Abstract
Spines are specialized neuronal membrane structures, often localized at sites where synaptic information is relayed from one cell to another in the central nervous system. By electron immunomicroscopy we have found that the mammalian Shaw family potassium channel Kv3.1 is localized on spine-like protrusions, adjacent to postsynaptic membranes of bushy cells in the cochlear nucleus. As direct characterization of the electrophysiological behavior of ion channels in such structures is difficult, we have used Kv3.1-transfected CHO cells to create artificial spine-like membrane compartments. Membrane patches were sucked into microelectrodes to form small, cell-attached vesicles with dimensions comparable to those of the neuronal structures. Currents mediated by the Kv3.1 channel in these vesicles undergo rapid and complete inactivation, in contrast to their noninactivating behavior in whole-cell recordings. This apparent inactivation is caused by the rapid depletion of K+ from the vesicle and the slow refilling of K+ into the vesicle compartment from the bulk cytoplasm. Our data provide evidence that compartmentalized ionic transients can be generated in spine-like membrane structures and support the view that the localization of ion channels in spine-like structures may influence responses to synaptic stimulation.
Voltage-gated potassium channels are important elements in determining neuronal excitability. Recent evidence shows that these channels are present in the dendrites of hippocampal pyramidal neurons and strongly influence dendritic integration and propagation of information (1). However, it is not known whether potassium channels exist in the dendritic spines, unique structural features thought to function as basic units of integration in central neurons (2–11). Using electron microscopy, we show here that the Kv3.1 potassium channel, a channel known to be expressed in neurons that are able to respond to synaptic inputs with very high rates of action potential firing (12, 13), is present at particularly high levels in spine-like protrusions, adjacent to postsynaptic membranes of bushy cells in the cochlear nucleus of the auditory brainstem. Furthermore, we demonstrate by using a membrane model that mimics the dimension of spine-like protrusions that, in this geometric configuration, the current through this delayed rectifier undergoes apparent inactivation because of potassium depletion from the spine-like compartment. This apparent inactivation may amplify the compartmentalized Ca2+ transients in spine-like structures and may thereby contribute to synaptic plasticity.
MATERIALS AND METHODS
Electron Microscopy.
Specific Kv3.1 antibodies were prepared and affinity-purified as described by Perney and Kaczmarek (12). Adult female (200–250 gm) Sprague–Dawley rats were deeply anesthetized with sodium pentobarbital (120 mg/kg, i.p.) and perfused through the ascending aorta with 250 ml PBS followed by 500 ml 4% paraformaldehyde/0.1% glutaraldehyde in 0.1 M NaPO4 buffer, pH 7.2. Brains were dissected, postfixed 1 hr in the same fix, and then washed for 2 hr in PBS. Fifty micron free-floating sections, cut on a vibratome (Vibratome 1000, TPI, St. Louis) were equilibrated with 20% sucrose in PBS and then subjected to two cycles of freeze-thawing in liquid N2. The sections were washed three times in PBS, blocked for 1 hr in staining media (DMEM/10% fetal bovine serum/0.02% sodium azide), and then incubated overnight with affinity-purified antibody (≈2 μg/ml). After washing in PBS the sections were incubated for 1 hr with biotinylated anti-rabbit IgG (1:200, Vector Laboratories). Immunoreactivity was visualized with avidin-biotin horseradish peroxidase complex and the chromogen 3,3′-diaminobenzidine-tetrahydrochloride according to the Vectastain Elite ABC kit instructions (Vector Laboratories). After immunolabeling, tissue sections were postfixed in 1% osmium tetroxide for 30 min to 2 hr, dehydrated in ethyl alcohol, and embedded in Epon-Araldite resin sandwiched between sheets of Aclar plastic. The embedded sections were viewed by using a light microscope, and areas of interest were excised, blocked, and thin-sectioned. Mounted sections were then viewed with a Phillips 300 electron microscope.
Stable Cell Line Expressing the Kv3.1 Channel.
Chinese hamster ovary cells with DHFR deficiency [CHO/DHFR(−)] were maintained in Iscove’s modified Aulbecco’s medium (Gibco) supplemented with 10% fetal bovine serum, 2 mM glutamine, 0.1 mM hypoxanthine, and 0.01 mM thymidine in a 5% CO2 air environment at 37°C. Cells were seeded 1 day before transfection at about 5 × 105 cells per 60-mm plate. Kv3.1b expression vector (pRC/CMV-Kv3.1, 5 μg) (18) was added 24 hr later to transfect CHO/DHFR(−) by using lipofectamine (Gibco). The cells were then grown in normal media for 48 hr to develop antibiotic resistance and subsequently exposed to geneticin (0.5 mg/ml, Gibco) for another 10–14 days. The geneticin-resistant cells were subjected to single-cell sorting by using FACSIV (Becton Dickinson) to generate individual stable cell lines.
Electrophysiology.
Patch-clamp recordings were made with electrodes (resistance 3 to 6 MΩ, tip diameter 0.4–1 μm) constructed from thin-wall borosilicate glass (1.5 mm diameter, World Precision Instruments, Sarasota, FL) by using a two-stage puller (Narishige PP83). Extracellular solution contained 140 mM NaCl, 10 mM CaCl2, 3 or 9 mM KCl, 25 mM Hepes, and 33 mM glucose (pH 7.4, 320–335 mosM). This solution was also used to fill pipettes for cell-attached recordings. Intracellular solution for whole-cell recordings contains 120 mM KCl, 10 mM Hepes, 2 mM MgCl2, and 11 mM EGTA (pH 7.3). An Axopatch-1D amplifier (Axon Instruments, Foster City, CA) was employed to acquire data on-line by using pClamp (Axon Instruments), and data was filtered at 5 kHz. Population data were expressed as the mean ± SE, and the Student’s t test was used to examine statistical significance.
Computer Simulation.
Kv3.1 potassium current across the membrane of the spine-like compartment was simulated by using the equations described by Kanemasa et al. (14), with a potassium conductance of gK = 8 nS. The equilibrium potential for potassium ions, EK, was determined by the concentration of potassium ions in the vesicle and in the unstirred layer, according to the Nernst equation. Potassium flux into and out of the vesicle, Js, was calculated by using the equation Js = kdiff([Kcyt] − Ki/Vs) − JKv3.1, where [Kcyt] is the bulk cytoplasmic potassium concentration (120 mM), Ki is the amount of potassium in the compartment, Vs is the volume of the vesicle, and kdiff is a constant determined by the diffusion constant for potassium ions in cytoplasm and by the diffusional neck resistance through the neck of the vesicle (15). JKv3.1 is the flux of potassium through Kv3.1 channels in the membrane. Potassium concentrations in the unstirred layer were calculated by integrating a similar equation, Je = kext([Ko] − Ku/Vu) + JKv3.1, where Je is the flux of potassium into a surrounding unstirred layer, [Ko] is the potassium concentration in the external medium or the pipette solution (3 mM), Ku is the amount of potassium in the unstirred layer, Vu is its volume, and kext is a constant that determines the rate of equilibration between the unstirred layer and the external medium.
RESULTS
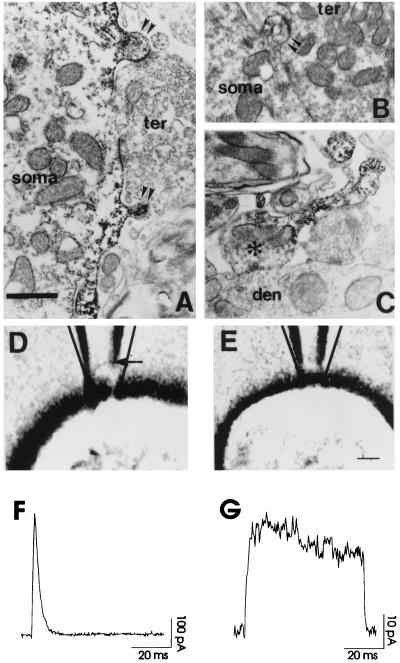
We first examined the distribution of the Kv3.1 channel in neurons of auditory brainstem nuclei by electron microscopic immunolocalization. Kv3.1 immunoreactivity is localized to both postsynaptic (Fig. 1 A and B) and presynaptic elements (Fig. 1C). A particularly high density of the Kv3.1 protein is, however, found at sites adjacent to postsynaptic membranes of some auditory neurons. Fig. 1 A and B show that, in the somata of bushy cells in the cochlear nucleus, much of the Kv3.1 immunoreactivity is found in the plasma membrane of spine-like protrusions. In contrast to typical neuronal synaptic spines, which are targets for small, discrete presynaptic endings, however, these structures on bushy cells are located at the edges of the large, calyx-like synapses that transmit the input from the auditory nerve (Fig. 1 A and B). Because recent imaging studies have indicated that in other cells, spines are important structures for neuronal integration (2–11) and because there is high expression of Kv3.1 channels in these spine-like protrusions, we have examined the possible consequences of localization of Kv3.1 channels to such structures by using CHO cells stably transfected with the Kv3.1b gene.
Figure 1.
Electron microscopic immunolocalization of Kv3.1 protein in restricted membrane compartments of auditory neurons. (A and B) Kv3.1 immunoreaction product found in the plasma membrane and underlying cytoplasm of spine-like protrusions (double arrowheads) of bushy cell somata (soma). The labeled spine protrusions are often present near or at the site of synaptic terminal (ter) contact. Note the difference in the neck geometry of the spine-like protrusions. (C) Photomicrograph of a Kv3.1 immunolabeled axon terminal (asterisk) (presumably from a bushy or MNTB neuron) synapsing on the dendrite of a parasuperior olivary neuron. Kv3.1 reaction product is concentrated in the neck region of the axon entering the synaptic terminal. [Bar = 0.75 μm (A and B), 0.91 μm (C)]. (D and E) A cell-attached vesicle or a vesicle-free patch following a seal in the patch pipette photographed through Nomarski optics. (Bar = 2.2 μm). (F and G) Currents recorded from the vesicle as in D and from the patch as in E. The calculated transmembrane potential was 140 mV (from −80 mV to +60 mV). The current density of CHO cells expressing Kv3.1 channels estimated from whole-cell recordings was about 10 pA/μm2, and single-channel conductance was 15 pS (14). This gave approximately 200 channels for the vesicle in F and 15 channels for the patch in G.
The shape of the spine-like protrusions on auditory neurons closely resembles the vesicles that are formed by suction of membrane into cell-attached patch pipettes (16–18). When negative pressure was gently and slowly applied to small patch pipettes in contact with CHO cells expressing Kv3.1 channels, the deformation of membrane in the tip of electrodes during seal formation could be clearly visualized by using Nomarski optics (Fig. 1D). Typically, a portion of cell membrane was withdrawn into the pipette to form a dome-like expansion with a narrow neck connecting this vesicle with the cell body. Depending on the amount of suction necessary to form a seal, the distance between the pipette-spanning dome and the tip ranged from 0 to 1.5 μm, within a factor of 1–3 of the size of the spine-like protrusions, although with increased suction it could extend up to 6 μm. The neck diameters (≈0.4–1.0 μm) were also comparable to those seen on the spine-like protrusions. Surprisingly, we found that the current recorded from patches with visible vesicles displayed rapid inactivation (Fig. 1F). In contrast, such inactivation was absent when vesicle formation was avoided by minimizing the negative pressure to generate a seal (Fig. 1 E and G). Notably, there was more than a 10-fold difference in the peak current amplitude with or without vesicle formation. These results suggest that the apparent inactivation depends on the geometry and area of the membrane patch in the pipette.
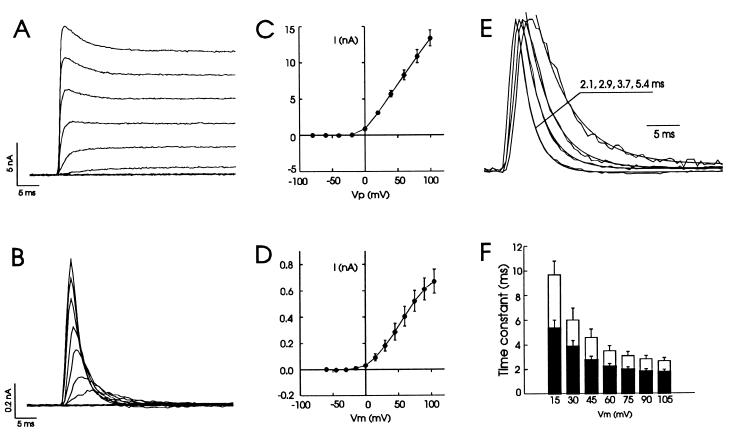
We further compared the properties of Kv3.1 channels recorded in the whole-cell configuration with those recorded in such cell-attached “vesicles.” Whole-cell patch-clamp recordings demonstrated that homomeric Kv3.1 channels conduct typical delayed rectifier outward currents (Fig. 2A), similar to those reported previously (14, 19). These currents have a threshold of activation of −20 mV and show little saturation in amplitude in response to large voltage steps (up to +140 mV) (Fig. 2 A and C). The inactivation of these currents is very limited and slow (Fig. 2A). In contrast, currents recorded in the cell-attached configuration with visible vesicles exhibited a transient activation that was followed by an apparent inactivation (Fig. 2B). In addition, the peak currents showed some degree of saturation at very positive potentials (Fig. 2D). The inactivation was very rapid and often complete within several milliseconds. This was strikingly different from the behavior of these channels in whole-cell recordings. Because the resting potential measured by whole-cell recordings without correcting the junctional potential was −44 ± 1.9 mV (n = 5), we assumed that the resting potentials were approximately −50 mV in intact CHO cells. Under such an assumption, the activation threshold was about the same as that in whole-cell recordings (Fig. 2D). Because currents recorded by using the nystatin-perforated patch technique (20) did not differ significantly from that by conventional whole-cell patch-clamping (14), the difference between whole-cell and cell-attached patch recordings is unlikely to involve intracellular mechanisms such as phosphorylation and dephosphorylation, which have been shown to regulate the inactivation of a number of voltage-gated ion channels (21, 22).
Figure 2.
Apparent inactivation of potassium currents mediated by Kv3.1 channels in cell-attached configuration. Superimposed current traces recorded in whole-cell (A) or cell-attached configuration (B) in response to a series of voltage steps. Voltage steps (Vp) ranged from −80 to +100 mV in increments of 20 mV for A. Assuming the resting potential was approximately −50 mV, the calculated transmembrane potentials (Vm) were from −60 to +105 mV with increments of 15 mV for B. Pipette holding potential was −70 mV for A and 0 mV for B. Averaged current–voltage relationships for those recordings as in A and B are shown in C (n = 12) and D (n = 8), respectively. (E) Current traces in B were normalized to the same height and superimposed. Smooth curves are single exponential fits to the decay phase of four representative traces recorded at +90, +45, +30, and +15 mV, and the time constants are shown from left to right. Also note that the rate of activation and inactivation were both accelerated as voltage steps increased. (F) A summary of time constants for apparent inactivation at voltages ranging from +15 to +105 mV in the presence of 3 mM (solid bars, n = 7; 5.33 ± 0.67 at +15 mV to 1.72 ± 0.23 ms at +105 mV) or 9 mM (open bars, 9.67 ± 1.13 ms to 2.65 ± 0.28) extracellular potassium. Leak currents were subtracted in this and subsequent figures.
The tip of recording pipettes imposes a diffusion restriction between the cell-attached vesicle and the soma. Exchange of ions between these two compartments would be expected to be affected not only by the size of the vesicle, but also by the size of the pipette tip that forms the neck, and by the density of intracellular organelles that may plug the neck region (17, 18). Although we do not have full control of all of these parameters, we determined whether apparent inactivation could be correlated with the size of pipette tips by making electrodes of different sizes with resistances that ranged from 2.8 to 12 MΩ. Cell-attached vesicles could be formed with all pipettes in this range, although, as noted above, their size depended on the amount of suction required for seal formation. As the electrode resistance, a parameter that partially reflects pipette tip size, was increased, the apparent inactivation became more rapid and there was a moderate correlation (γ = 0.55, n = 19) between electrode resistances and decay time constants.
To characterize the kinetics of this apparent inactivation, we fit the decay phase of these currents with single exponential functions. We found that the time constants decreased as the transmembrane potential was increased (Fig. 2 E and F). We also found that the decay time constants increased significantly at all test voltages when K+ in the recording pipette was raised from 3 to 9 mM (Fig. 2F). These observations suggest that the alteration in the behavior of potassium currents in the cell-attached mode could be related to the dynamic reequilibrium of K+ between the two sides of the membrane patch.
We hypothesized that the apparent inactivation results from a depletion of K+ from the vesicular compartment through Kv3.1 channels in the patch and that recovery from inactivation requires the slower refilling of this compartment by K+ flux from the cell body through the diffusion barrier bridging the two compartments. The apparent inactivation could also reflect the transient accumulation of K+ ions on the extracellular surface of vesicle in the recording pipette, reducing the driving force for K+ ions across the membrane.
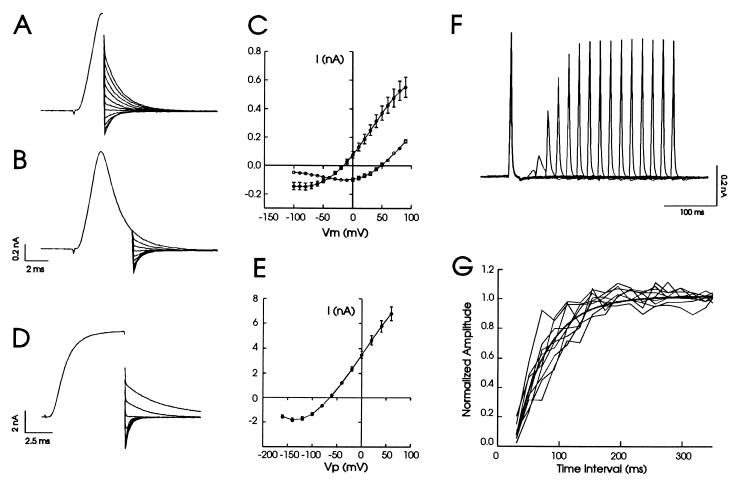
Comparison of tail currents measured immediately after the peak of the evoked currents with those recorded after currents had decayed by more than 75%, revealing that there was a large shift in the reversal potential of the current, from −20 to +50 mV (Fig. 3 A–C). This shift indicated that the rapid flux through potassium channels had caused a depletion of K+ ions in the vesicle and possibly an increase in K+ on the extracellular surface of the patch. The reversal potential at the peak shifted 50 mV from the true reversal of −70 mV obtained from whole-cell recording (Fig. 3 D and E), suggesting that significant redistribution of K+ ions had already taken place before the peak current was reached.
Figure 3.
The shift in the reversal potential associated with depletion of K+ and time course of K+ refill from cell body to vesicle. After a single voltage step from −50 to +90 mV, the transmembrane potential (Vm) was stepped to voltages ranging from −100 to +90 mV at the peak of the current (A) or at 5 ms after onset of voltage step. (B). Eleven tail traces around reversal potential are shown in each case, and the initial part of the current preceding tail currents are averaged and shown as a single trace for clarity. (C) A plot of the amplitudes of tail currents at the peak (solid circles, reversal potential −20 mV) and at 5 ms after onset of voltage step (open circles, reversal potential +50 mV) against individual repolarizing voltages. These curves were generated by paired comparisons in the same cells (n = 8). (D) Tail currents recorded in whole-cell configuration by using similar protocol as in A and B except the initial voltage step was from −70 to +40 mV and repolarizing voltages ranged from −160 to +60 mV. The holding potential was 0 mV for A and B and −70 mV for D. (E) A plot of the amplitude of whole-cell tail currents against repolarizing voltages revealed a reversal potential of −70 mV. Data from 14 cells were pooled to construct this graph. (F) An example recording using double-pulse protocol showed that as the interval between the first and second test pulse (20.5 ms per each pulse) was increased the current amplitude increased. Fifteen separate sweeps were superimposed. (G) The current amplitude to the second test pulse was normalized to that of the first test pulse and plotted against the time interval between two pulses. The smooth curve represents single exponential fit to the averaged data from nine cells. The time constant was 51.3 ± 4.9 ms.
To characterize the kinetics of refilling the vesicle by K+ diffusion from cell body, we used a double-pulse protocol, in which the first test pulse was used to deplete K+ in the vesicle and the second test pulse was delivered at increasing time intervals after the first test pulse. Fig. 3F shows a typical recording in which 15 test sweeps were superimposed. As the time interval was increased, the current amplitude to the second test pulse increased and reached the same height as control within about 100–200 ms. Fig. 3G plots the normalized peak amplitude of the second test pulse against the time interval between the first and second test pulse from a number of recordings. The refill kinetics could be fit by a single exponential curve with an average time constant of 51.3 ms. These experiments indicate that refilling is a relatively slow process compared with redistribution of K+ ions across the Kv3.1 channels.
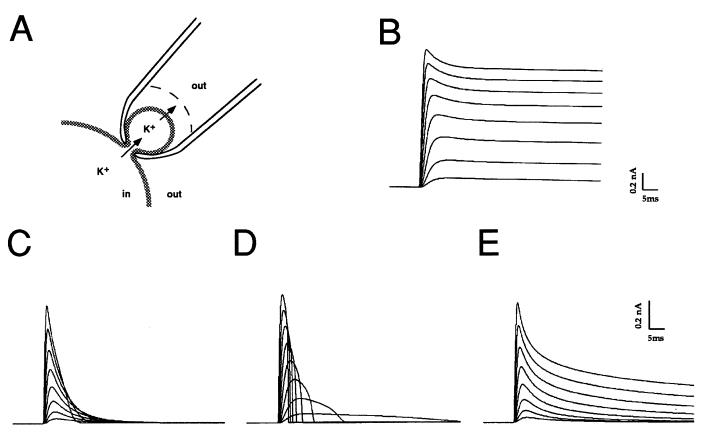
To test the relative roles of potassium ion depletion and accumulation, we constructed a computer model that simulates the flux of potassium ions from a spine-like structure into an unstirred layer in the extracellular space (Fig. 4A). The flux of potassium across the plasma membrane was simulated by using a model that we have used previously to model the voltage dependence and kinetics of Kv3.1 channels (14). Simulations were carried out for vesicles with neck diameters of 0.2–1.0 μm, with Kv3.1 current densities similar to those recorded in cell-attached patches. The diffusional flux of potassium ions through the neck of the vesicle (Jn) was given by the equation Jn = kdiff([Kcyt] − [Kv]), where [Kcyt] and [Kv] represent potassium concentrations in bulk cytoplasm and in the vesicle respectively, and kdiff is a constant determined by the bulk diffusion coefficient in the cytoplasm and the diffusional resistance of the vesicle neck (15). A similar equation, with diffusional constant kext, represented flux between the external medium and the unstirred layer around the vesicle. If the potassium concentrations inside and outside the vesicle were maintained equal to the bulk cytoplasmic and extracellular values (120 mM and 3 mM respectively, by giving very high values to kdiff and kext), the time course of the simulated currents (Fig. 4B) closely matched those recorded using the whole-cell patch clamp technique (Fig. 1A). If, however, either the diffusional current across the neck of the vesicle or exchange with the bulk external medium was reduced, Kv3.1 currents across the vesicle decayed more rapidly (Fig. 4 D and E). Neither condition alone, however, was found to mimic the decay of currents observed in the present experiments. Restricted potassium flux across the vesicle neck alone resulted in currents that did not decay exponentially during a depolarization to positive potentials (Fig. 4D). In contrast, restricted exchange between the unstirred layer and bulk external medium produced currents that decayed with an approximately exponential time course, but the time course of decay was independent of the voltage step (Fig. 4E). Only when depletion of potassium from within the vesicle was accompanied by a build-up at the external face of the vesicle membrane could currents that matched the cell-attached patch-clamp data be simulated (Fig. 4C). These currents decayed exponentially during a maintained depolarization, and, as in the experimental data (Fig. 2B), the time constant of decay increased markedly as the potential across the spine was increased from +10 mV to +150 mV. This behavior was observed with values for kdiff < 0.017 μm3 ms−1, which correspond to the rates of diffusional reequilibration measured experimentally in spines of hippocampal pyramidal cells (15). Currents also decayed more rapidly with increasing density of Kv3.1 channels in the vesicle. A 10-fold increase in Kv3.1 current density (gK = 80 nS) over that in Fig. 4C (gK = 8 nS) caused a greater than 8-fold increase in the rate of apparent inactivation. In contrast, a 10-fold reduction in current density (gK = 0.8 nS) markedly slowed the inactivation but did not eliminate it. These simulations suggest, therefore, that the behavior of the potassium currents in the spine-like compartments is determined by dynamic reequilibrium of potassium concentrations on both sides of the membrane.
Figure 4.
Simulations of Kv3.1 current in a spine-like compartment. (A) Drawing of the geometry of the simulated configuration of the spine-like compartment, with potassium ion flux from the bulk cytoplasm into the head of the spine, and across the membrane into an unstirred layer on the surface of the external face of the membrane. (B) Simulations of Kv3.1 currents with fixed intracellular and intracellular potassium concentrations (120 and 3 mM, respectively). (C) Simulations of Kv3.1 current in a spine with a diameter of 0.9 μM, with restricted diffusion from bulk cytoplasm to spine and from the unstirred layer (thickness 50 nm) and bulk extracellular medium (kdiff and kext = 0, see Methods). (D) Simulations of Kv3.1 current in a spine of the same size with a fixed extracellular potassium concentration (3 mM, thickness of unstirred layer = 500 μm, kext =1.0) but with restricted diffusion from bulk cytoplasm to spine (kdiff = 0). (E) Simulations of Kv3.1 current with a fixed intracellular potassium concentration (120 mM) within the spine, but with restricted diffusion between the unstirred layer (thickness 50 nm) and bulk extracellular medium (kext = 0). Currents in all cases are shown in response to voltage steps from +10 mV to +150 mV in 20 mV increments.
DISCUSSION
We have described a morphological structure that exists at the somatic membranes of bushy cells of the cochlear nucleus. The spine-like structures in these cells have characteristics similar to the classical spines seen on the dendrites of other central neurons, but they appear to be located perisynaptically rather than directly opposed to the presynaptic terminals. We have shown that these structures are particularly enriched in Kv3.1 channels. Using Kv 3.1-transfected cells, we have demonstrated that the behavior of ionic currents in such spine-like membrane compartments may differ from that of the whole-cell. In particular, the restricted availability of conducting ions in the vicinity of these channels can produce rapidly inactivating currents from normally noninactivating channels. Such apparent inactivation requires a relatively slow diffusion rate (time constant, ≈50 ms) for ions to equilibrate between the spine-like vesicle and the bulk cytoplasm, similar to that observed directly for spines in hippocampal neurons (15). Moreover, we have created a mathematical model simulating potassium ion flux through structures that match those of the postsynaptic membranes. We have found that there is a close agreement of experimental and theoretical data. Taken together, our results strongly suggest that activation of Kv3.1 channels in these spine-like protrusions can cause a rapid depletion of potassium ions from the “vesicle” compartment.
Although it is not possible to measure potassium currents directly in the perisynaptic structures of bushy cells or in other restricted compartments such as presynaptic boutons, the dimension of these structures is similar to or smaller than those created by our cell-attached patch pipettes. In common with our artificial spine-like vesicles, these structures have a long and thin neck and a relatively large surface/volume ratio. Thus, compartmentalized ionic transients may occur in these structures independent of events in cellular compartments such as the soma or dendritic shafts (2–11). Depletion of potassium ions from such spine-like vesicles during normal firing is unlikely to influence the initial activation of the high-threshold Kv3.1 currents or to delay repolarization on the time scale of single synaptic inputs. It may, however, influence the response of the synapses to repetitive stimulation.
The specific function of the Kv3.1-containing spine-like structures on bushy cells is not yet known. One possible function for localized inactivating potassium transients is to cause a broadening of action potentials in response to a train of stimuli, and, as a consequence, localized changes in intracellular calcium levels may be induced. For example, for certain neurons, the postsynaptic calcium signal in response to synaptic inputs can be very heterogeneous in different dendritic spines from the same cell (2–11), and synaptic specificity associated with long-term potentiation (LTP) (23) may be achieved thorough spines with particularly high neck resistivity. Presynaptically, action potential broadening may also increase the release of neurotransmitter. Although it is not yet known whether potassium depletion from postsynaptic spines or presynaptic boutons contributes to the long-lasting changes in synaptic strength, a blockade of potassium channels has been shown to induce LTP (23). Furthermore, during neuronal development or following acutely induced LTP, dendritic spines have been reported to undergo profound modifications in the size and diameter of their heads and necks as well as in the length of their necks (24, 25). The spine head usually becomes enlarged whereas the length of the neck is reduced. These morphological changes are likely important for synaptic plasticity. It remains to be determined whether similar changes occur in the Kv3.1-containing spine-like protrusions of the bushy cells and whether these contribute to localized ionic transients during synaptic transmission or to regulating synaptic development and plasticity.
Acknowledgments
We thank Drs. I. B. Levitan, and F. J. Sigworth, for their critical comments. This work was supported by National Institutes of Health grants (to T.M.P., I.S., and L.K.K.). L.-Y.W. was supported in part by postdoctoral fellowships from the James Hudson Brown–Alexander B. Coxe foundation and the Eppley foundation for Research Inc.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Hoffman D A, Magee J C, Colbert C M, Johnston D. Nature (London) 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 2.Yuste R, Denk W. Nature (London) 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- 3.Petrozzino J J, Miller L D P, Connor J A. Neuron. 1995;14:1223–1231. doi: 10.1016/0896-6273(95)90269-4. [DOI] [PubMed] [Google Scholar]
- 4.Guthrie P B, Segal M, Kater S B. Nature (London) 1991;354:76–79. doi: 10.1038/354076a0. [DOI] [PubMed] [Google Scholar]
- 5.Muller W, Connor J A. Nature (London) 1991;354:73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- 6.Gamble E, Koch C. Science. 1987;236:1311–1315. doi: 10.1126/science.3495885. [DOI] [PubMed] [Google Scholar]
- 7.Malinow R, Otmakhov N, Blum K I, Lisman J. Proc Natl Acad Sci USA. 1994;91:8170–8174. doi: 10.1073/pnas.91.17.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy T H, Baraban J M, Wier W G, Blatter L A. Science. 1994;263:529–532. doi: 10.1126/science.7904774. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe D B, Fisher S A, Brown T H. J Neurobiol. 1994;25:220–233. doi: 10.1002/neu.480250303. [DOI] [PubMed] [Google Scholar]
- 10.Roberts W M, Jacobs R A, Hudspeth A J. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon S M, Linas R R. Biophys J. 1985;48:485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perney T M, Kaczmarek L K. J Comp Neurol. 1997;386:178–202. [PubMed] [Google Scholar]
- 13.Perney T M, Marshall J, Martin K A, Hockfield S, Kaczmarek L K. J Neurophysiol. 1992;68:756–766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- 14.Kanemasa T, Gan L, Perney T M, Wang L-Y, Kaczmarek L K. J Neurophysiol. 1995;74:207–217. doi: 10.1152/jn.1995.74.1.207. [DOI] [PubMed] [Google Scholar]
- 15.Svoboda K, Tank T D, Denk W. Science. 1996;272:716–719. doi: 10.1126/science.272.5262.716. [DOI] [PubMed] [Google Scholar]
- 16.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch Eur J Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 17.Sokabe M, Sachs F. J Cell Biol. 1990;111:599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruknudin A, Song M J, Sachs F. J Cell Biol. 1991;112:125–134. doi: 10.1083/jcb.112.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Critz S D, Wible B A, Lopez H S, Brown A M. J Neurochem. 1993;60:1175–1178. doi: 10.1111/j.1471-4159.1993.tb03273.x. [DOI] [PubMed] [Google Scholar]
- 20.Horn R, Marty A. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levitan I B. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 22.Jonas E A, Kaczmarek L K. Curr Opinion Neurobiol. 1996;6:318–323. doi: 10.1016/s0959-4388(96)80114-0. [DOI] [PubMed] [Google Scholar]
- 23.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 24.Papa M, Bundman M C, Greenberger V, Segal M. J Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K S, Schottler F, Oliver M, Lynch G. J Neurophysiol. 1980;44:247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]