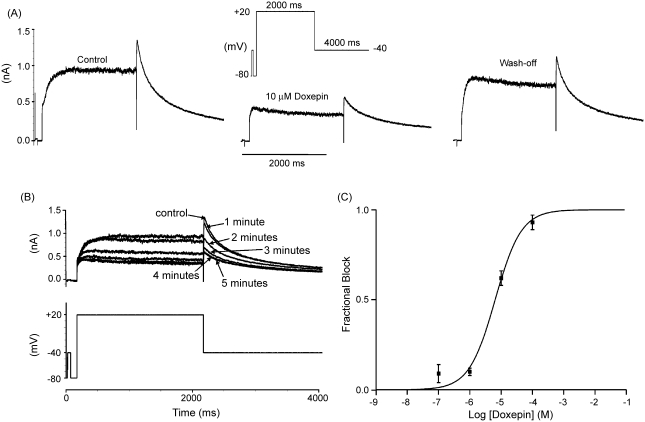
IHERG was elicited by the protocol shown in the inset of Fig. 1A, which is a standard protocol used to study IHERG pharmacology in this laboratory (e.g. [28,29,32]). Membrane potential was stepped from −80 to +20 mV for 2 s followed by a 4-s step to −40 mV to elicit IHERG tails. A brief (50 ms) pulse to −40 mV preceded the step to +20 mV in order to monitor the instantaneous current without activation of IHERG (peak outward IHERG tails on repolarization to –40 mV were compared with the instantaneous current during the 50 ms pulse to −40 mV, in order to measure IHERG tail amplitude). This voltage protocol was applied repeatedly (at 20 s intervals) prior to and during the application of doxepin. Fig. 1A shows a representative recording of IHERG in control, 5 min after the addition of 10 μM doxepin and 5 min following wash-out of the drug. Doxepin produced a substantial inhibition of IHERG; this was largely reversible upon washout (to 73 ± 9% of control in six cells to which 10 μM doxepin was applied). Fig. 1B shows records for the same cell before and at increasing times during doxepin exposure, indicating that blockade was maximal within 4–5 min of drug application. Fig. 1C shows a plot of mean ± S.E.M. fractional block of IHERG tails by four different concentrations of doxepin, fitted by Eq. (2). The IC50 for doxepin inhibition of IHERG with this protocol was 6.5 ± 1.4 μM and the Hill coefficient for the fit was 1.0 ± 0.2. IHERG blockade by doxepin was not accompanied by statistically significant changes to the time-course of deactivation of the time-dependent component of IHERG tails on repolarization to −40 mV: bi-exponential fitting of the time-dependent tail current decline yielded fast time-constants of deactivation (τfast) of 203 ± 15 and 273 ± 21 ms in control solution and 10 μM doxepin, respectively (n = 6; p > 0.05, paired t-test), and slow time-constants of deactivation (τslow) of 1164 ± 141 and 1499 ± 131 ms (p > 0.1). Doxepin did not influence significantly the relative proportions of deactivating current described by the fast and slow time-constants of deactivation (the proportion of deactivating current fitted by the τfast was ∼0.6 in both control and doxepin).
Fig. 1.
Concentration dependent inhibition of IHERG by doxepin. (A) Representative currents in the absence, presence (5 min) and following wash off (5 min) of 10 μM doxepin, elicited by the voltage protocol shown in the inset. (B) Representative currents (upper traces; lower trace shows voltage protocol), from the same cell as in A, in the absence and during doxepin exposure. ‘Control’ refers to current prior to doxepin exposure, whilst numbered time-points indicate currents sampled at 1–5 min of doxepin application. (C) Mean fractional block produced by four different concentrations of doxepin fitted with Eq. (2), which yielded an IC50 value of 6.5 μM (± 1.4 μM) with a Hill coefficient of 1.0 (± 0.2). Each drug concentration was applied to a minimum of five cells.
It has been suggested that pharmacological inhibition of IHERG by some drugs may vary between different stimulus protocols [33]. Therefore, we also investigated IHERG blockade by doxepin using an action potential (AP) voltage waveform [32,34]. The voltage-command used (shown in the lower panel of Fig. 2A) was a previously acquired, digitised AP from a rabbit ventricular myocyte. This was applied repeatedly (at 4 s intervals) from a holding potential of −80 mV [32]. The upper panel of Fig. 2A shows traces in the absence and presence of doxepin. Peak outward IHERG during the repolarizing phase of the AP was inhibited 60 ± 12% (n = 5) by 10 μM doxepin, which does not differ significantly from the extent of IHERG tail current blockade by this concentration obtained using the protocol shown in Fig. 1 (62 ± 4%; n = 6; p > 0.8; unpaired t-test).
In order to determine effects of doxepin on native IKr, three drug concentrations (1, 10 and 100 μM) were applied to ventricular myocytes under whole-cell patch clamp. The command protocol for these experiments (similar to [32]) is shown in Fig. 2B and IKr tails were monitored on repolarization from +20 to −40 mV. Under our conditions, the outward tail currents observed on repolarization to −40 mV were almost completely abolished by 1 μM dofetilide (91 ± 3% blockade; n = 7), verifying that these were carried by IKr with little or no contamination from overlapping currents. Fig. 2C shows representative IKr tails on repolarization from +20 to −40 mV in the absence and presence of 100 μM doxepin; at this concentration the current was largely abolished. Fig. 2D contains mean ± S.E.M fractional block data for the tested doxepin concentrations, fitted by Eq. (2). The derived IC50 for doxepin inhibition of native IKr tails was 4.4 ± 0.6 μM, in good agreement with the observed inhibitory potency of doxepin on IHERG.
3.2. Voltage dependence of IHERG blockade by doxepin
Voltage dependence of IHERG blockade by doxepin was determined by the application, in control and doxepin-containing solutions, of a series of 2 s duration depolarizing commands to a range of test potentials up to +40 mV [32]. Successive command pulses were applied at 20 s intervals. Representative control currents at selected command voltages are shown in Fig. 3Ai; currents from the same cell following equilibration in 10 μM doxepin are shown in Fig. 3Aii (upper traces; lower traces in each panel show corresponding voltage commands). At all test potentials, IHERG was inhibited by doxepin. For each of five similar experiments, fractional inhibition of IHERG tails following each voltage command was calculated using Eq. (1); the mean ± S.E.M. fractional block of IHERG tails is plotted against command voltage in Fig. 3B. Also shown in Fig. 3B are voltage-dependent activation relations for IHERG in control solution and in the presence of doxepin (see Section 2). The derived mean V0.5 and k values were: control V0.5 = −21.0 ± 3.1; doxepin V0.5 = −24.0 ± 2.2, p > 0.1; control k = 5.9 ± 0.2; doxepin k = 7.5 ± 1.7, p > 0.3, with the IHERG activation relations in control and doxepin closely overlying one another (Fig. 3B). Fractional block of IHERG showed no statistically significant dependence on voltage over the range from −40 to + 40 mV (p > 0.2; Anova).
The voltage dependence of IHERG availability/inactivation was assessed using a 3-step protocol similar to those used in previous IHERG investigations from our laboratory ([32,35], and shown schematically as an inset to Fig. 3C). Mean data from five experiments were corrected for deactivation [35] and the resulting values were plotted to give availability plots in the absence and presence of doxepin (Fig. 3C); the data-sets were fitted with Eq. (4) (see Section 2). In control and doxepin the inactivation V0.5 values were, respectively, −37.9 ± 2.4 and −43.5 ± 4.1 mV (ns, paired t-test), with corresponding k values of −16.2 ± 2.4 and −17.3 ± 8.8 mV (ns, paired t-test). Thus, doxepin did not alter the voltage-dependence of IHERG inactivation. Although there was a trend towards an acceleration in the time-course of IHERG inactivation, this did not attain statistical significance (inactivation time–constant of 3.3 ± 1.2 ms in control versus 1.4 ± 0.6 ms in doxepin, p > 0.1; obtained from exponential fits to the inactivating phase of the current elicited during the third step of the protocol, following a brief hyperpolarizing step to −80 mV).
3.3. Time dependence of IHERG inhibition by doxepin
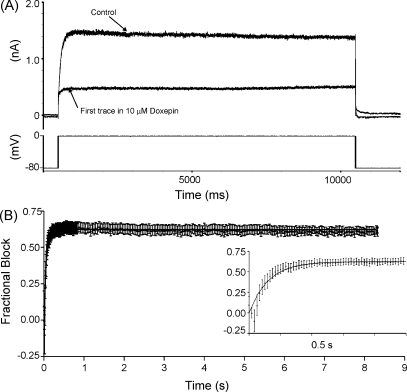
Gating-dependence of IHERG inhibition by doxepin was investigated further by the use of two protocols to examine the time-dependence of development of IHERG blockade. The first protocol used a sustained (10 s) depolarizing step to 0 mV from a holding potential of −80 mV (shown in Fig. 4A). This protocol was applied first in the absence of doxepin to elicit control IHERG. It was then discontinued whilst cells were equilibrated in 10 μM doxepin (for 7 min), after which it was re-applied. The first current trace recorded on resumption of stimulation was used to determine development of fractional blockade throughout the applied depolarization. Fig. 4A shows representative traces in control and doxepin; these traces diverged rapidly following depolarization, suggesting that IHERG blockade developed rapidly with time. Fig. 4B shows plots of mean ± S.E.M. fractional block of IHERG at various time intervals throughout the applied depolarization (main panel; n = 8) and on an expanded time-scale to show development of blockade over the first 0.5 s (inset). These plots show clearly that IHERG blockade developed rapidly on depolarization, with little change in blockade after 200–300 ms following the onset of the voltage-command. The time-course of development of blockade was well-described by a mono-exponential fit to the data, with a rate constant (K) for the fit of 16.33 s−1 (equivalent to a time-constant (1/K) of 61 ms).
Fig. 4.
Time-dependence of development of IHERG inhibition during a sustained depolarization. (A) Lower trace shows voltage-protocol (10 s depolarizing step from −80 to 0 mV) used to elicit IHERG in the absence of doxepin and following a 7-min exposure to 10 μM doxepin in the absence of pulsing. (B) Mean fractional block of IHERG (n = 8) produced by 10 μM doxepin during the 10-s depolarizing step to 0 mV, following 7-min of drug application in the absence of pulsing. Inset shows, on an expanded time-scale, the development of blockade over the first 500 ms of the protocol. Monoexponential fitting of the fractional block time relationship yielded a rate constant (K) for the fit of 16.33 ± 0.73 s−1 (equivalent to a time-constant (1/K) of 61 ms).
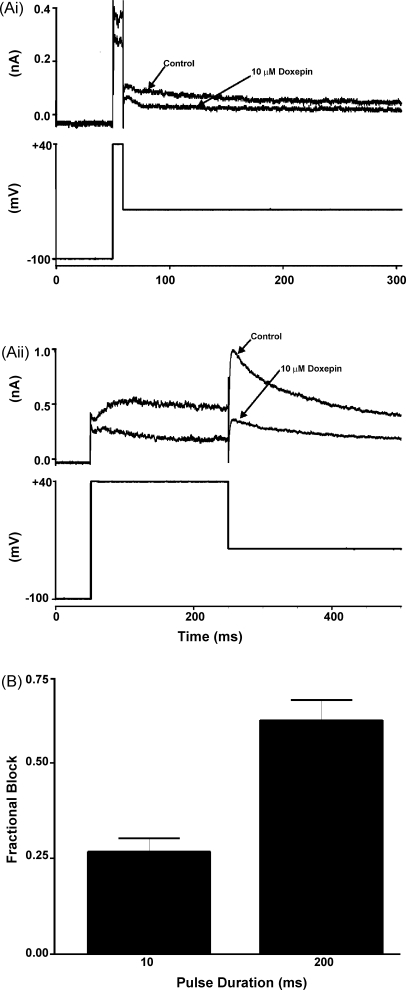
Although the protocol used in Fig. 4 is well suited for examining the development of IHERG inhibition over a period of seconds following membrane depolarization, it is less well suited for accurate assessment of blockade of IHERG immediately following membrane depolarization than are protocols based on tail current measurements [29,32]. Therefore, a second protocol was also used [29]. Membrane potential was held at –100 mV and, from this, 10 and 200 ms duration depolarizations to +40 mV were applied, each followed by a period at −40 mV to monitor IHERG tails [29]. Fig. 5A shows representative currents from the same cell activated by 10 ms (Fig. 5Ai) and 200 ms (Fig. 5Aii) commands in the absence and presence of doxepin. Some IHERG tail inhibition was evident following the 10 ms duration command, with further blockade evident after the 200 ms command. Fig. 5B shows mean fractional block of IHERG tails for five similar experiments. Whilst blockade for 200 ms commands was significantly greater than that for 10 ms commands (p < 0.02), the occurrence of some blockade with only a very brief duration depolarizing command is concordant with either a very rapidly developing gating-dependent blockade on depolarization or with a contribution of closed-channel block to the overall effect of doxepin [25,29,36].
Fig. 5.
IHERG inhibition by doxepin with brief-duration voltage-commands. (A) For each of (Ai) and (Aii), the upper traces show IHERG elicited by the voltage protocol shown in the lower trace: (Ai) shows representative traces of currents elicited by a 10-ms duration voltage command from −100 to +40 mV in the absence and presence of 10 μM doxepin; (Aii) shows data from the same cell with a 200 ms duration command. (B) Plot of mean (±S.E.M.) fractional block of IHERG tails following the 2 different duration commands (n = 5; p < 0.02).
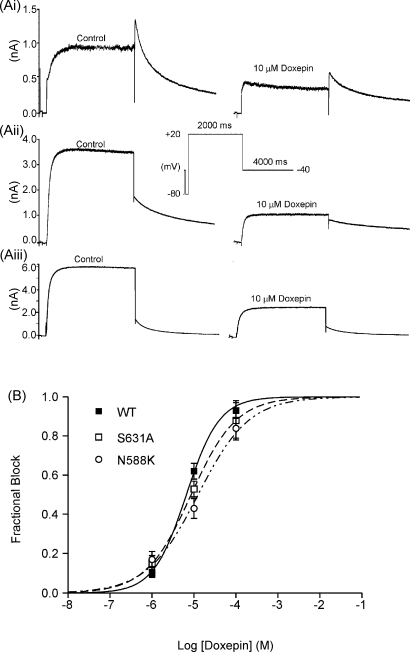
3.4. Effect of inactivation-attenuating mutants on IHERG inhibition by doxepin
In order to investigate further the gating-dependence of IHERG blockade by doxepin, experiments were performed using two inactivation-attenuating HERG mutants: S631A and N588K. Residue S631 is located towards the outer mouth of the HERG channel pore, and the S631A (serine → alanine) mutation has been reported to shift IHERG inactivation by ∼+100 mV [26]. Residue N588 is located in the external S5-Pore linker of the channel, and the N588K (asparagine → lysine) mutation, which is responsible for one form of the recently identified genetic short QT syndrome [27], has been reported to shift IHERG inactivation by ∼+60 to +100 mV [35,37]. The effect of each mutation on the potency of IHERG blockade by doxepin was determined using the same protocol as was used to establish concentration-dependence of WT IHERG blockade (Fig. 1). Fig. 6Ai shows representative currents in control and 10 μM doxepin for WT–HERG, whilst Fig. 6Aii and Aiii show similar records for S631A–HERG and N588K–HERG, respectively. In contrast to WT–HERG, for both S631A–HERG and N588K–HERG the IHERG tail magnitude was substantially smaller than the maximal current during the voltage-command (Fig. 6Aii and Aiii, respectively) reflecting the greatly attenuated IHERG inactivation of these HERG mutants [26,35,37]. Both mutant channels retained the ability to be inhibited by doxepin. Exponential fits to currents activated on depolarization to +20 mV for both mutants showed similar time-courses of current activation in control solution and following equilibration with 10 μM doxepin (S631A control: 60.6 ± 5.5 ms; doxepin: 64.5 ± 8.0 ms, p > 0.5; N588K control: 49.8 ± 6.2; doxepin: 41.7 ± 9.9 ms, p > 0.5; n = 5 for both). Three doxepin concentrations (1, 10 and 100 μM) were tested to obtain concentration-response data. Fig. 6B shows concentration-response relations (determined using Eq. (2)) for IHERG tail inhibition for both S631A–HERG and N588K–HERG, with corresponding data for WT–HERG plotted for comparison. Although the S631A and N588K mutations produced small and modest increases, respectively, in the IC50 derived from the fits to the concentration-response relations (from 6.6 μM for WT to 8.6 μM for S631A–HERG and 12.6 μM for N588K–HERG), these differences did not attain statistical significance (p > 0.05; Anova). These observations indicate the IHERG blockade by doxepin was not highly sensitive to attenuation of HERG channel inactivation.
Fig. 6.
(A) Effect of inactivation mutants on IHERG inhibition by doxepin. (Ai) Representative WT-HERG. (Aii) S631A-HERG and (Aiii) N588K-HERG currents in the absence (control, left hand traces) and presence of 10 μM doxepin (right hand traces). The voltage protocol is shown as an inset. (B). Mean fraction block of IHERG by 1, 10 and 100 μM doxepin (each drug concentration was applied to at least five cells; data for WT IHERG replotted from Fig. 1) fitted with Eq. (2). The derived IC50 values for WT IHERG, S631A–HERG and N588K–HERG were, respectively, 6.6 ± 0.6, 8.6 ± 0.0 and 12.6 ± 2.7 μM (p > 0.05; Anova); Hill coefficients for the fits to WT, S631A and N588K data were, respectively, 1.1 ± 0.1, 0.8 ± 0.0, and 0.7 ± 0.l (p < 0.05 between WT and N588K).
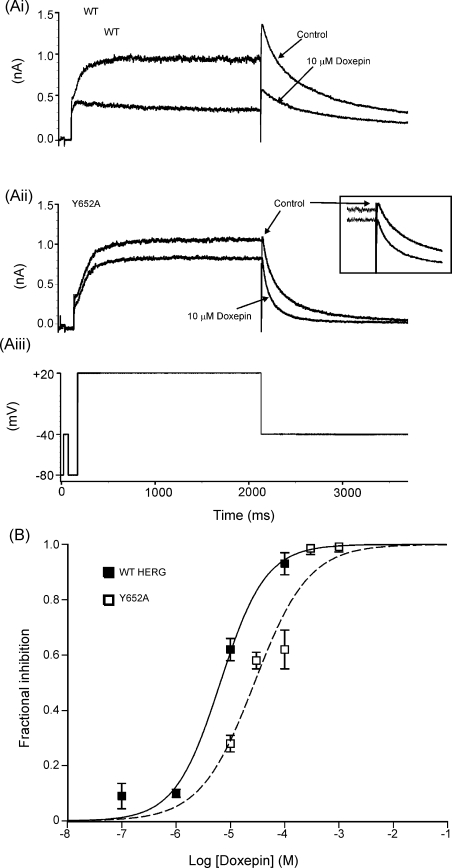
3.5. Sensitivity of IHERG inhibition by doxepin to the S6 mutations Y652A and F656A
Two aromatic amino-acid residues, Y652 and F656, have been shown to be important components of the drug-binding site for a variety of HERG channel blockers [5,9,38]. Accordingly, we investigated whether or not doxepin inhibition of IHERG was sensitive to mutation of either residue, adopting a similar approach and protocols to those used in other recent IHERG pharmacology studies [29,32,39–41]. Fig. 7 shows the effects on doxepin inhibition of IHERG of the mutation Y652A (tyrosine → alanine). The experimental voltage protocol used (Fig. 7Aiii) was identical to that used for WT IHERG in Fig. 1 and to investigate the S631A and N588K mutants in Fig. 6. Representative traces showing the effect of 10 μM doxepin on WT IHERG and Y652A IHERG are shown in Fig. 7Ai and Aii. WT IHERG was inhibited substantially by this doxepin concentration (Fig. 7Ai). In contrast, inhibition of Y652A IHERG was noticeably reduced (Fig. 7Aii; 28 ± 3% peak tail current inhibition compared to 62 ± 4% for WT IHERG; p < 0.001). Four other doxepin concentrations (30, 100, 300μM and 1 mM) were also tested in order to construct a concentration response relation for inhibition of Y652A IHERG tails (Fig. 7B). A fit to the data with Eq. (2) (dashed line) yielded an IC50 for inhibition of Y652A IHERG of 27.8 ± 8.8 μM (with a Hill coefficient of 0.9 ± 0.3), which represented a modest, though significant (p < 0.05), ∼4.2 fold increase in IC50 over that for WT IHERG.
Fig. 7.
(A) Effect of the Y652A mutation on IHERG inhibition by doxepin. (Ai) Representative WT-HERG and (Aii) Y652A-HERG currents in the absence and (Aiii) the presence of 10 μM doxepin, elicited by voltage protocol. Inset in (Aii) shows expanded tail-currents to highlight attenuation of blockade for the Y652A mutant.(B) Concentration-response relations for inhibition of IHERG by doxepin for WT–HERG and Y652A–HERG, fitted with Eq. (2). Data for WT IHERG are replotted from Fig. 1. For Y652A–HERG the following concentrations were tested: 10 μM (n = 4), 30 μM (n = 4); 100 μM (n = 12); 300 μM (n = 4) and 1 mM (n = 4). The derived IC50 values for WT IHERG and Y652A IHERG were, respectively, 6.5 ± 1.4 and 27.8 ± 8.8 μM (p < 0.05); Hill coefficients for the fits to WT IHERG and Y652A IHERG of 1.0 ± 0.2 and 0.9 ± 0.3 (p > 0.05).
IHERG carried by F656A–HERG channels was studied using the protocol shown in the inset of Fig. 8Aiii and inward tails were measured at −120 mV [25,28,29]. Initial experiments employed a doxepin concentration of 100 μM (shown in Fig. 1 to produce extensive inhibition of WT IHERG with a standard [K+]e). Representative traces showing effects of this concentration on WT and F656A IHERG are shown in Fig. 8Ai and Aii. The F656A mutation produced a modest attenuation of IHERG inhibition by this concentration of doxepin. However, as observed previously for some other drugs (e.g. [28]), the extent of inhibition of WT IHERG by 100 μM doxepin under conditions of high [K+]e (see Section 2) was significantly smaller (56 ± 5%) than that observed with the same concentration in the experiments with a standard [K+]e in Fig. 1 (93 ± 4%; p < 0.001). Therefore, the effects of two further concentrations of doxepin (500 μM and 1 mM) were also examined. Fig. 8B shows bar-chart plots of the mean levels of inhibition of WT and F656A IHERG by the three doxepin concentrations. These concentrations resulted in progressive increases in the level of blockade of WT IHERG. In contrast, although Anova comparison of the data with the three drug concentrations confirmed that doxepin inhibition of F656A IHERG was concentration-dependent (p < 0.001), the observed concentration-dependence was unusual: there was no statistically significant difference between the observed level of blockade of F656A IHERG between 100 and 500 μM, whilst at 1 mM the observed level of inhibition of F656A IHERG was markedly increased (p < 0.001 compared to each of 100 and 500 μM, Bonferroni post-test) and approached that of WT IHERG. Taken together, the data with the three doxepin concentrations suggest that the F656A mutation exerted some influence on the ability of doxepin to inhibit IHERG, though the F656A data did not appear to follow a conventional monotonic concentration dependence.
4. Discussion
Despite a strong association between TCA use and QT interval lengthening [42], and although doxepin itself has been linked with both QT interval prolongation and TdP [18–20], the effects of this drug on HERG K+ channels have not hitherto been reported. Moreover, whilst IHERG blockade has been investigated previously for the TCAs imipramine and amitriptyline [21–23], to our best knowledge the present study is the first in which molecular determinants of IHERG inhibition have been investigated for any member of the TCA family.
4.1. Characteristics of IHERG blockade by doxepin
Previously, imipramine has been reported to inhibit IHERG recorded in experiments using a mammalian expression system with an IC50 of 3.4 μM [23] whilst an IC50 of 10 μM was reported for amitriptyline [21]. A different study of amitriptyline, using the Xenopus oocyte expression system, reported IC50's of ∼3.3–4.8 μM (depending on [K+]e) [22]. Thus, the potency of doxepin as an IHERG inhibitor found here (IC50 of 6.5 μM) is broadly comparable to that seen previously for these other two TCAs. However, in terms of the characteristics of observed IHERG blockade, doxepin appears to be closer to imipramine than to amitriptyline: amitriptyline block of IHERG has been reported to show significant voltage-dependence [22] whereas imipramine showed only weak voltage-dependence [23]. Moreover, no significant effects of doxepin on the voltage-dependence of activation or inactivation were seen in this study. Unfortunately, comparative data for the TCAs imipramine and amitriptyline are lacking [21–23] and, therefore, a direct comparison between doxepin and these agents cannot be made in this regard. However, the atypical tetracyclic antidepressant maprotiline has recently been reported to show no alteration to the voltage-dependence of activation and inactivation [43], though another study contradicted this with regard to activation [44]. The lack of a significant leftward shift in the voltage-dependence of inactivation with doxepin in our study suggests that this agent does not act to stabilise IHERG inactivation, and the time-course of WT IHERG inactivation was not significantly accelerated by the drug. Moreover, the results with inactivation-attenuating mutations to two residues from distinct parts of the channel (S631A: outer mouth of the channel pore; N588K: in the S5-Pore linker) provide evidence that IHERG inactivation does not play an obligatory role in doxepin's binding to the channel. This is further supported by unaltered levels of WT IHERG blockade with progressive depolarization over voltages at which IHERG was maximally activated, but over which inactivation increased (Fig. 3C and D). Doxepin is quite distinct in this regard from a number of other drugs, including the archetypal high affinity HERG blocking methanesulphonanilide drugs E-4031 and dofetilide, for which IHERG inactivation exerts a strong influence on blocking potency – either as a direct consequence of inactivation-state dependent block, or due to conformational changes during inactivation facilitating optimal orientation of S6 helical residues to which drugs bind [10,45–48].
Blockade of IHERG by imipramine has been reported to develop rapidly during a sustained depolarization, with a component of inhibition visible even for comparatively brief depolarizing voltage commands [23]. These features of imipramine's action correspond well with those seen for doxepin in the present study. They are also similar to those reported for the serotonin-selective reuptake inhibitors (SSRIs) fluvoxamine and citalopram [25,49], but differ significantly from the methanesulphonanilides, for which little blockade is observed immediately upon depolarization, with blockade then increasing progressively during the maintained depolarization [24,49,50]. The lack of a strong dependence of HERG channel blockade by doxepin on IHERG inactivation suggests that gating-dependent blockade by this drug is likely to arise predominantly to activated/open channels. Accordingly, the observed time-dependence of IHERG inhibition by doxepin in this study is consistent with either a mixed state-dependence of blockade (with components of both closed and open channel blockade) or with the presence of a very rapidly developing component of activation-dependent inhibition immediately on depolarization.
For the majority of drugs that have been studied, one or both of the Y652 and F656 aromatic amino acid residues in the S6 helices of the HERG channel comprise key elements of the drug binding site [5,9,38,51]. For example, the Y652A and F656A mutations increased the IC50 for HERG blockade by the methanesulphonanilide MK-499 by 94-fold and 650-fold, respectively, and that for terfenadine by ∼100-fold [8]. In comparison, the ∼4-fold increase in IC50 for doxepin produced by the Y652A mutation in this study, is rather modest, suggesting that this residue is less influential for binding of doxepin than for either of these high affinity blockers. The unusual concentration dependence seen with the F656A mutation indicated that at a high doxepin concentration of 1 mM IHERG blockade was little affected by mutation of this residue, whilst at concentrations of 100 and 500 μM significant attenuation of blockade occurred with no significant increase in block at 500 μM compared to 100 μM. It was not possible to obtain an adequate fit of these data with Eq. (2). A cautious interpretation of the lack of a conventional concentration-dependence of F656A–HERG inhibition by doxepin is that blockade may depend partly, but incompletely, on this residue. Neither the Y652A nor F656A mutations attenuated blockade by doxepin concentrations producing very high levels (>90%) of blockade of WT IHERG, suggesting that neither residue is absolutely obligatory for doxepin binding to HERG channels to occur. Whilst unusual, this is not unprecedented; IHERG block by both the SSRI fluvoxamine and the antiarrhythmic agent dronedarone has been reported to be only partially attenuated by the Y652A and F656A mutations [25,28]. Comparative data for other TCAs are lacking, though recently these residues have been implicated in IHERG inhibition by the tetracyclic drug maprotiline [43,44]; one of these two studies obtained IC50 values for the Y652A and F656T mutants, with respective (modest) 3-fold and 7-fold increases in IC50 [43]. Whilst obligatory molecular determinants of doxepin-binding to HERG remain to be found, the fact that IHERG inhibition by doxepin developed progressively over several minutes following rapid external solution exchange is consistent with the drug crossing the cell membrane to reach its site of action. The reduced IHERG inhibition by doxepin of WT IHERG in the presence of raised [K+]e may also be of significance. Since IHERG inhibition by doxepin appears not to be critically dependent on channel inactivation, decreased blockade with high [K+]e is unlikely to result from any effect of [K+]e on IHERG inactivation. Rather, reduced inhibition in high [K+]e may be accounted for by an interference with drug-binding due to an electrostatic repulsion or “knock-off” process [28,52], consistent with drug binding to the channel at a site close to the ion conduction pathway. It remains to be determined whether this site would need to reside within the channel pore. However, in a limited series of experiments using the D540K mutant [53], we did not find evidence that doxepin can readily unbind on hyperpolarization-induced channel opening (data not shown); though not all drugs that bind within the pore exhibit marked ‘untrapping’ [54]. Nevertheless, given the presence of a significant component of IHERG blockade with brief depolarization and incomplete attenuation of inhibition by the Y652A and F656A mutations, we cannot exclude the possibilities that a proportion of the observed blockade with doxepin involves binding outside of the channel pore, or binding to closed HERG channels.
4.2. Clinical significance of IHERG blockade by doxepin
TCAs are associated with QTc interval lengthening in clinical use [42], and IHERG inhibition by doxepin observed in the present study is consistent both with documented cases of QT interval prolongation and TdP with doxepin [18–20] and with QT interval increases seen in anaesthetised guinea-pigs receiving doxepin infusion [15]. Our study was conducted at a physiologically relevant temperature and the extent of IHERG blockade by doxepin was similar between ventricular action potential and conventional voltage step protocols. A lack of relief of IHERG blockade on membrane hyperpolarization for the D540K mutant (mentioned in Section 4.1, above) is suggestive of relatively poor drug unbinding at negative voltages in the maintained presence of drug, whilst the data shown in Fig. 5 indicate that at 10 μM (the same concentration as used for the AP clamp experiment in Fig. 2) doxepin was able to bind and inhibit IHERG rapidly on membrane depolarization to a positive voltage. These factors may combine to account for the similar levels of IHERG inhibition by doxepin observed with step and AP clamp protocols. Although we did not co-express HERG with MiRP1, a putative β subunit suggested to be necessary to recapitulate native IKr [55], it has recently been suggested that MiRP1 is unlikely to interact with HERG outside of the cardiac conduction system [51] and, additionally, the pharmacological sensitivity of HERG channels expressed in mammalian cells without MiRP1 co-expression has been found to be similar to that of native IKr [56]. This notion is reinforced by the close concordance of inhibitory potency of doxepin on IHERG and native IKr in our experiments (IC50 values of 6.5 and 4.4 μM, respectively). A question therefore arises as to the relationship between the potency of IHERG/IKr blockade seen in this study and plasma concentrations of doxepin in patients. As a class, the TCAs are lipophilic and are known to become concentrated in some tissues, including the myocardium [57]. In the case of doxepin, one experimental study has reported doxepin concentration in cardiac muscle to be 41-fold higher than plasma levels [58]. This makes it difficult to extrapolate with accuracy from known plasma concentrations to likely levels of IHERG/IKr blockade by doxepin in vivo. The therapeutic plasma level of doxepin is thought to be between 50 and 250 ng/ml (0.16–0.8 μM), although a wide variety of recommendations from university psychiatric departments and laboratories (up to 1000 ng/ml; 3.2 μM) have been reported [59]. Whilst IHERG (or IKr) blockade at the lower end of this range might be anticipated to be modest, inhibition at higher concentrations would be significant and, taking into account also potential cardiac accumulation, the observed potency of IHERG inhibition by doxepin in this study is likely to be clinically relevant, particularly in overdose. Such an effect may be exacerbated in individuals exhibiting pre-existing QT interval prolongation (congenital or acquired), electrolyte abnormalities or with impaired drug metabolism. Thus, as for other IHERG blocking medications, caution is warranted in its use in patients with pre-existing QT interval prolongation or with risk factors likely to exacerbate the effects of IHERG blocking medications.
The findings of this study have further clinical relevance in a second, perhaps less expected, respect. The attenuated-inactivation N588K–HERG mutant used in this study has been shown recently to underlie the SQT1 familial form of the recently identified genetic ‘Short QT syndrome’, which carries a risk of cardiac arrhythmia and sudden death [27,60]. Pharmacological approaches to correcting the QT-interval of SQT1 patients are currently very limited. These patients are comparatively insensitive to Class III IKr/HERG blocking drugs [27,61] and the N588K–HERG blocking potencies of the IKr/HERG blockers E-4031and D-sotalol are reduced ∼12–20-fold compared to their effects on WT–HERG [62,63], presumably due to a role (direct or otherwise) of channel inactivation in facilitating drug binding to the HERG channel. To date, only the Class Ia antiarrhythmic drug quinidine has been found both to inhibit N588K–HERG effectively and to correct the QT interval in such patients [27,62,63]; however, very recently, another Class Ia antiarrhythmic, disopyramide, has been shown to be effective against N588K–HERG in vitro [63]. The present study identifies doxepin as both an IHERG-blocker for which channel inactivation does not play a major role in drug binding and as an additional drug that is an effective inhibitor of N588K–HERG. Whilst the sedative effects of doxepin may make it unsuitable as a corrective treatment for SQT1 patients, our findings prompt the question as to whether chemical structures related to doxepin might feasibly offer viable IHERG blocking agents in SQT1.
Acknowledgements
RSD was funded by a BBSRC/Pfizer ‘CASE’ studentship. The authors also acknowledge support from the British Heart Foundation (PG/03/121, PG/04/090, PG/06/139) and thank Mrs Lesley Arberry for technical assistance.
Contributor Information
H.J. Witchel, Email: harry.witchel@bristol.ac.uk.
J.C. Hancox, Email: jules.hancox@bristol.ac.uk.
References
- 1.Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354:1625–1633. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]
- 2.Haverkamp W., Breithardt G., Camm A.J., Janse M.J., Rosen M.R., Antzelevitch C. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications report on a policy conference of the European Society of Cardiology. Cardiovasc Res. 2000;47:219–233. doi: 10.1016/s0008-6363(00)00119-x. [DOI] [PubMed] [Google Scholar]
- 3.Shah R.R. Drugs QT interval prolongation and ICH E14—the need to get it right. Drug Safety. 2005;28:115–125. doi: 10.2165/00002018-200528020-00003. [DOI] [PubMed] [Google Scholar]
- 4.Witchel H.J., Hancox J.C. Familial and acquired long QT syndrome and the cardiac rapid delayed rectifier potassium current. Clin Exp Pharmacol Physiol. 2000;27:753–766. doi: 10.1046/j.1440-1681.2000.03337.x. [DOI] [PubMed] [Google Scholar]
- 5.Vandenberg J.I., Walker B.D., Campbell T.J. HERG K+ channels: friend and foe. Trends Pharmacol Sci. 2001;22:240–246. doi: 10.1016/s0165-6147(00)01662-x. [DOI] [PubMed] [Google Scholar]
- 6.Sanguinetti M.C., Jiang C., Curran M.E., Keating M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 7.Trudeau M.C., Warmke J.W., Ganetzky B., Robertson G.A. HERG a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 8.Mitcheson J.S., Chen J., Lin M., Culberson C., Sanguinetti M.C. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitcheson J.S., Perry M.D. Molecular determinants of high-affinity drug binding to HERG channels. Curr Opin Drug Discov Devel. 2003;6:667–674. [PubMed] [Google Scholar]
- 10.Lees-Miller J.P., Duan Y.J., Teng G.Q., Duff H.J. Molecular determinant of high-affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: Involvement of S6 sites. Mol Pharmacol. 2000;57:367–374. [PubMed] [Google Scholar]
- 11.Webster R., Leishman D., Walker D. Towards a drug concentration effect relationship for QT prolongation and torsades de pointes. Curr Opin Drug Discov Devel. 2002;5:116–126. [PubMed] [Google Scholar]
- 12.Bridgland-Taylor M.H., Hargreaves A.C., Easter A., Orme A., Henthorn D.C., Ding M. Optimisation and validation of a medium-throughput electrophysiology-based hERG assay using IonWorks(TM) HT. J Pharmacol Toxicol Meth. 2006;54:189–199. doi: 10.1016/j.vascn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Redfern W.S., Carlsson L., Davis A.S., Lynch W.G., MacKenzie I., Palethorpe S. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 14.Pinder R.M., Brogden R.N., Speight T.M., Avery G.S., Doxepin Up-To-Date Review of its pharmacological properties and therapeutic efficacy with particular reference to depression. Drugs. 1977;13:161–218. doi: 10.2165/00003495-197713030-00001. [DOI] [PubMed] [Google Scholar]
- 15.Dumovic P., Burrows G.D., Vohra J., Davies B., Scoggins B.A. The effect of tricyclic antidepressant drugs on the heart. Arch Toxicol. 1976;35:255–262. doi: 10.1007/BF00570267. [DOI] [PubMed] [Google Scholar]
- 16.Luchins D.J. Review of clinical and animal studies comparing the cardiovascular effects of doxepin and other tricyclic antidepressants. Am J Psychiatry. 1983;140:1006–1009. doi: 10.1176/ajp.140.8.1006. [DOI] [PubMed] [Google Scholar]
- 17.Roose S.P., Dalack G.W., Glassman A.H., Woodring S., Walsh B.T., Giardina E.G. Is doxepin a safer tricyclic for the heart? J Clin Psychiatry. 1991;52:338–341. [PubMed] [Google Scholar]
- 18.Baker B., Dorian P., Sandor P., Shapiro C., Schell C., Mitchell J. Electrocardiographic effects of fluoxetine and doxepin in patients with major depressive disorder. J Clin Psychopharmacol. 1997;17:15–21. doi: 10.1097/00004714-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Alter P., Tontsch D., Grimm W. Doxepin-induced torsade de pointes tachycardia. Ann Intern Med. 2001;135:384–385. doi: 10.7326/0003-4819-135-5-200109040-00026. [DOI] [PubMed] [Google Scholar]
- 20.Rademacher S., Dietz R., Haverkamp W. QT prolongation and syncope with methadone, doxepin, and a beta-blocker. Ann Pharmacother. 2005;39:1762–1763. doi: 10.1345/aph.1G277. [DOI] [PubMed] [Google Scholar]
- 21.Tie H., Walker B.D., Valenzuela S.M., Breit S.N., Campbell T.J. The heart of psychotropic drug therapy. Lancet. 2000;355:1825–11825. doi: 10.1016/s0140-6736(05)73083-x. [DOI] [PubMed] [Google Scholar]
- 22.Jo S.H., Youm J.B., Lee C.O., Earm Y.E., Ho W.K. Blockade of the HERG human cardiac K+ channel by the antidepressant drug amitriptyline. Br J Pharmacol. 2000;129:1474–1480. doi: 10.1038/sj.bjp.0703222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teschemacher A.G., Seward E.P., Hancox J.C., Witchel H.J. Inhibition of the current of heterologously expressed HERG potassium channels by imipramine and amitriptyline. Br J Pharmacol. 1999;128:479–485. doi: 10.1038/sj.bjp.0702800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z., Gong Q., Ye B., Fan Z., Makielski J.C., Robertson G.A. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milnes J.T., Crociani O., Arcangeli A., Hancox J.C., Witchel H.J. Blockade of HERG potassium currents by fluvoxamine: incomplete attenuation by S6 mutations at F656 or Y652. Br J Pharmacol. 2003;139:887–898. doi: 10.1038/sj.bjp.0705335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou A., Xu Q.P., Sanguinetti M.C. A mutation in the pore region of HERG K+ channels expressed in Xenopus oocytes reduces rectification by shifting the voltage dependence of inactivation. J Physiol. 1998;509:129–137. doi: 10.1111/j.1469-7793.1998.129bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brugada R., Hong K., Dumaine R., Cordeiro J., Gaita F., Borggrefe M. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 28.Ridley J.M., Milnes J.T., Witchel H.J., Hancox J.C. High affinity HERG K+ channel blockade by the antiarrhythmic agent dronedarone: resistance to mutations of the S6 residues Y652 and F656. Biochem Biophys Res Commun. 2004;325:883–891. doi: 10.1016/j.bbrc.2004.10.127. [DOI] [PubMed] [Google Scholar]
- 29.Duncan R.S., Ridley J.M., Dempsey C.E., Leishman D.J., Leaney J.L., Hancox J.C. Erythromycin block of the HERG K+ channel: accessibility to F656 and Y652. Biochem Biophys Res Commun. 2006;341:500–506. doi: 10.1016/j.bbrc.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Hancox J.C., Levi A.J., Lee C.O., Heap P. A method for isolating rabbit atrioventricular node myocytes which retain normal morphology and function. Am J Physiol. 1993;265:H755–H7666. doi: 10.1152/ajpheart.1993.265.2.H755. [DOI] [PubMed] [Google Scholar]
- 31.Howarth F.C., Levi A.J., Hancox J.C. Characteristics of the delayed rectifier K current compared in myocytes isolated from the atrioventricular node and ventricle of the rabbit heart. Pflugers Arch. 1996;431:713–722. doi: 10.1007/BF02253834. [DOI] [PubMed] [Google Scholar]
- 32.Ridley J.M., Milnes J.T., Hancox J.C., Witchel H.J., Clemastine A conventional antihistamine, is a high potency inhibitor of the HERG K+ channel. J Mol Cell Cardiol. 2006;40:107–118. doi: 10.1016/j.yjmcc.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Kirsch G.E., Trepakova E.S., Brimecombe J.C., Sidach S.S., Erickson H.D., Kochan M.C. Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J Pharmacol Toxicol Meth. 2004;50:93–101. doi: 10.1016/j.vascn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Ridley J.M., Milnes J.T., Zhang Y.H., Witchel H.J., Hancox J.C. Inhibition of HERG K+ current and prolongation of the guinea-pig ventricular action potential by 4-aminopyridine. J Physiol. 2003;549:667–672. doi: 10.1113/jphysiol.2003.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McPate M.J., Duncan R.S., Milnes J.T., Witchel H.J., Hancox J.C. The N588K-HERG K+ channel mutation in the ‘short QT syndrome’: mechanism of gain-in-function determined at 37 °C. Biochem Biophys Res Commun. 2005;334:441–449. doi: 10.1016/j.bbrc.2005.06.112. [DOI] [PubMed] [Google Scholar]
- 36.Milnes J.T., Dempsey C.E., Ridley J.M., Crociani O., Arcangeli A., Hancox J.C. Preferential closed channel blockade of HERG potassium currents by chemically synthesised BeKm-1 scorpion toxin. FEBS Lett. 2003;547:20–26. doi: 10.1016/s0014-5793(03)00662-8. [DOI] [PubMed] [Google Scholar]
- 37.Cordeiro J.M., Brugada R., Wu Y.S., Hong K., Dumaine R. Modulation of IKr inactivation by mutation N588K in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc Res. 2005;67:498–509. doi: 10.1016/j.cardiores.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Sanguinetti M.C., Mitcheson J.S. Predicting drug-HERG channel interactions that cause acquired long QT syndrome. Trends Pharmacol Sci. 2005;26:119–124. doi: 10.1016/j.tips.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Ridley J.M., Dooley P.C., Milnes J.T., Witchel H.J., Hancox J.C. Lidoflazine is a high affinity blocker of the HERG K+ channel. J Mol Cell Cardiol. 2004;36:701–705. doi: 10.1016/j.yjmcc.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Alexandrou A.J., Duncan R.S., Sullivan A., Hancox J.C., Leishman D.J., Witchel H.J. Mechanism of hERG K+ channel blockade by the fluoroquinolone antibiotic moxifloxacin. Br J Pharmacol. 2006;147:905–916. doi: 10.1038/sj.bjp.0706678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridley J.M., Milnes J.T., Duncan R.S., McPate M.J., James A.F., Witchel H.J. Inhibition of the HERG K+ channel by the antifungal drug ketoconazole depends on channel gating and involves the S6 residue F656. FEBS Lett. 2006;580:1999–2005. doi: 10.1016/j.febslet.2006.02.073. [DOI] [PubMed] [Google Scholar]
- 42.Reilly J.G., Ayis S.A., Ferrier I.N., Jones S.J., Thomas S.H.L. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–1052. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- 43.Kiesecker C., Alter M., Kathofer S., Zitron E., Scholz E., Thomas P.D. Atypical tetracyclic antidepressant maprotiline is an antagonist at cardiac hERG potassium channels. Naunyn-Schmiedebergs Arch Pharmacol. 2006;373:212–220. doi: 10.1007/s00210-006-0068-z. [DOI] [PubMed] [Google Scholar]
- 44.Ferrer-Villada T., Navarro-Polanco R.A., Rodriguez-Menchaca A.A., Benavides-Haro D.E., Sanchez-Chapula J.A. Inhibition of cardiac HERG potassium channels by antidepressant maprotiline. Eur J Pharmacol. 2006;531:1–8. doi: 10.1016/j.ejphar.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Ficker E., Jarolimek W., Kiehn J., Baumann A., Brown A.M. Molecular determinants of dofetilide block of HERG K+ channels. Circ Res. 1998;82:386–395. doi: 10.1161/01.res.82.3.386. [DOI] [PubMed] [Google Scholar]
- 46.Herzberg I.M., Trudeau M.C., Robertson G.A. Transfer of rapid inactivation and sensitivity to the class III antiarrhythmic drug E-4031 from HERG to M-eag channels. J Physiol. 1998;511:3–14. doi: 10.1111/j.1469-7793.1998.003bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang B.F., Xu D.H., Xu C.Q., Li Z., Du Z.M., Wang H.Z. Inactivation gating determines drug potency: a common mechanism for drug blockade of HERG channels. Acta Pharmacologica Sinica. 2004;25:554–560. [PubMed] [Google Scholar]
- 48.Chen J., Seebohm G., Sanguinetti M.C. Position of aromatic residues in the S6 domain, not inactivation, dictates cisapride sensitivity of HERG and eag potassium channels. Proc Nat Acad Sci USA. 2002;99:12461–12466. doi: 10.1073/pnas.192367299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witchel H.J., Pabbathi V.K., Hofmann G., Paul A.A., Hancox J.C. Inhibitory actions of the selective serotonin re-uptake inhibitor citalopram on HERG and ventricular L-type calcium currents. FEBS Lett. 2002;512:59–66. doi: 10.1016/s0014-5793(01)03320-8. [DOI] [PubMed] [Google Scholar]
- 50.Snyders D.J., Chaudhary A. High affinity open channel block by dofetilide of HERG expressed in a human cell line. Mol Pharmacol. 1996;49:949–955. [PubMed] [Google Scholar]
- 51.Sanguinetti M.C., Tristan-Firouzi M. HERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 52.Wang S., Morales M.J., Liu S., Strauss H.C., Rasmusson R.L. Modulation of HERG affinity for E-4031 by [K+]o and C-type inactivation. FEBS Lett. 1997;417:43–47. doi: 10.1016/s0014-5793(97)01245-3. [DOI] [PubMed] [Google Scholar]
- 53.Mitcheson J.S., Chen J., Sanguinetti M.C. Trapping of a Methanesulfonanilide by closure of the HERG potassium channel activation gate. J Gen Physiol. 2000;115:229–240. doi: 10.1085/jgp.115.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry M., de Groot M.J., Helliwell R., Leishman D., Tristani-Firouzi M., Sanguinetti M.C. Structural determinants of HERG channel block by clofilium and ibutilide. Mol Pharmacol. 2004;66:240–249. doi: 10.1124/mol.104.000117. [DOI] [PubMed] [Google Scholar]
- 55.Abbott G.W., Sesti F., Splawski I., Buck M.E., Lehmann W.H., Timothy K.W. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 56.Weerapura M., Nattel S., Chartier D., Caballero R., Hebert T.E. A comparison of currents carried by HERG, with and without coexpression of MiRP1, and the native rapid delayed rectifier current Is MiRP1 the missing link. J Physiol. 2002;540:15–27. doi: 10.1113/jphysiol.2001.013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marshall J.B., Forker A.D. Cardiovascular effects of tricyclic antidepressant drugs: therapeutic usage, overdose, and management of complications. Am Heart J. 1982;103:401–414. doi: 10.1016/0002-8703(82)90281-2. [DOI] [PubMed] [Google Scholar]
- 58.Elonen E., Linnoila M., Lukkari I., Mattila M.J. Concentration of tricyclic antidepressants in plasma, heart and skeletal muscle after their intravenous infusion to anaesthetized rabbits. Acta Pharmacol Toxicol. 1975;37:274–281. doi: 10.1111/j.1600-0773.1975.tb00845.x. (Copenh) [DOI] [PubMed] [Google Scholar]
- 59.Leucht S., Steimer W., Kreuz S., Abraham D., Orsulak P.J., Kissling W. Doxepin plasma concentrations: is there really a therapeutic range? J Clin Psychopharmacol. 2001;21:432–439. doi: 10.1097/00004714-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Brugada R., Hong K., Cordeiro J.M., Dumaine R. Short QT Syndrome. CMAJ. 2005;173:1349–1354. doi: 10.1503/cmaj.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaita F., Giustetto C., Bianchi F., Schimpf R., Haissaguerre M., Calo L. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol. 2004;43:1494–1499. doi: 10.1016/j.jacc.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 62.Wolpert C., Schimpf R., Giustetto C., Antzelevitch C., Cordeiro J., Dumaine R. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol. 2005;16:54–58. doi: 10.1046/j.1540-8167.2005.04470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McPate M.J., Duncan R.S., Witchel H.J., Hancox J.C. Disopyramide is an effective inhibitor of mutant HERG K+ channels involved in variant 1 short QT syndrome. J Mol Cell Cardiol. 2006;41:563–566. doi: 10.1016/j.yjmcc.2006.05.021. [DOI] [PubMed] [Google Scholar]