Abstract
A simple and new simultaneous fourth derivative spectrophotometric method is proposed for the analysis of a two-component system containing cobalt(II) and nickel(II) without separation using 2-hydroxy-3-methoxy benzaldehyde thiosemicarbazone (HMBATSC) as a chromophoric reagent. The reagent reacts with cobalt(II) and nickel(II) at pH 6.0, forming soluble brown and yellow colored species, respectively. Cobalt(II) and nickel(II) present in themixture are simultaneously determined without solving the simultaneous equations bymeasuring the fourth derivative amplitudes at 468.5 nm and 474.5 nm, respectively. The derivative amplitudes obey Beer's law at 468.5 nm and 474.5 nm for Co(II) and Ni(II) in the range 0.059–3.299 μg mL−1 and 0.058–3.285 μg mL−1 respectively. A large number of foreign ions do not interfere in the present method. The present simultaneous method is used for the determination of micro amounts of cobalt in biological samples, nickel in plant samples, and in some alloy steels and soil sample.
1. INTRODUCTION
The direct spectrophotometric determination of metal ions in multicomponent systems is often complicated by interferences from the formulation matrix and spectral overlapping. Such interferences have been treated in many ways, such as solving two simultaneous equations [1] or using absorbance ratios at certain wavelengths [2–4]. However, during the application of these methods [1–4], the presence of spectral interferences or spectral overlap would certainly lead to erroneous results [5]. Other approaches aimed at solving this problem have been used; including pH-induced differential spectrophotometry [6], least squares [7], and orthogonal function [5, 7, 8 ] methods.
Derivative spectrophotometry is a useful means of resolving two overlapping spectra and eliminating matrix interferences in the assay of two-component mixtures using the zero-crossing technique [9–11]. In the absence of a zerocrossing point, two simultaneous equations can be solved to determine the components in such a mixture [12]. The latter method is based on criteria for selecting the optimumworking wavelength [2]. In addition, the component being determined should make a reasonable contribution to the total derivative reading of the mixture at the selected wavelength. Derivative spectrophotometric analysis of twocomponent mixtures is also carried out without the need to solve simultaneous equations. The compensation method [13] is also used for the purpose. It is a nonmathematical method for the detection and elimination of unwanted absorption during photometric analysis.
Cobalt alloys are used in some industrial products because of their sufficient hardness and resistivity against oxidation at high temperatures, for example, in the manufacturing of turbine blades and cutting tools. Cobalt-60 is used as an efficient radioactive tracer and an anticancer treatment agent in medicine. Some cobalt compounds, such as vitamin B12 (cyanocobalamine), are noted for their biological activities. Therefore, the determination of cobalt is valuable for the quality control of artificial and biological samples in a simple, selective, and sensitive manner. Nickel usually occurs in +2 oxidation state. It is one of the important alloying elements for steel and cast iron. The possibility that nickel may be an essential micro nutrient [14–16] for plants arose from urease, which is a nickel metallo-enzyme. Nickel is an important metal both industrially and biologically. It is one among the essential trace elements along with cobalt, copper, zinc, and manganese in the human diet [17]. Bertrand and Nakamure [18] observed in their experiments on synthetic nutrition that nickel and cobalt play a direct role in nutritional phenomenon. Nickel, which is bound to ribonucleic acid, has a special affinity for bone and skin and has been suggested to play an important role in pigmentation [19].
Thiosemicarbazones [20–26] are one of the important classes of reagents widely used for the spectrophotometric determination of metal ions. In the present paper, 2-hydroxy- 3-methoxy benzaldehyde thiosemicarbazone (HMBATSC) is used as a spectrophotometric reagent for simultaneous determination of cobalt(II) and nickel(II).
In the present paper, 2-hydroxy-3-methoxy benzaldehyde thiosemicarbazone (HMBATSC) reacts with cobalt(II) and Ni(II) forming brown and yellow colored complexes at pH 6.0, respectively. The fourth-order derivative spectrum of Co(II) gives sufficient amplitude at 468.5 nm with zero amplitude at 474.5 nm at pH 6.0. On the other hand, Ni(II) complex with HMBATSC shows sufficient and measurable derivative amplitude at 474.5 nm and zero amplitude at 468.5 nm. This difference in amplitudes was utilized to develop a fourth-order derivative spectrophotometric method for the simultaneous determination of cobalt(II) and nickel(II) in a mixture without solving simultaneous equations by measuring the fourth-order derivative amplitude at 468.5 nm and 474.5 nm, respectively.
2. EXPERIMENTAL
The absorbance and pH measurements were made on a Shimadzu UV-visible spectrophotometer (model UV-160A) fitted with 1 cm Quartz cells and Phillips digital pH meter (model L1 613), respectively. Fourth-order derivative spectra were recorded with a scan speed of fast (nearly 2400 nm min−1); slit width of 1 nm with nine degrees of freedom, in the required wavelength range (nm). The derivative amplitudes measured at required wavelengths were plotted against amount of cobalt(II) or nickel(II) to obtain the calibration plots.
3. REAGENTS
3.1. Preparation of 2-hydroxy-3-methoxy benzaldehyde thiosemicarbazone
The reagent (HMBATSC) was prepared by the reported procedure [27] and characterized by IR and NMR spectral data.
A 0.01 M solution of HMBATSC in dimethyl formamide (DMF) was used in the present studies.
3.2. Cobalt(II) solution
Stock solution of Co(II) (1 × 10−2 M) was prepared by dissolving appropriate amount of Co(NO3)2 6H2O in doubly distilled water containing a few drops of conc. HNO3 in a 100 mL volumetric flask and standardized gravimetrically [28].
3.3. Nickel(II) solution
A 0.2808 g of NiSO4 · 7H2O was dissolved in doubly distilled water containing few drops of conc. H2SO4 in a 100 mL standard flask to get 1 × 10−2 M solution which is then standardized gravimetrically using dimethyl glyoxime [29].
The working solutions were prepared daily by diluting the stock solution to an appropriate volume. All other chemicals used were of analytical grade.
3.4. Buffer solutions
The buffer solutions were prepared by mixing 1 M hydrochloric acid, 1 M sodium acetate (pH 1.0–3.0), 0.2 M acetic acid, and 0.2 M sodium acetate (pH 3.5–7.0). The pH of these solutions was checked with a pH meter.
3.5. Preparation of alloy steel sample solutions [30]
A 0.1–0.5 g of steel sample was dissolved completely in minimumamount of aqua-regia by slow heating on sand bath and then heated to fumes of oxides of nitrogen. After cooling, 5–10 mL of 1 : 1 H2O : H2SO4 mixture was added and evaporated to dryness. Sulphuric acid treatment was repeated three times to remove all the nitric acid. The residue was dissolved in 20 mL of distilled water, filtered, and the filtrate was made up to 100 mL in a calibrated volumetric flask with distilled water. The sample solution was appropriately diluted to obtain the concentrations in the required range.
3.6. Preparation of soil sample solution
Soil sample (S-18) was obtained from Geological Survey of India (GSI), Bangalore, India. 500 mg of the soil sample was treated with 1.0 mL of concentrated HCl. When the reaction was almost over, the mixture was heated on a hot plate and evaporated to dryness. The contents were dissolved in distilled water and filtered. The filtrate was collected in a 50 mL standard flask and made up to the mark with distilled water.
3.7. Biological samples (tea leaf and vehicle exhaust)
The tea leaf samples were supplied by Andra Pradesh Agricultural Research Institute (APARI), Hyderabad, India. The vehicle exhaust sample was collected from Environment Protection Training and Research Institute (EPTRI), Hyderabad, India. A 0.1 g of tea leaf sample was taken in a beaker and dissolved in conc. nitric acid (≈ 5 mL) with heating. The solution was cooled, diluted, and filtered. The filtrate wasmade up to 100 mL with water in a calibrated flask. Vehicle exhaust particles (1 g) were dissolved in a mixture of 18 mL of conc. nitric acid, 18 mL of conc. perchloric acid, and 2 mL of conc. hydrofluoric acid in a 100 mL teflon beaker, evaporated to a small volume, filtered through a filter paper, and made up to 100 mL with distilled water. An aliquot (10–50 mL) of the sample solution was taken individually and cobalt was determined from predetermined calibration plot. The results are presented in Table 5.
Table 5.
Analysis of cobalt in biological samples.
| Sample | Amount of cobalt (mg/g) | |
| Certified value | Present method* | |
| Tea leaves | 0.12 ± 0.008 | 0.117 ± 0.04 |
| Vehicle exhaust | 3.3 ± 0.3 | 3.25 ± 0.05 |
*Average of five determinations.
3.8. Preparation of plant samples
Freshly collected samples were cleaned and dried for one hour in open air protecting from mineral contamination. The dried samples were finely powdered in a mortar. The powdered material was brought into solution by wet ashing method according to the procedures given in the literature [31]. The results are presented in Table 6.
Table 6.
Determination of nickel in plant leaves.
| Sample | AAS method [32] | Present method* |
| Pisum sativum (Hulls) | 2.060 ± 0.003 | 2.065 ± 0.004 |
| Mangifera indica leaves | 2.150 ± 0.004 | 2.152 ± 0.002 |
| Eucalyptus leaves | 1.038 ± 0.002 | 1.033 ± 0.005 |
| Azadirachta indica leaves | 1.481 ± 0.005 | 1.485 ± 0.003 |
*Average of five determinations.
4. RESULTS AND DISCUSSION
2-Hydroxy-3-methoxy benzaldehyde thiosemicarbazone (HMBATSC) reacts with cobalt(II) forming brown-colored complex and with vanadium(V) forming yellow-colored complex at pH 6.0. The fourth derivative spectra of [Co(II)-HMBATSC] and [Ni(II)-HMBATSC] at pH 6.0 were recorded in the wavelength region 420–550 nm and shown in Figure 1. From the figure, it can be seen that the thirdorder derivative spectrum of Co(II) complex shows zero amplitude at 474.5 nm and sufficient derivative amplitude at 468.5 nm, while Ni(II) complex has sufficient amplitude at 474.5 nm and zero amplitude at 468.5 nm, respectively. This will allow simultaneous determination of Co(II) and Ni(II) by fourth-order derivative spectrophotometric method by measuring the derivative amplitudes at 468.5 nm and 474.5 nm, respectively.
Figure 1.
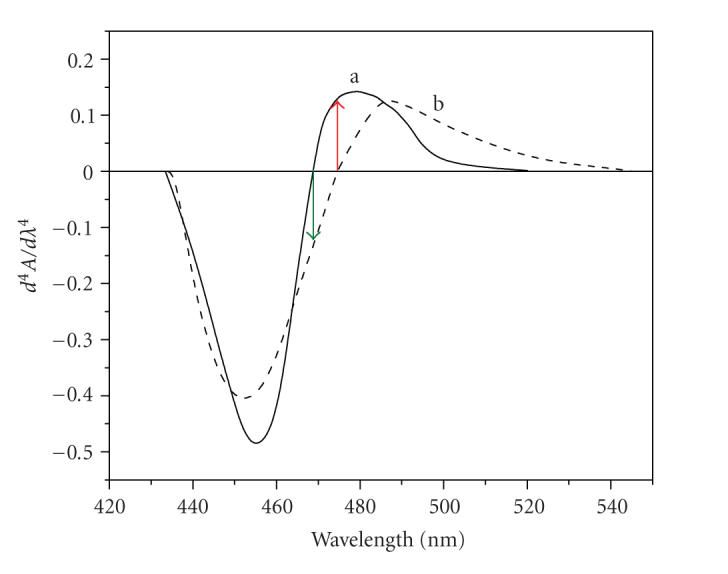
Fourth derivative spectra of (a) Co(II)-HMBATSC system versus reagent blank, (b) Ni(II)-HMBATSC system versus reagent blank; [Ni(II)] = [Co(II)] = 1.2 × 10−5 M; pH = 6.0.
4.1. Verification of Beer's law validity
Individual calibration plots were constructed by plotting the fourth derivative amplitudes measured at 468.5 nm against the amount of Co(II) and plotting those measured at 474.5 nm against the amount of Ni(II) as shown in Figures 2 and 3, respectively. The plots reveal that Beer's law is obeyed in the range of 0.059–3.299 μg mL−1 of Co(II) and 0.058–3.285 μg mL−1 of Ni(II).
Figure 2.
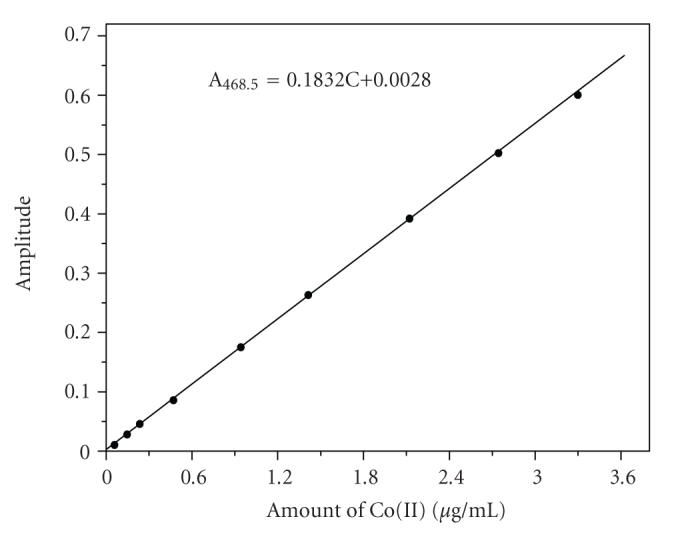
Calibration plot of Co(II)-HMBATSC, [HMBATSC] = 8 × 10−4 M; wavelength = 468.5 nm; pH = 6.0.
Figure 3.
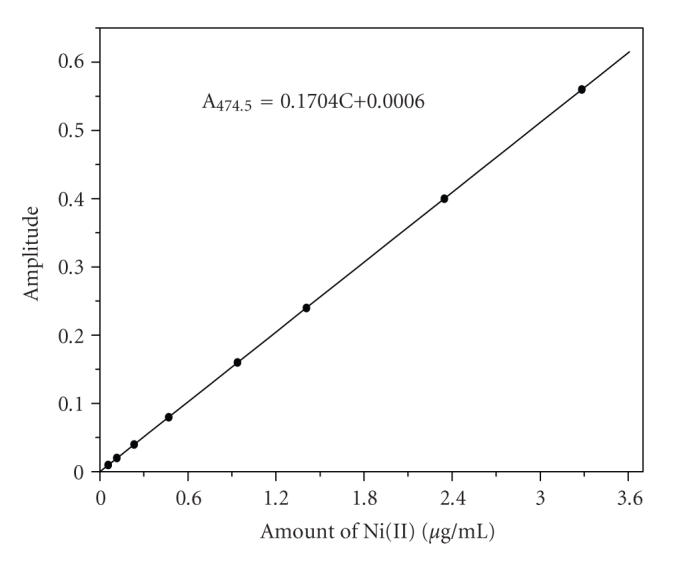
Calibration plot of Ni(II)-HMBATSC, [HMBATSC] = 8 × 10−4 M; wavelength = 474.5 nm; pH = 6.0.
4.2. Simultaneous determination of Co(II) and Ni(II)
Variable amounts of Co(II) and Ni(II) were treated with the required volume of the reagent at pH 6.0 in total volume of 10mL and the fourth derivative spectra were recorded in the range of 420–550 nm. The derivative amplitudes were measured at 468.5 nm and 474.5 nm and the amounts of Co(II) and Ni(II) present in the mixture were computed from their respective calibration plots. The results are presented in Table 1.
Table 1.
Simultaneous fourth-order derivative determination of Co(II) and Ni(II) in synthetic binary mixtures.
| Amount taken (μg mL−1) | Amount found* (μg mL−1) | Relative error (%) | |||
| Co(II) | Ni(II) | Co(II) | Ni(II) | Co(II) | Ni(II) |
| 0.2357 | 0.2347 | 0.2362 | 0.2340 | +0.21 | −0.29 |
| 0.2357 | 0.4694 | 0.2352 | 0.4685 | −0.21 | −0.23 |
| 0.2357 | 0.7041 | 0.2354 | 0.7049 | −0.12 | +0.11 |
| 0.2357 | 0.9388 | 0.2361 | 0.9382 | +0.16 | −0.06 |
| 0.2357 | 1.1785 | 0.2365 | 1.1780 | +0.33 | −0.04 |
| 0.2357 | 0.2347 | 0.2362 | 0.2352 | +0.21 | +0.21 |
| 0.4714 | 0.2347 | 0.4708 | 0.2344 | −0.10 | −0.12 |
| 0.7071 | 0.2347 | 0.7081 | 0.2354 | +0.14 | +0.29 |
| 0.9428 | 0.2347 | 0.9420 | 0.2340 | −0.08 | −0.29 |
| 1.1735 | 0.2347 | 1.1742 | 0.2362 | +0.05 | +0.63 |
*Average of five determinations.
4.3. Effect of foreign ions
The effect of various foreign ions on the determination of Co(II) and Ni(II) was studied to find out the tolerance levels of these foreign ions in the present method. The results are presented in Table 2.
Table 2.
Tolerance limits of diverse ions.
| Diverse ion | Tolerance limit (μg mL−1) | Diverse ion | Tolerance limit (μg mL−1) | ||
| In the presence of 1.173 μg mL−1 of Ni(II) | In the presence of 1.18 μg mL−1 of Co(II) | In the presence of 1.173 μg mL−1 of Ni(II) | In the presence of 1.18 μg mL−1 of Co(II) | ||
| Ascorbic acid | 3000 | 3200 | Pb(II) | 2200 | 2000 |
| Tartrate | 2800 | 2700 | W(VI) | 1900 | 1800 |
| Citrate | 2600 | 2500 | U(VI) | 1800 | 1750 |
| EDTA | 2400 | 2400 | Zr(IV) | 1500 | 1400 |
| Thiourea | 2000 | 2100 | Cd(II) | 1300 | 1200 |
| Formate | 1900 | 2000 | Li(I) | 1200 | 1100 |
| Urea | 1700 | 1800 | Th(IV) | 1000 | 950 |
| Bromate | 1650 | 1700 | Na(I) | 950 | 900 |
| Oxalate | 1600 | 1650 | Te(IV) | 800 | 800 |
| Bromide | 1500 | 1400 | K(I) | 750 | 800 |
| Phosphate | 1450 | 1400 | Cu(II) | 700 | 750 |
| Nitrate | 1400 | 1350 | Al(III) | 650 | 600 |
| Chloride | 1200 | 1300 | Zn(II) | 550 | 450 |
| Sulphate | 1100 | 1150 | Pt(IV) | 400 | 400 |
| Acetate | 1050 | 1000 | Fe(II) | 350 | 400 |
| Thiosulphate | 1000 | 900 | Pd(II) | 300 | 350 |
| Iodide | 850 | 800 | V(V) | 250 | 300 |
| Fluoride | 800 | 700 | Ru(III) | 250 | 250 |
| — | — | — | Ti(IV) | 220 | 200 |
| — | — | — | Cr(III) | 200 | 180 |
| — | — | — | Ce(IV) | 180 | 150 |
| — | — | — | Mo(VI) | 150 | 140 |
| — | — | — | Mn(II) | 130 | 125 |
5. APPLICATIONS
The present simultaneous method was used for the determination of cobalt and nickel in alloy steels and soil samples.
A known aliquot of the sample was treated with 5 mL of buffer solution (pH 6.0), 0.5 mL of HMBATSC (1 × 10−2 M) and made up to the volume in a 10 mL volumetric flask with distilled water. The fourth derivative curves were recorded and the derivative amplitudes were measured at 468.5 nm and 474.5 nm. The amounts of Co(II) and Ni(II) were computed from the measured amplitudes with the help of predetermined calibration plots. The results obtained are given in Tables 3 and 4.
Table 3.
Simultaneous determination of Co(II) and Ni(II) in alloy steel samples.
| Sample | Certified value (%) | Amount found by present method* (%) | Relative error (%) | |||
| Co(II) | Ni(II) | Co(II) | Ni(II) | Co(II) | Co(II) | |
| Eligiloy M-1712(a) | 40.00 | 15.00 | 39.94 | 15.10 | −0.15 | +0.67 |
| BCS 406/1(b) | 0.016 | 0.14 | 0.017 | 0.128 | +0.63 | −1.54 |
| Alloy steel(c) | 23.72 | 11.22 | 23.68 | 11.30 | −0.16 | +0.71 |
*Average of five determinations;
(a)20% Cr, 40% Co, 15% Ni, 0.15% C, 15% Fe, 2% Mn, 7% Mo, 0.05% Be.
(b)0.066% Mn, 1.06% Cr, 0.05% Mo, 0.14% Ni, 0.016% Co, 0.091% Cu, 0.19% Vz.
(c)51.15% Fe, 11.22% Ni, 5.09% Cu, 23.72% Co, 6.9% Al, 0.79% Ti, 0.235% Mn, 0.57% Si.
Table 4.
Analysis of soil sample.
| Sample and composition (ppm) | Certified (GSI) value (ppm) | Amount found by present method* (ppm) | Relative error (%) | |||
| Co(II) | Ni(II) | Co(II) | Ni(II) | Co(II) | Ni(II) | |
| S-18 | 40.35 | 50.50 | 39.98 | 50.84 | −0.92 | +0.67 |
| 20.20 Pb(II) | ||||||
| 20.30 Zn(II) | ||||||
| 88.85 Cu(II) | ||||||
*Average of five determinations.
6. CONCLUSIONS
The present method is simple, sensitive, and highly selective for the simultaneous determination of Co(II) and Ni(II) in admixtures without separation and without solving simultaneous equations.
References
- 1.Heilmayer L. Spectrophotometry in Medicine. London, UK: Adam Hilger; 1943. [Google Scholar]
- 2.Glenn AL. The importance of extinction ratios in the spectrophotometric analysis of mixtures of two known absorbing substances. The Journal of Pharmacy and Pharmacology. 1960;12:595–608. doi: 10.1111/j.2042-7158.1960.tb12715.x. [DOI] [PubMed] [Google Scholar]
- 3.Pernarowski M, Knevel AM, Christian JE. Application of absorbancy ratios to the analysis of pharmaceuticals I. Theory of the analysis of binary mixtures. Journal of Pharmaceutical Sciences. 1961;50(11):943–945. doi: 10.1002/jps.2600501113. [DOI] [PubMed] [Google Scholar]
- 4.Cho MJ, Pernarowski M. Application of absorbance ratios to analysis of pharmaceuticals VI: analysis of binary mixture using a reference spectrum. Journal of Pharmaceutical Sciences. 1970;59(9):1333–1335. doi: 10.1002/jps.2600590926. [DOI] [PubMed] [Google Scholar]
- 5.Glenn AL. The use of orthogonal functions to correct for irrelevant absorption in two component spectrophotometric analysis. The Journal of Pharmacy and Pharmacology. 1963;15 (supplement):123–130. doi: 10.1111/j.2042-7158.1963.tb11198.x. [DOI] [PubMed] [Google Scholar]
- 6.Wahbi AM, Farghaly AM. Application of the delta-A method to the determination of morphine. The Journal of Pharmacy and Pharmacology. 1970;22(11):848–850. doi: 10.1111/j.2042-7158.1970.tb08451.x. [DOI] [PubMed] [Google Scholar]
- 7.Wahbi AM, Barary M. Spectrophotometric determination of atropine sulfate in the presence of phenylmercury (II) acetate. Journal of the Association of Official Analytical Chemists. 1981;64(4):855–859. [PubMed] [Google Scholar]
- 8.Wahbi AM, Belal S, Abdine H, Bedair M. Spectrophotometric determination of methylphenobarbitone by use of orthogonal polynomials. Talanta. 1982;29(11) supplement 1:931–935. doi: 10.1016/0039-9140(82)80154-9. [DOI] [PubMed] [Google Scholar]
- 9.Wahbi AM, Abdine H, Blaih SM. Spectrophotometric determination of some preservatives in milk. Journal of the Association of Official Analytical Chemists. 1977;60(5):1175–1179. [PubMed] [Google Scholar]
- 10.Fell AF. Analysis of pharmaceutical dosage form by second derivative ultraviolet-visible spectrophotometry. Proceedings of the Analytical Division of the Chemical Society. 1978;15:260–267. [Google Scholar]
- 11.Bedair M, Korany MA, El-Yazbi FA. UV-derivative spectrophotometric determination of hydralazine hydrochloride in combination with hydrochlorothiazide or propranolol hydrochloride. Scientia Pharmaceutica. 1986;54(1):31–36. [Google Scholar]
- 12.Korany MA, Wahbi AM, Elsayed MA, Mandour S. First derivative spectrophotometric determination of certain drugs in two-component mixtures. Analytical Letters. 1984;17(12):1373–1389. [Google Scholar]
- 13.Jones JH, Clark GR, Harrow LS. Journal of Association of Official Agricultural Chemists. 1951;34:135. [Google Scholar]
- 14.Dixon NE, Gazzola C, Watters JJ, Blakeley RL, Zerner B. Inhibition of jack bean urease (EC 3.5.1.5) by acetohydroxamic acid and by phosphoramidate. An equivalent weight for urease. Journal of the American Chemical Society. 1975;97(14):4130–4131. doi: 10.1021/ja00847a044. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt EJ. Chemistry and Agriculture. London, UK: Chemical Society; 1979. special publication no. 36. [Google Scholar]
- 16.Welch RM. The biological significance of nickel. Journal of Plant Nutrition. 1981;3:345–356. [Google Scholar]
- 17.Bell GH, Davidson JN, Scarborough H. Text Book of Physiology and Biochemistry. 2nd ed. Edinburgh, UK: E. S. Living stone; 1953. [Google Scholar]
- 18.Bertrand G, Nakamure H. Bulletin de la Société de Chimie Biologique. 1934;16:1366. [Google Scholar]
- 19.Bernard L. Hawk's Physiological Chemistry. 14th ed. New York, NY, USA: McGraw-Hill; 1965. [Google Scholar]
- 20.Singh RB, Garg BS, Singh RP. Analytical applications of thiosemicarbazones and semicarbazones: a review. Talanta. 1978;25(11-12):619–632. doi: 10.1016/0039-9140(78)80163-5. [DOI] [PubMed] [Google Scholar]
- 21.Reddy KH, Reddy DV. Q. chem., Rev. 1985;1:47. [Google Scholar]
- 22.Garg BS, Jain VK. Analytical applications of thiosemicarbazones and semicarbazones. Microchemical Journal. 1988;38(2):144–169. [Google Scholar]
- 23.Ramanjaneyulu G, Reddy PR, Reddy VK, Reddy TS. Spectrophotometric determination of iron in trace amount using 5-bromo-salicylaldehyde thiosemicarbazone. Indian Journal of Chemistry—Section A Inorganic, Physical, Theoretical and Analytical Chemistry. 2002;41(7):1436–1437. [Google Scholar]
- 24.Reddy KH, Prasad NBL, Reddy TS. Analytical properties of 1-phenyl-1,2-propanedione-2-oxime thiosemicarbazone: simultaneous spectrophotometric determination of copper(II) and nickel(II) in edible oils and seeds. Talanta. 2003;59(3):425–433. doi: 10.1016/s0039-9140(02)00543-x. [DOI] [PubMed] [Google Scholar]
- 25.Ramanjaneyulu G, Reddy PR, Reddy VK, Reddy TS. Direct and derivative spectrophotometric determination of cobalt with 5-bromosalicylaldehydethiosemicarbazone. Journal of the Indian Chemical Society. 2003;80(8):773–776. [Google Scholar]
- 26.Reddy BK, Reddy KJ, Kumar JR, Kumar AK, Reddy AV. Highly sensitive extractive spectrophotometric determination of palladium(II) in synthetic mixtures and hydrogenation catalysts using benzildithiosemicarbazone. Analytical Sciences. 2004;20(6):925. doi: 10.2116/analsci.20.925. [DOI] [PubMed] [Google Scholar]
- 27.Sah PT, Peoples SA. Isonicotinyl hydrazones as antitubercular agents and derivatives for identification of aldehydes and ketones. Journal of the American Pharmaceutical Association. 1954;43(9):513–524. doi: 10.1002/jps.3030430902. [DOI] [PubMed] [Google Scholar]
- 28.Vogel AI. A Text Book of Quantitative Inorganic Analysis. 4th ed. New York, NY, USA: ELBS and Longman; 1985. [Google Scholar]
- 29.Vogel AI. A Text Book of Quantitative Inorganic Analysis. 3rd ed. London, UK: ELBS and Longman; 1962. [Google Scholar]
- 30.Buseve AI, Ivanov VM. Zh. Annal. Chim. 1963;18:208. [Google Scholar]
- 31.Piper CS. Soil and Plant Analysis. Bombay, India: Hans Publishers; 1966. [Google Scholar]
- 32.Folin M, Contiero E, Calliari J. Determination of Nickel in different samples with AAS. Analytica Chimica. 1991;81:39–42. [Google Scholar]


