Abstract
ATF4 is an essential regulator in osteogenesis as well as in stress responses to the endoplasmic reticulum (ER). We addressed a question: Does ER stress to osteoblasts upregulate ATF4 expression? If so, do they exhibit ATF4-mediated bone remodeling or apoptosis? ER stress, induced by Thapsigargin and tunicamycin, elevated a phosphorylated form of eIF2α and ATF4, but the cellular fate depended on treatment duration. The treatment for 1 h, for instance, activated Runx2, and type I collagen, while the treatment for 24 h induced apoptosis. Our observations suggest that there is a threshold for ER stress and osteoblasts present a bi-phasic pattern of their fate.
Keywords: osteoblasts, ER stress, ATF4, apoptosis, bone remodeling
1. Introduction
Differentiation of osteoblasts results from a complex molecular interplay involving the action of various transcription factors such as Runx2, Osterix, and ATF4. In osteoblasts, whose primary role in bone formation is building a calcium enriched ECM, expression of ATF4 is coordinately regulated during the course of their differentiation [1]. It has been shown that a mutant mouse lacking the ATF4 gene is unable to form any part of a mature cytoskeleton [1].
ER stress is induced in many eukaryotic cells by a multitude of causes including protein misfolding, UV, viral infection, and nutritional deprivation [2]. It leads to preferential translational expression by a mechanism involving phosphorylation of eukaryotic translation initiation factor-2α (eIF2α) [3]. The phosphorylated eIF2α acts - in turn - to activate the translation of ATF4. When ER stress is excessive, activation of ATF4 expression results in apoptosis. An intriguing question is the fate of osteoblasts that utilize ATF4 as a critical transcription factor during osteogenesis.
ATF4 is known to stimulate ATF3 as well as osteoblast specific genes, while it can elevate expression of CHOP and facilitates to apoptosis [4]. CHOP is necessary for bone development and its anabolic effects have been reported to occur in response to bone morphogenetic protein [5, 6], although overexpression of CHOP resulted in impaired bone formation. A specific question addressed herein is whether ER stress would stimulate ATF4 expression in osteoblasts, and if so, whether upregulation of ATF4 would result in ATF4/CHOP-mediated apoptosis or activation of ATF4-dependent bone remodeling. We hypothesized that the fate of osteoblasts would be bi-phasic depending on stress intensity analogous to mechanical stimulation, where excessive forces negatively influence anabolic responses but appropriate stimulations can enhance bone formation [7]. In the present studies we used Thapsigargin and tunicamycin as an inducer of ER stress.
2. Materials and methods
2.1. Cell culture
MC3T3 osteoblast-like cells and their C4 clone [8] were cultured in αMEM containing10% FBS and antibiotics. We used these two lines, since the former was reported less sensitive to an osteogenic agent than the latter [9]. In our PCR assay the C4 clone presented 1240 fold (N = 4) higher expression of Osteocalcin mRNA. Note that MC3T3 cells and the C4 clone were used in the experiments for Figs. 1–3 and Figs. 4–6, respectively. Approximately 4 x 105 cells were treated in a plastic dish with Thapsigargin (Santa Cruz Biotech.) or tunicamycin (MP Biomedicals) at varying concentrations. The treatment duration was continuous for up to 24 h (T 24h), or in the beginning for 5 min to 3 h.
Figure 1.
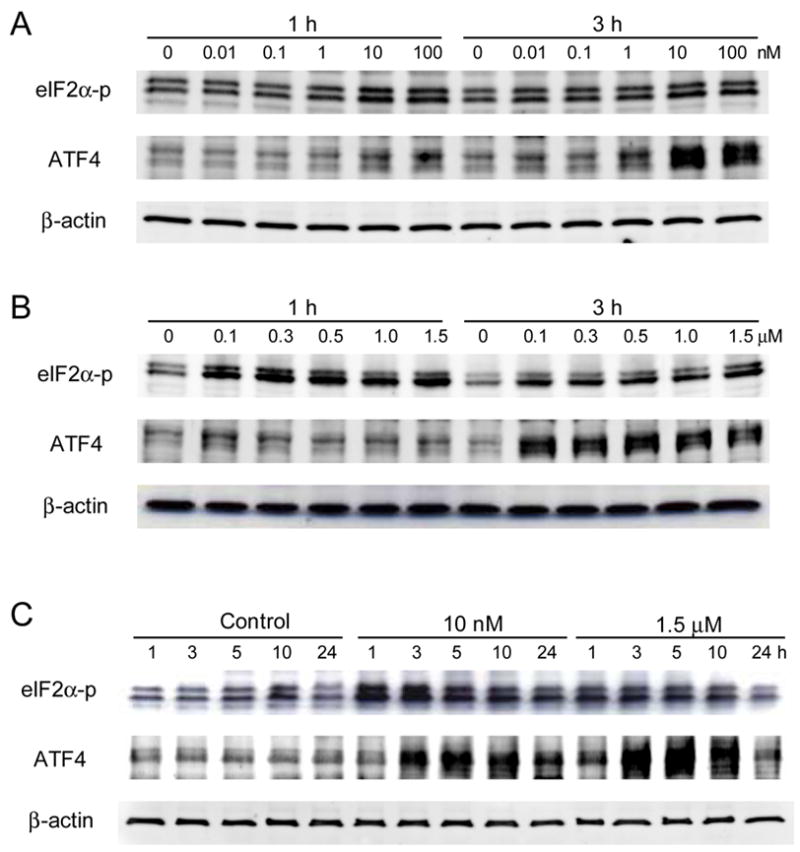
Protein expression of ATF4 and eIF2α-p in response to Thapsigargin in MC3T3 cells. (A) Responses at 0.01 to 100 nM for 1 and 3 h. (B) Responses at 100 nM to 1.5 μM for 1 and 3 h. (C) Responses at 10 nM and 1.5 μM for 1 to 24 h.
Figure 3.
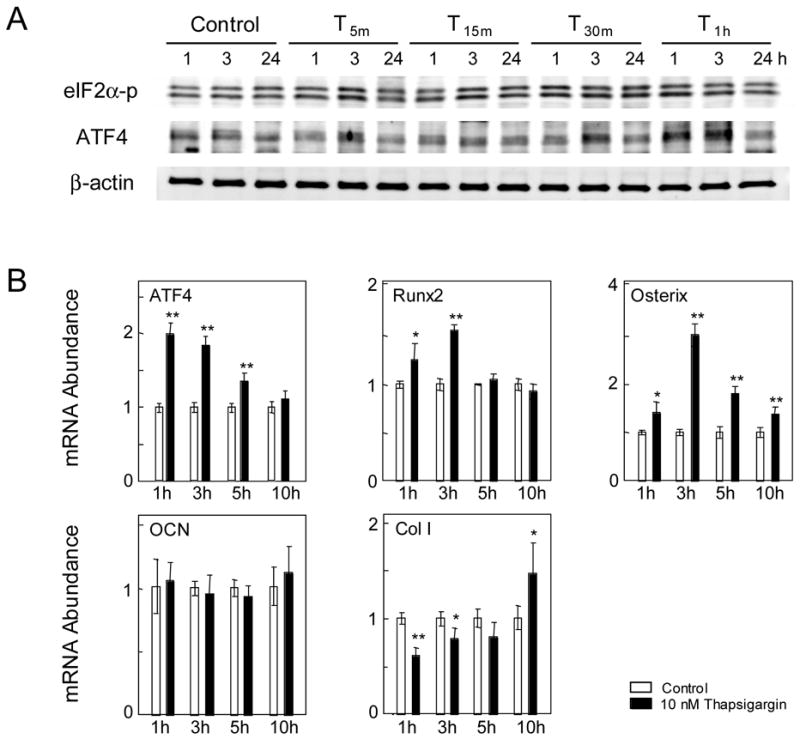
Responses to short exposure to Thapsigargin in MC3T3 cells. (A) ATF4 and eIF2α-p proteins in response to T 5m, T 15m , T 30m, and T 1h. (B) Levels of ATF4, Runx2, Osterix, Osteocalcin (OCN), and type I collagen (Col I) mRNA in response to T 1h.
Figure 4.
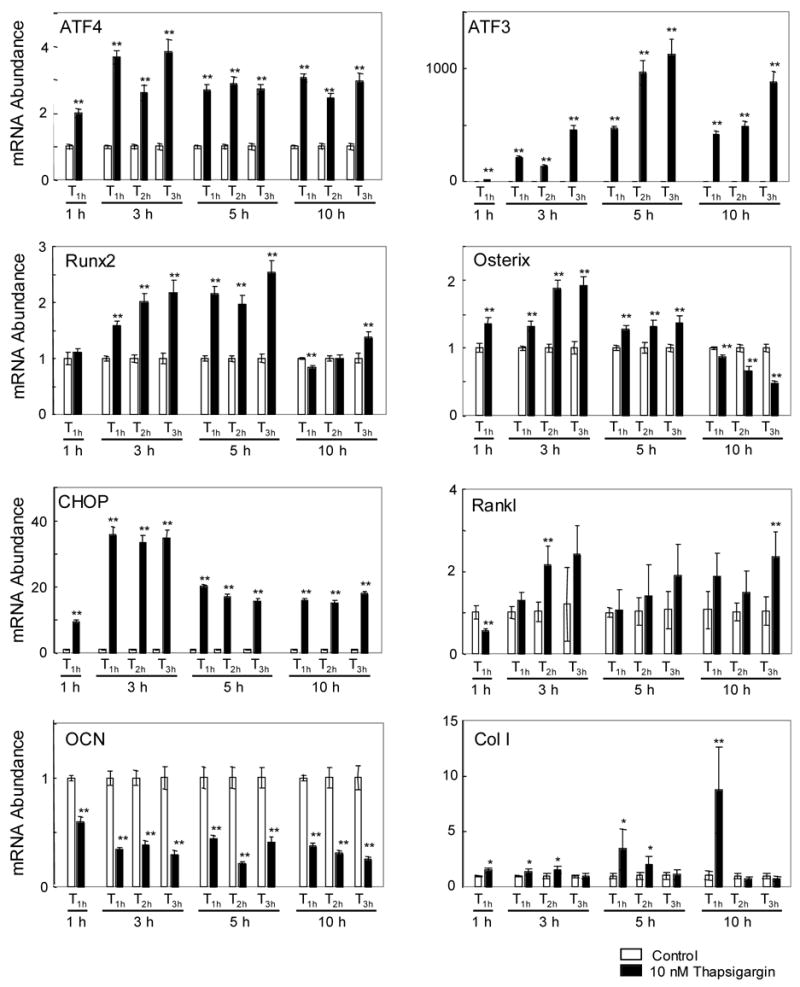
Messenger RNA levels of ATF4, ATF3, Runx2, Osterix, CHOP, Rankl, Osteocalcin (OCN), and type I collagen (Col I) for the C4 clone in response to Thapsigargin (T1h, T2h, and T3h).
Figure 6.
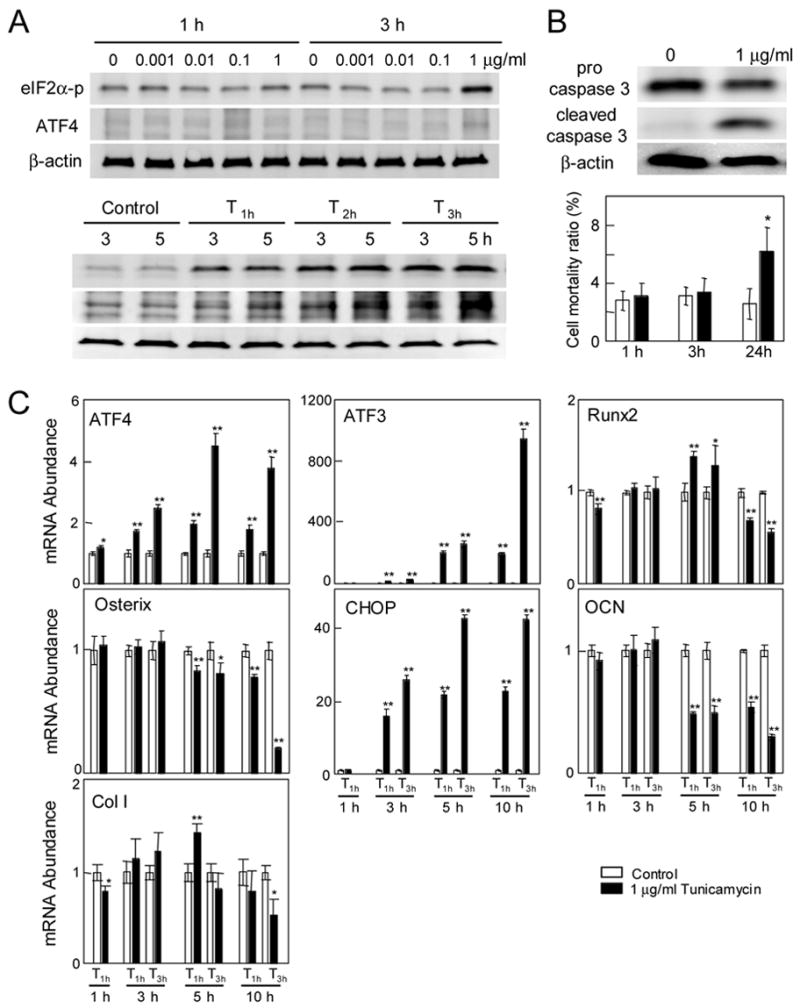
Response to tunicamycin in the C4 clone. (A) Responses to tunicamycin at 0–1 μg/ml, and at 1 μg/ml (T1h, T2h, and T3h). (B) Expression of pro- and cleaved caspase 3 for T 24h, and cell motility ratio in response to 1 μg/ml tunicamycin. (C) Messenger RNA levels of ATF4, ATF3, Runx2, Osterix, CHOP, Osteocalcin (OCN), and type I collagen (Col I) for the C4 clone in response to tunicamycin (T1h, and T3h).
2.2. Real-time PCR
Total RNA was extracted using an RNeasy plus mini kit (Qiagen). Reverse transcription was conducted, and real-time PCR was performed using ABI 7500 with SYBR green PCR kits (Applied Biosystems). The mRNA level of the genes listed Table 1 was determined. GAPDH was used for internal control, and the results were interpreted using a ΔCT method.
Table 1.
Real-Time PCR primers
| Gene | forward primer | backward primer |
|---|---|---|
| ATF3 | 5′-CGAAGACTGGAGCAAAATGATG-3′ | 5′-CAGGTTAGCAAAATCCTCAAATAC-3′ |
| ATF4 | 5′-TGGCGAGTGTAAGGAGCTAGAAA -3′ | 5′-TCTTCCCCCTTGCCTTACG-3′ |
| CHOP | 5′-CCACCACACCTGAAAGCAGAA-3′ | 5′-GGTGCCCCCAATTTCATCT-3′ |
| Col I | 5′-AAACTCCCTCCACCCCAATCT-3′ | 5′-TTTGGGTTGTTCGTCTGTTTCC-3′ |
| Osteocalcin | 5′-CCGGGAGCAGTGTGAGCTTA-3′ | 5′-AGGCGGTCTTCAAGCCATACT-3′ |
| Osterix | 5′-CCCTTCTCAAGCACCAATGG -3′ | 5′-AGGGTGGGTAGTCATTTGCATAG -3′ |
| RANKL | 5′-CCGAGACTACGGCAAGTACC-3′ | 5′-GCGCTCGAAAGTACAGGAAC-3′ |
| Runx2 | 5′-AAATGCCTCCGCTGTTATGAA-3′ | 5′-GCTCCGGCCCACAAATCT-3′ |
| GAPDH | 5′-TGCACCACCAACTGCTTAG-3′ | 5′-GGATGCAGGGATGATGTTC-3′ |
2.3. Immunoblots
Cells were lysed in a RIPA lysis buffer containing inhibitors for proteases and phosphatases. Isolated proteins were fractionated using 10–15 % SDS gels and electro-transferred to Immobilon-P membranes (Millipore). Anti-rabbit IgG conjugated with HRP, and anti caspase-3 antibodies (Cell Signaling Tech.), and antibodies against ATF4, ATF3, and CHOP (Santa Cruz Biotech.) were used. Anti eIF2α (pS52), anti mouse IgG conjugated with HRP, and anti β-actin were obtained from Biosource, Amersham, and Sigma, respectively.
2.4. Assay for cellular proliferation and cell death
Cells were treated with Thapsigargin or tunicamycin for 24 h. The ratio of cell numbers was defined as the number of harvested cells divided by the number of seeded cells. Cells were stained with trypan blue, and the number of live and dead cells was counted using a hemacytometer.
3. Results
3.1. Induction of eIF2α-p and ATF4 proteins by Thapsigargin in MC3T3 cells
A series of Thapsigargin concentrations were employed to evaluate expression of eIF2α-p and ATF4 proteins. At lower dosages of 0.01 and 0.1 nM, no detectable upregulation was observed (Fig. 1A). At 10 and 100 nM, however, their levels were elevated after 1 h and 3 h (Fig. 1A). At 100 nM and the higher concentrations, they were further increased at 1 h and 3 h (Fig. 1B). Using the lowest (10 nM) and the highest (1.5 μM) concentrations that enhanced the level of eIF2α-p and ATF4, the 24-h expression profile was determined. The level of eIF2α-p was increased at 1, 3, 5, and 10 h, and ATF4 elevated at 3, 5, and 10 h (Fig. 1C).
3.2. Cell death and apoptosis by Thapsigargin in MC3T3 cells
After 24-h treatment some cells appeared to be dead, and the number of cells was reduced by 25.3 ± 4.0% in response to 10 nM Thapsigargin (Figs. 2A & 2B). In addition, the number of dead cells with respect to the total number of cells was increased with Thapsigargin: 2.5 ± 0.5% (control), and 20.1 ± 1.6% (10 nM) (Fig. 2C).
Figure 2.
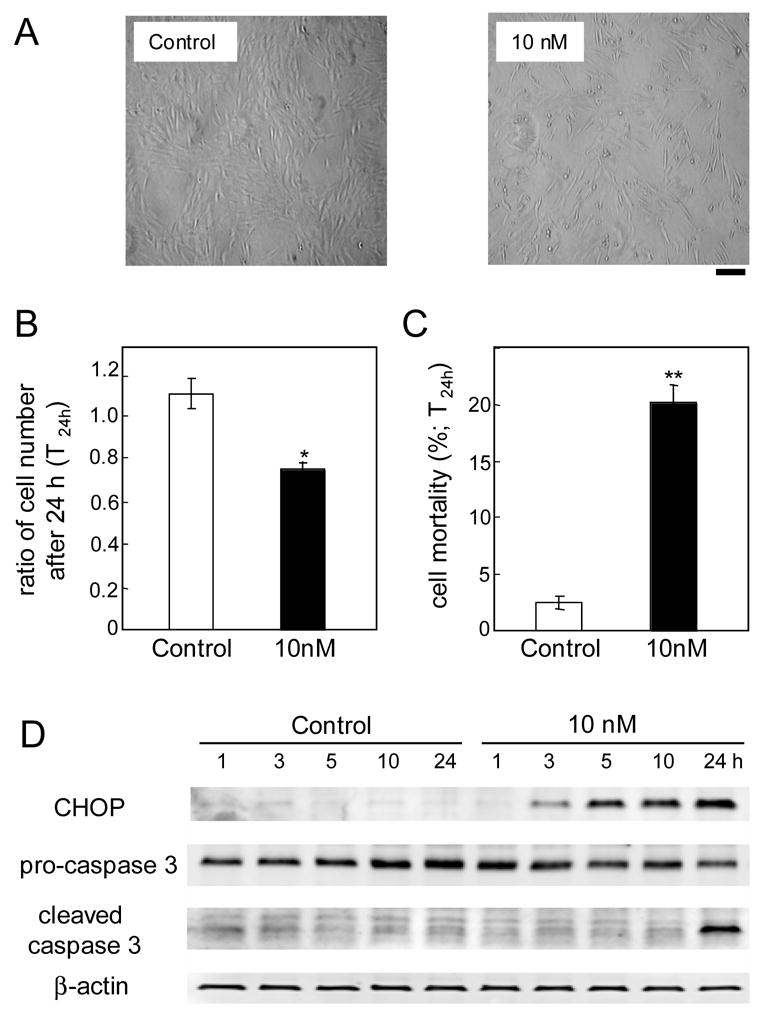
Apoptotic cell death for continuous Thapsigargin treatments in MC3T3 cells. (A) Cellular images. Black bar = 100 μm. (B) Ratio of cell numbers 24 h after a continuous treatment. (C) Ratio of the number of dead cells with respect to the total number of cells. (D) CHOP, pro-caspase 3, and cleaved caspase 3 proteins in response to 10 nM Thapsigargin.
To examine induction of apoptosis, we determined the protein levels of CHOP, pro-caspase 3 (inactive), and cleaved caspase 3 (active) (Fig. 2D). The level of CHOP started to increase after 3 h and it became higher for a longer incubation. Pro-caspase 3 level was not significantly altered, but the level of an active form was increased after 24 h.
3.3. Non apoptotic pathway with short exposure to Thapsigargin in MC3T3 cells
To examine the effect of shorter exposure to Thapsigargin, cells were incubated at 10 nM for 5 min (T 5m), 15 min (T 15m), 30 min (T 30m), and 1 h (T 1h). All treatments raised the level of eIF2α-p, while ATF4 was elevated only in T 30m and T 1h (Fig. 3A). In response to T 1h, the mRNA level of ATF4, Runx2, Osterix, and type I collagen was elevated 1, 3, 5, or 10 h after the onset of the treatment while the level of osteocalcin mRNA was unchanged (Fig. 3B).
3.4. Transcriptional activation with short exposure to Thapsigargin in C4 cells
Unlike MC3T3 cells, the C4 clone has a strong osteogenic potential and therefore a possibility of pursuing a different cellular fate. We therefore re-examined the effects of Thapsigargin on transcriptional activation using the C4 clone (Fig. 4). In response to T1h at 10 nM, no significant difference in the cell number or mortality was observed between the control cells and the treated cells (data not shown). Out of 4 transcription factors in Table 1, the level of ATF4 mRNA was elevated 2 – 4 fold throughout the treatments. ATF3 mRNA was upregulated 100 – 1000 times. An increase in Runx2 mRNA started at 3 h and peaked at 5 h, while Osterix mRNA was enhanced at 1 h. The mRNA level of CHOP was raised 10 – 35 fold, and Rankl mRNA was significantly up 3h for T2h and 10 h for T3h. . The mRNA level of type 1 collagen was elevated 5h and 10 h for T1h. In spite of upregulation of ATF4 and Runx2 mRNA, the level of Osteocalcin mRNA was decreased down to 20 to 60% of the control level
3.5. Western analysis in response to 10 nM Thapsigargin in C4 cells
Like MC3T3 cells, C4 cells showed upregulation of eIF2α-p and ATF4 in response to T 1h (Fig. 5A). Note that MC3T3 cells and their C4 clone cells exhibited two bands and a single band for eIF2α-p, respectively. The protein level of ATF3 and CHOP was transiently elevated for T 1h treatment and diminished at 24 h. When the treatment was continuous (T 24h), the level of ATF3 and CHOP was sustained elevated (Fig. 5A). The temporal alteration of ATF4 mRNA and protein levels supports translational regulation, showing that the increase in the protein level was significantly higher than that in the mRNA level (Fig. 5B).
Figure 5.
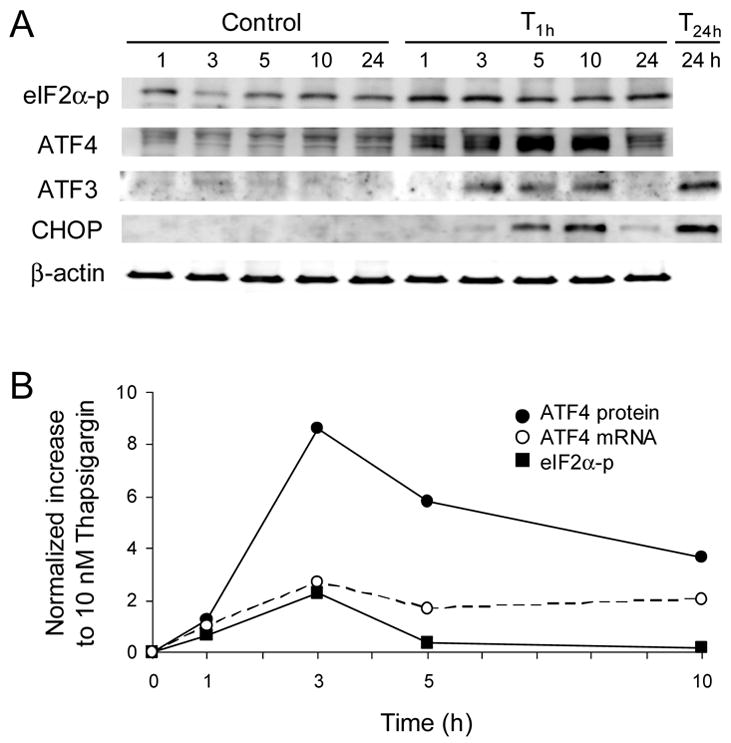
Protein and mRNA expression for the C4 clone in response to 10 nM Thapsigargin (T1h). (A) Expression of eIF2α-p, ATF4, ATF3, and CHOP proteins. (B) Temporal fold changes of ATF4 protein, eIF2α-p protein, and ATF4 mRNA.
3.6. Response to tunicamycin in C4 cells
Thapsigargin is an inhibitor of Ca2+ ATPase, while tunicamycin induces ER stress by inhibiting protein glycosylation. The Western analysis revealed that the protein level of eIF2α-p and ATF4 was elevated by 1 μg/ml tunicamycin (Fig. 6A). Induction of eIF2α-p took place 3 h after the treatment with tunicamycin, while it happened 1 h with Thapsigargin. The treatment with 1 μg/ml tunicamycin for short time (T 1h, and T 3h) did not stimulate cell death, but the continuous treatment (T 24h) activated caspase 3 and increased cell death (Fig. 6B). The mRNA expression of ATF4, ATF3, CHOP, and Osteocalcin was altered similarly to the response to Thapsigargin (Fig. 6C). However, the increase in Runx2 and type I collagen was smaller and limited to 5 h after the treatment. The mRNA level of Osterix, which was elevated approximately 2 fold with Thapsigargin, was not upregulated with tunicamycin.
4. Discussion
The present study demonstrates that ER stress by Thapsigargin and tunicamycin leads to two distinctive pathways – apoptosis and regulation of osteoblastogenesis. This bi-phasic response was dependent on exposure time to ER stress: 1 h treatment favored the pathway toward transcriptional activation of the selected genes such as Runx2 and type I collagen as opposed to 24 h for the apoptotic pathway. Regulation of ATF4 appeared to be involved at both transcriptional and translational levels. Induction of ATF3 mRNA and CHOP mRNA was observed in all treatments. These results indicate a molecular mechanism that is sensitive to intensity of ER stress and differentially regulates a pathway of osteoblasts.
The unique feature of ER stress to osteoblasts, revealed in this study, was induction of genes involved in bone remodeling. Unlike a continuous exposure of ER stress leading to apoptosis, a short exposure to Thapsigargin enhanced not only ATF4 but also an mRNA level of Rankl and transcriptional regulators such as Runx2 and Osterix. Rankl is a critical cytokine required for bone resorption through activation of osteoclasts. Our results are consistent with a previous report showing that Rankl expression is elevated through the binding of ATF4 to its regulatory DNA sequences [10]. Runx2 dictates osteoblastogenesis through interactions with numerous regulatory proteins including Osterix, which is essential for calcification and bone formation. It is reported that the ATF3 mRNA level was upregulated during chondrocyte differentiation and ATF3 overexpression activated a Runx2-dependent promoter [11]. Therefore, the observed upregulation of Runx2 and Osterix might be linked to elevation of ATF3.
Thapsigargin is a known inhibitor of Ca2+ATPase in ER, while tunicamycin is an inhibitor of pretein glycosylation [12]. Induction of apoptosis by the two agents has been reported in various cells, and the results here are consistent with the ATF4/CHOP medicated apoptosis [4, 13]. Regarding transcriptional activation for bone remodeling, our results on mRNA expression of Osterix and type I collagen indicate that Thapsigargin is a more potent activator than tunicamycin.
Despite the observed upregulation of ATF4 and Runx2, Osteocalcin transcription was not activated in either cell line by Thapsigargin or tunicamycin. The promoter of the mouse Osteocalcin gene contains two binding motifs (OSE1, and OSE2), and its transcription is activated through synergistic binding of ATF4 and Runx2 to them [14]. ATF3 is a negative regulator that shares the consensus binding motif with a family of ATF factors [15], and therefore elevation of ATF3 might abolish the stimulatory role of ATF4. Although the level of Osteocalcin mRNA was decreased from 1 h in the C4 clone, its further downregulation from 3 h is consistent with an expression pattern of ATF3.
Phosphorylation of eIF2α is important in skeletal development, since a mutation in the ER kinase responsible for its phosphorylation is shown to cause Wolcott-Rallison syndrome that exhibit skeletal dysplasias [16]. In summary, this study demonstrates that ER stress driven by Thapsigargin and tunicamycin leads to ATF4/CHOP mediated apoptosis or stimulation of the critical genes involved in bone development. Understanding the bi-phasic molecular mechanism would contribute to future disease treatments and therapies for the promotion of bone health.
Acknowledgments
The authors appreciate George Malacinski’s critical reading of the manuscript. This study was supported NIHR01AR50008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and essential regulator of osteoblast biology: Implication for Coffin-Lowry Syndrome. Cell. 2004;117:387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 2.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 3.Vattem KM, Wek RC. Reinitation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes & Development. 2006;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira RC, Stadmeyer LE, Smith DL, Rydziel S, Canalis E. CCAAT/Enhancer binding protein homologous protein (CHOP) decreases bone formation and causes osteopenia. Bone. 2006 doi: 10.1016/j.bone.2006.09.028. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirakawa K, Maeda S, Gotoh T, Hayashi M, Shinomiya K, Ehata S, Nishimura R, Mori M, Onozaki K, Hayashi H, Uematsu S, Akira S, Ogata E, Miyazono K, Imamura T. CCAAT/Enhancer binding protein homologous protein (CHOP) regulates osteoblasts differentiation. Mol Cell Biol. 2006;26:6105–6116. doi: 10.1128/MCB.02429-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jee WSS. Principles in bone physiology. J Musculoskel Neuron Interact. 2000;1:11–13. [PubMed] [Google Scholar]
- 8.Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11:1103–1113. doi: 10.1210/mend.11.8.9955. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 10.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Valsse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 11.James CG, Woods A, Underhill TM, Beier F. The transcription factor ATF3 is upregulated during chondrocyte differentiation and represses cyclin D1 and A gene transcription. BMC Mol Biol. 2006;7:30. doi: 10.1186/1471-2199-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279:49420–49429. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- 13.Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gonzalez-Feria L, Piris MA, Iovanna JL, Guzman M, Velasco G. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9:301–312. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995;15:1858–1869. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez AB, Wang C, Huang CC, Yaman I, Li Y, Chakravarty K, Johnson PF, Chiang CM, Snider MD, Wek RC, Hatzoglou M. A feedback transcriptional mechanism controls the level of the arginine/lysine transporter cat-1 during amino acid starvation. Biochem J. 2007;402:163–173. doi: 10.1042/BJ20060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2a kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


