Abstract
Neu differentiation factor (NDF/neuregulin) is widely expressed in the central and peripheral nervous systems, where it functions as a mediator of the interactions between nerve cells and Schwann, glia, oligodendrocyte, and muscle cells, to control cellular proliferation, differentiation, and migration. NDF binds to two receptor tyrosine kinases, ErbB-3 and ErbB-4. Here we demonstrate that NDF and its ErbB-4 receptor are highly reactive to changes in ambient neuronal activity in the rodent brain in a region-selective manner. Generation of epileptic seizures by using kainic acid, a potent glutamate analog, elevated levels of NDF transcripts in limbic cortical areas, hippocampus, and amygdala. Concomitantly, ErbB-4 mRNA was increased with a similar spatial distribution, but transcription of the other NDF receptor, ErbB-3, did not change. A more moderate stimulation, forced locomotion, was accompanied by an increase in NDF transcripts and protein in the hippocampus and in the motor cortex. Similar changes were found with ErbB-4, but not ErbB-3. Last, a pathway-specific tetanic stimulation of the perforant path, which produced long-term potentiation, was followed by induction of NDF expression in the ipsilateral dentate gyrus and CA3 area of the hippocampus. Taken together, these results indicate that NDF is regulated by physiological activity and may play a role in neural plasticity.
Keywords: central nervous system, neural plasticity, tyrosine kinase
Polypeptide growth factors play a central role in a variety of environmentally induced structural changes in the cortex and hippocampus of adult brain (1–3). For example, various treatments that induce seizure cause an increase in the expression of NGF in hippocampus (4). An even more dramatic and rapid elevation of brain-derived neurotrophic factor (BDNF) mRNA was observed after limbic seizures (1). These observations raise the question as to whether other polypeptide factors that are expressed in the central nervous system are also affected by physiological activity. This possibility is especially relevant to a family of soluble factors, variably named Neu differentiation factors (NDFs) (5), heregulins (6), acetylcholine receptor inducing protein (ARIA) (7), and GGF (8), collectively renamed neuregulins (9), that has been recently identified as ligands of ErbB receptor tyrosine kinase. All of the isoforms of NDF/neuregulin are encoded by a single gene located on human chromosome 8 (10). However, alternative splicing generates more than 13 isoforms that differ in their mosaic structure. Most important is an intrinsic EGF-like domain that is sufficient for receptor binding and has two variants: α and β. Both groups of isoforms bind directly to two types of receptors, ErbB-3 (11, 12) and ErbB-4 (12, 13). Whereas the cytoplasmic domain of ErbB-4 displays a tyrosine-specific kinase activity, the corresponding portion of ErbB-3 is devoid of catalytic function (14). Nevertheless, because NDF/neuregulin induces heterodimerization between ErbB-3 and ErbB-2, the latter can reconstitute signaling by the neuregulins even in cells that do not express ErbB-4 (15, 16).
The NDF/neuregulin family is widely expressed during embryonic development in two major locations: the nervous systems and the mesenchymal portion of parenchymal organs (10, 17). Both α and β isoforms are expressed in mesenchymal cells, where they presumably function as mediators of mesenchyme–epithel interactions. Likewise, the neural isoforms of the neuregulins, which are mostly of the β isoform, also play a role in cell-to-cell interactions. Thus, the ARIA promotes synapse-specific gene expression, thereby augmenting nerve–muscle interactions (7). Other isoforms of NDF/neuregulin promote maturation of astroglia and Schwann cells (8, 18), as well as oligodendrocytes (19), and at the same time inhibit neuronal differentiation (20, 21). These observations, together with the abundant expression of NDF/neuregulins by brain neurons and astroglia (18, 22), suggest that neuregulins are involved in the response of the brain to environmental stimuli. Here we show that brain NDF/neuregulin, along with one of its surface receptors, namely ErbB-4, is induced by three types of stimuli: activation of general excitatory neurotransmission with a glutamate receptor agonist, locomotion, and tetanic stimulation. These observations imply that the NDF/neuregulin family of ligands plays an important role in activity-dependent brain processes and in neuronal plasticity.
MATERIALS AND METHODS
Materials.
A polyclonal rabbit antibody was raised against bacterial recombinant NDF-α2 and found to recognize all isoforms of the factor. The antiserum was purified over a column of recombinant rat NDF, and the acid-eluted Ig fraction was used after dilution (23). Rabbit antibodies to a recombinant extracellular portion of ErbB-3 and an antibody to a synthetic peptide derived from human ErbB-4 were purchased from Santa Cruz Biotechnology. In preliminary experiments we verified that these antisera do not cross-react. MK-801 and CPP were from Tocris Neuramin (Bristol, U.K.). All other chemicals were from Sigma.
Animals.
Adult male Hooded or Wistar rats (3 months old, 200–250 g body weight) were used in all experiments. Between 5 and 10 animals were used in each experimental group.
Immunohistochemistry.
Following perfusion of the animals with 2.5% paraformaldehyde, brains were removed and stored for 5–10 days in 1% paraformaldehyde. Serial 35-mm-thick coronal or sagittal sections were subjected to immunohistochemical analysis by using the avidin–biotin peroxidase technique (Vector Laboratories), as described (24).
In Situ Hybridization Analysis.
NDF-specific primers with the following nucleotide sequences were synthesized: 5′-oligonucleotide: 5′-TGAAGAGCCAGGAGTCAGCTGCAGG-3′ and 3′-oligonucleotide: 5′-GGCTCGAGACTCTGAGGACACATAGG-3′. These were used as primers to amplify a 0.3-kb-long fragment of the rat NDF coding region from cDNA clone 44 (5). The amplification procedure was exactly as described before (10), and the resulting DNA was cloned into the Bluescript plasmid (Stratagene). T3 and T7 RNA polymerases were used to generate [α-(35S)thio]UTP-labeled sense and antisense transcripts that were used as probes. Embedding, sectioning, postfixation, and hybridization were performed as described (25). To prepare cRNA probes specific to erbB-3 and to erbB-4, we extracted RNA from the cerebellum of a 22-day-old rat by using the guanidinium thiocyanate/LiCl method (26). erbB-3- and erbB-4-specific DNA sequences were amplified by using the following oligonucleotides: erbB-3-specific primers: 5′-TCCTGGCCGCCCCACATGCACAAC 3′ [sense; nucleotides 1300–1323 of human erbB-4 (27)] and 5′-GTCACATTTGCCCTCTGCCA-3′ (antisense; nucleotides 1580–1599), and erbB-4-specific primers: 5′-TGTGCGTGCCTGCCCTAGTTCCAAGATGG-3′ [sense; nucleotides 945–973 of human erbB-4 (28)] and 5′-CCTGTTATCTCTCTGACTGTCCG-3′ (antisense; nucleotides 1210–1232). These primers were used to amplify 299-bp and 288-bp fragments of rat erbB-3 and erbB-4 coding regions, respectively. The amplification procedure was performed as described (18), and the resulting DNA was cloned into the Bluescript plasmid (Stratagene). Sections were exposed either for 10 days (e.g., Figs. 3A and 5C) or for 30 days (e.g., Fig. 1) to detect differences in cases where the control signals were low.
Figure 3.
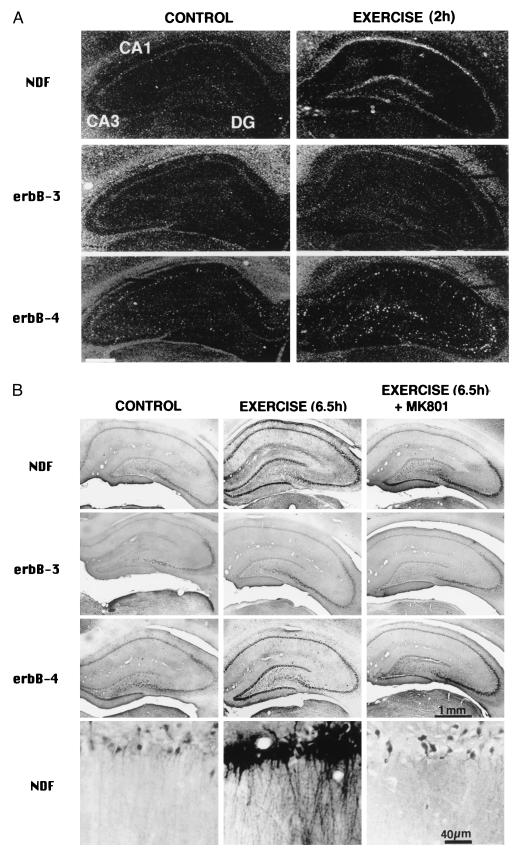
Effect of locomotion on NDF/neuregulin expression in the hippocampus. The brains of rats that walked for 1 hr were analyzed by in situ hybridization (A) with cRNA probes of either NDF, erbB-3, or erbB-4, as indicated, or by immunostaining (B) with antibodies specific to either recombinant rat NDF, or to the indicated ErbB receptor. Control rats were similarly analyzed. RNA was analyzed 2 hr after walking, and proteins were assayed after 6.5 hr. The lower row in B shows high-power images of the CA1 region. Note the homogenous increase in NDF message in the pyramidal layer of region CA1 in A, and the more punctate appearance of labeling in the DG. Only punctate, more diffused labeling is seen with the probe for ErbB-4, indicating that the probe labeled interneurons, in both CA1 and CA3 regions. Control animals, which were pretreated with the NMDA receptor blocker MK-801, lacked the dense staining in CA1, but still expressed a higher staining intensity than control, in CA3 area (B Right). [Bar = 1.7 mm (A).]
Figure 5.
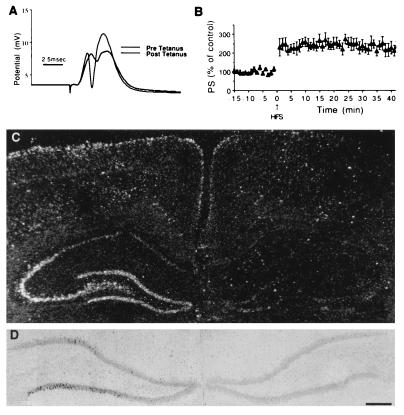
NDF induction by an LTP-producing tetanic stimulation. Unilateral LTP was induced in the left perforant path of rats, and the brain was removed 1 hr later. In situ hybridization analysis of NDF expression was then performed. (A) Sample traces of population EPSPs and spikes taken before and after application of tetanic stimulation, illustrating a marked change in EPSP slopes and size of population spikes. (B) Persistence of enhancement of population spike size after the tetanic stimulation. The 2-fold change in population spike size lasted at least 40 min of recording past the tetanic stimulation. (C) Dark-field photomicrograph of coronal section through the hippocampus, indicating the increase in NDF message in the ipsilateral DG and CA3 of the hippocampus. (D) Higher-power, bright-field photomicrographs of the ipsilateral and contralateral sides showing NDF expression in individual granule cells of the ipsilateral dentate gyrus. [Bar = 3.75 mm (D) and 2.5 mm (C).]
Figure 1.
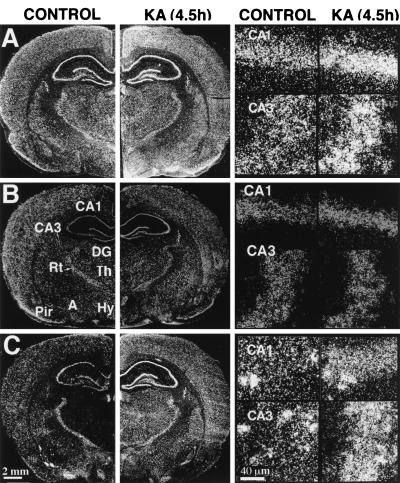
Effect of kainic acid on the expression of NDF/neuregulin and its ErbB-3 and ErbB-4 receptors in rat brain. Coronal brain sections were prepared from rats 4.5 hr after intraperitoneal injection of kainate (KA) and hybridized to the following 35S-labeled cRNA probes: NDF (A), erbB-3 (B), and erbB-4 (C). Dark-field photomicrographs are shown in comparison with sections that were prepared from brains of untreated rats (CONTROL). The far-right panels show high magnification of cells in the hippocampal areas CA1 and CA3. Note the punctate appearance of ErbB-4 staining corresponding to single cells especially in control brains. A, amygdala; CA1 and CA3, fields of Ammon’s horn; DG, dentate gyrus; Hy, hypothalamus; Rt, reticular thalamic nucleus; Pir, piriform cortex; and Th, thalamus. Scales in C also are for the corresponding images in A and B.
Image Analysis.
To quantify the relative levels of specific mRNA molecules in rat brain we prepared computer images of dark-field emulsion photomicrographs of thin sections by using a video camera attached to a Universal Zeiss microscope. Frames were recorded by using the software screenplay (VideoSpring, SuperMac Technology, Sunnyvale, CA) and transferred as PICT files to photoshop 3.0 for processing and analysis. The optical density of the autoradiograms was measured by using the nih-image 1.41 software (Bethesda, MD).
Kainic Acid (KA)-Induced Epilepsy.
KA (adjusted to pH 7.4, 12 mg/kg of body weight) was injected intraperitoneally, and animals were monitored to detect the typical development of epileptic seizures. Treated rats were sacrificed at the indicated time points after injection.
Locomotion.
Rats were placed in a wire mesh wheel (15-cm radius), that was rotated mechanically for 1 hr at a speed of 4.7 turns per min. Rats were sacrificed at various time intervals after termination of the locomotor activity, as indicated. Five control animals were injected with the N-methyl-d-aspartate (NMDA) receptor antagonist MK-801 (0.5 mg/kg) 30 min before the behavioral treatment. Other control rats were injected with saline.
In Vivo Long Term Potentiation (LTP).
LTP was induced in the hippocampal dentate gyrus (DG) by high-frequency stimulation (HFS) of the perforant path. Rats were anesthetized with urethane (1.5 g/kg) and placed in a Kopf stereotaxic apparatus as described before (33). The stimulation intensity of test pulses (100 μs, 0.066 Hz) to the perforant path was adjusted to yield a population spike amplitude of about 1.0 mV in the DG, and baseline responses were collected for 15 min. Both the initial slope of the positive-going excitatory postsynaptic potential (EPSP) and the amplitude of the granule cell population spike were measured. HFS, at an intensity sufficient to elicit the maximum granule cell population spike, consisted of 10 trains (1 per min) of 50 pulses delivered in 5 bursts, each of 25-ms duration and 400 Hz; bursts were separated by 1 s. After tetanic stimulation, the responses to test pulses were monitored for 60 min, and the rats were sacrificed 1 hr later. The brains were removed and kept frozen at −70°C until sectioning for in situ hybridization analysis. In two control rats, 3-[(RS)-2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid (CPP) (10 mg/kg) was injected 20 min before stimulation, which led to blockade of LTP expression.
Statistics.
Both changes in optical density values obtained for mRNA levels and changes in the number of NDF-immunopositive cells were determined by using a two-way ANOVA. The accepted level of significance was 0.05.
RESULTS
Induction of NDF and ErbB-4 by Epileptic Seizures.
As an initial test of the possibility that transcription of brain NDF/neuregulin is dynamically regulated we used massive stimulation of brain activity with the seizure-producing glutamate analog, kainate, which is involved in de novo gene transcription (29, 30). KA was injected intraperitoneally, and changes in the expression of NDF, as well as its receptors, ErbB-3 and ErbB-4, were followed. In accordance with previous reports (18, 22), in situ hybridization localized NDF transcripts to multiple sites in the brain, including cerebral cortex, thalamus, hypothalamus, and amygdala (Fig. 1). Likewise, transcripts of erbB-3 and erbB-4 were found to be widely expressed in adult rat brain, in agreement with previous reports (21, 31): major sites of erbB-4 expression are the cerebral cortex, hippocampus, and amygdala, whereas erbB-3 displayed low and uniform expression. KA injection resulted in a complex pattern of changes in the expression of NDF and ErbB-4 receptors (Figs. 1 and 2A). These were quantified and their time dependency was analyzed. Evidently, NDF expression was increased primarily in limbic cortical areas, including the cingulate and piriform cortex. High levels were also detected in CA1 and CA3 and the dentate granular region of hippocampus, as well as in the amygdala and the medial hypothalamus. erbB-4 transcripts were elevated by 2- to 5-fold in neocortical areas, hippocampus, and the thalamus. Despite elevation of both types of transcripts already 1 hr after KA treatment, and an apparently shared biphasic response in cortical areas, sustained elevation of erbB-4, but not NDF, was exhibited in the CA1 region of the hippocampus. NDF elevation was most sustained in the amygdala, lasting 4.5 hr. Statistical analyses indicated that erbB-3 did not change significantly in any of the areas studied (Fig. 1). Elevated expression of NDF and erbB-4 was localized to the pyramidal layers of CA1 and CA3 areas of the hippocampus, as well as to large pyramidal cells of the neocortex. We found no hybridization signals in cells with small, darkly stained nuclei characteristic of microglia or oligodendroglial cells. In addition, hybridization was not detected in astrocyte-rich regions, such as layer I of the neocortex, the corpus callosum, and the molecular layer of the hippocampus.
Figure 2.
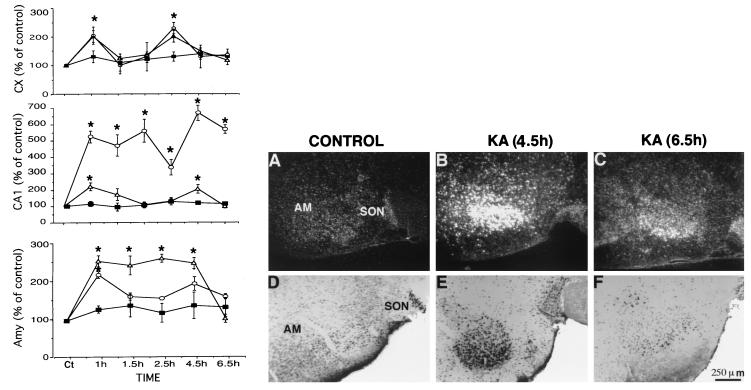
Time-dependent changes in kainate-induced effects in rat brain. (Left) Three brain regions were analyzed: the neocortex (Cx, Top), CA1 area of the hippocampus (Middle), and the amygdala (Amy, Bottom). Thin sections were subjected to in situ hybridization analysis with probes of NDF/neuregulin (triangles), erbB-3 (squares), and erbB-4 (circles). Three animals were tested at each time point, and six measurements were made with each probe and brain area. Asterisks indicate a significant effect relative to the control (P < 0.05). (Right) Changes in the amygdala detected by immunocytochemistry (Lower) and by in situ hybridization (Upper). Brain slices from control (A and D) or kainate-treated rats (B, C, E, and F) were processed at the indicated time intervals after kainate injection. Both in situ hybridization analysis (A–C) with an NDF/neuregulin probe, and immunocytochemistry with anti-NDF antibodies (D–F) were performed. AM, amygdala; SON, supraoptic nucleus.
Immunohistochemical examination of the brains of KA-treated rats with affinity-purified antibodies to NDF confirmed that transcriptional up-regulation was coupled to local increase in the NDF protein. An example of this coupling in the amygdala is shown in Fig. 2B; the low level of NDF transcript and protein in untreated animals was replaced by a punctate pattern of high expressing cell bodies 4.5 hr posttreatment, but both RNA and protein returned to near basal levels 2 hr later. We observed a general lag of approximately 3.5 hr between the peak of mRNA and that of NDF protein; the peptide peaked at 4.5–6.5 hr in the cortex, at 6.5 hr in the hippocampus, and at 4.5 hr in the amygdala, and the induced levels persisted for about 3 hr before being reduced to control levels. Morphologically, NDF immunoreactivity was found in large pyramidal cells in the cortex (primarily in layer V) and in the hippocampus. This implies that the cells undergoing up-regulation of NDF expression are neurons and not glial cells, in accordance with the basal NDF expression exhibited by primary neuronal cultures (18, 21).
Forced Locomotion Elevates NDF and ErbB-4 in Hippocampus and in Motor Cortex.
KA activates extensive regions in the brain. In trying to use more restricted stimuli that will activate only functionally relevant areas of the brain, we resorted to the motor system. During locomotion, the motor cortex is assumed to be active, and, in addition, a large theta rhythm is recorded from the dorsal hippocampus (32), but other brain regions are not assumed to be activated as much. Using in situ hybridization analysis with specific probes, we found elevated transcription of NDF and erbB-4, but not erbB-3, in hippocampal areas CA1–3 and DG of rats that underwent a 1-hr-long exercise on a running wheel (Fig. 3A). Unlike NDF, which localized to all cells in the pyramidal cell layer, erbB-4 expression was punctate and limited to fewer cells, possibly interneurons, laying below the NDF-positive layer. Immunohistochemical analysis with antibodies to NDF, ErbB-3 and ErbB-4, was performed with brains of animals sacrificed 6 hr after forced locomotion. This confirmed up-regulation of the peptide in hippocampus and in DG, along with up-regulation of ErbB-4 (Fig. 3B). ErbB-3 expression was not affected by the forced locomotion. To test whether glutamate plays a role in coupling physical activity to gene regulation, we injected rats with MK-801, a noncompetitive NMDA-receptor antagonist, 30 min before the start of locomotion. The NMDA antagonist prevented the activity-induced elevation in staining for NDF and its receptor, consistent with glutamate involvement (Fig. 3B).
The other brain region that underwent massive changes in both NDF and ErbB-4 expression is the motor cortex. Using immunohistochemistry, we observed a striking increase in the number of NDF- and ErbB-4-immunopositive pyramidal cells in the superficial and deep layers II, III, and VI, respectively, after forced locomotion (Fig. 4, and data not shown). There was no significant effect on the number of stained cells in layer V. In addition, the number of stained cells in layers II and III peaked 3.5 h after termination of locomotion, and in layer VI, after 6.5 hr (Fig. 4B). Elevation of NDF was most pronounced in the motor cortex; in other areas substantial staining was observed only in scattered neurons of all layers. Staining was reduced in all cortical layers at 7.5 hr after the locomotion training.
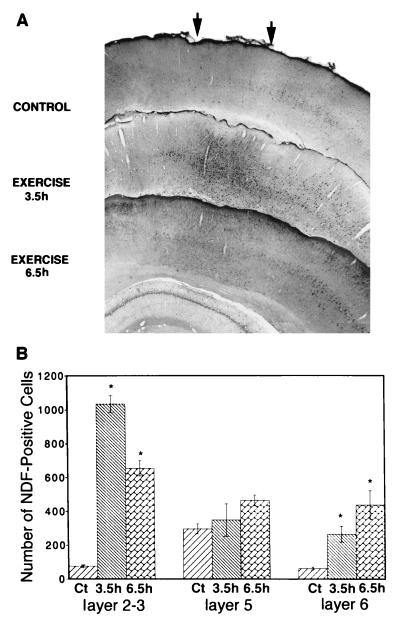
Figure 4.
Forced locomotion enhances NDF staining in the motor cortex. (A) Rats were sacrificed 3.5 hr or 6.5 hr after walking for 1 hr on a revolving wheel, and the corresponding areas of the motor cortex (marked by arrows) were analyzed by immunostaining with an affinity-purified antibody to NDF. (B) Quantification of the effect of locomotion on NDF expression was performed by counting the number of NDF-positive cells in the indicated layers of the motor cortex. A large increase in cell staining is seen already at 3.5 hr in layers 2–3 of the neocortex, whereas in the deeper layers, including the large pyramidal cells of layers 5 and 6, immunoreactivity increased more slowly. Asterisks indicate an increase above control at a significance level of P < 0.05.
LTP Triggers Unilateral Increases in Hippocampal NDF.
LTP, a persistent, synapse-specific increase in synaptic efficacy, which is a candidate mechanism for memory formation in mammalian brain (33), involves activation of many immediate early gene proteins, as well as neurotrophic factors like BDNF (34). To address possible effects of LTP on NDF induction we used tetanic stimulation of the perforant path input to the dentate gyrus, and followed LTP in this monosynaptic pathway. The results of this experiment are presented in Fig. 5. Evidently, LTP induction led within 1 hr to an ipsilateral increase in NDF transcripts in DG and in CA3, but not in CA1 of the hippocampus (Fig. 5). The contralateral hippocampus displayed no induction of NDF (Fig. 5A). In addition, at this early time point we observed no significant increase in erbB-3 or in erbB-4 transcripts (data not shown). Bright-field microscopy of brain sections localized NDF transcripts to the pyramidal cells of CA3 and to granular cells of the dentate gyrus (Fig. 5B). Injection of CPP before the tetanic stimulation blocked the effect on NDF in both CA3 and DG (data not shown). These results suggest that the association between NDF and induction of LTP in these regions is mediated by activation of NMDA receptors.
DISCUSSION
The present results demonstrate that NDF/neuregulin and one of its receptors, ErbB-4, are differentially up-regulated in several parts of rat brain after epileptic seizure (Figs. 1 and 2), locomotor activity (Figs. 3 and 4), and induction of LTP (Fig. 5). Although both the transcripts of the ligand and the respective receptor were elevated after treatment with KA, their kinetics were different (Fig. 2A). Nevertheless, enhanced transcription was transient in both cases and displayed some regional specificity. Additional experiments confirmed that the elevation of mRNAs leads to similar increases in NDF and ErbB-4 proteins after KA-induced seizure (Fig. 2B) and simple locomotion (Figs. 3B and 4). Once again, transient rather than persistent enhanced expression was observed, indicating a short half-life of the induced NDF in brain. For example, maximal elevation of the NDF transcript after locomotion was observed between 2 and 3 hr after the behavioral test, and the protein lagged by 2.5 hr and returned to basal level after 6–7 hr. This may be due to regulatory sequences in the 3′ untranslated region of the corresponding mRNAs (6, 5, 28). Interestingly, the Drosophila ortholog of NDF, Vein, carries a PEST domain characteristic of rapidly proteolized proteins (35), but a similar domain in mammalian neuregulins has not yet been reported.
Enhanced expression of NDF and ErbB-4 after stimuli involved in neural plasticity may not be surprising in view of the vast literature reporting induction of extensive gene expression in experimentally activated brain or in the developing central nervous system (33, 36, 37). A plethora of genes, including not only immediate early response genes but also trophic factors, transcription factors, enzymes, vesicular, and structural proteins, were found to be induced by plasticity-related stimuli such as treatment with KA (38), or after LTP induction (39, 40). Perhaps most relevant to the case reported in this paper is the pair of a neurotrophic factor, BDNF, and its receptor, TrkB. BDNF, which functions as a survival factor for several types of neurons, along with its receptor, is elevated after mechanical damage, seizure (1), kindling (41), visual stimulation (3), and exercise (42). Interestingly, like ErbB-3, whose expression is not affected by activity-related stimuli, other Trk proteins undergo only limited changes upon brain insults (43). Consistent with ErbB-4 being the more dynamically regulated receptor in the central nervous system, its expression precedes that of ErbB-3 during embryonic development of the brain, and analysis of primary brain cultures indicated that ErbB-4 is expressed in many types of brain cells, whereas ErbB-3 expression is restricted to oligodendrocytes in late gestation (21). In addition, targeted inactivation of erbB-4 in mice resulted in mistargeting of axons of specific rhombomers in the hindbrain (31), attributing a role for ErbB-4 in axonal guidance. Moreover, when expressed in glial cells, a dominant-negative mutant of ErbB-4 can block migration of overlying cerebellar granular cells (44). These observations in the developing brain are relevant to the possible role of NDF and ErbB-4 in activity-dependent processes in adult rats. Related to this question is the exact localization of the up-regulated transcripts. The more prominent sites are the hippocampal areas, the DG, and the motor cortex. These are highly plastic areas, responsive to external stimuli (1, 3). Close inspection of the patterns of expression, however, suggests that NDF and ErbB-4 are induced by adjacent, yet distinct, cells (Fig. 3A). Conceivably, as in other cellular systems in which NDF plays a mediator role, such as mesenchyme–epithel, muscle–neuron, and neuron–Schwann cells (9), a paracrine rather than an autocrine mechanism is operative in the induced hippocampus.
One possible function of NDF up-regulation may be to protect brain cells against the excitotoxic effects of glutamate-like molecules (e.g., NMDA) (45). However, because the granular neurons of the DG are not susceptible to excitotoxic damage induced by KA (46), alternative roles that are related to potential mechanisms of neural plasticity may be considered. Examples include a postsynaptic effect on transmission efficacy, similar to the role of ARIA/neuregulin in strengthening neuromuscular synapses (47). Alternatively, retrograde transport of hippocampal NDF, analogous to that of BDNF (48), may support survival of forebrain cholinergic neurons, similar to the effect observed on cultured retinal cells (49). Independent of its functional role, NDF secretion by hippocampal neurons is apparently under glutamatergic control. Thereby, activity-dependent expression of this trophic factor and its ErbB-4 receptor may act as the target for use-dependent competition between afferent nerves, similar to the requirement for NDF, when developing Schwann cells attain the characteristic 1:1 ration with nerve cells (50). In Schwann cells, however, NDF receptor consists of a heterodimer between ErbB-3 and ErbB-2. Whether or not ErbB-2 expression is modulated in activity-dependent processes remains to be seen, as is possible physiological regulation of the recently identified second family of neuregulins (51). Despite these open questions, our results attribute to neuregulins a role in the mechanism by which exercise can influence the structure and function of the nervous system. Because NDF resides primarily in pyramidal neurons whereas ErbB-4 is found in interneurons, and both are up-regulated, it is tempting to speculate that NDF secretion will affect local hippocampal circuits in a manner similar to the potentiating effect of the motor neuron-derived ARIA on the neuromuscular junction (47). The implications of this model to cognitive and motoric brain functions will have to await further studies.
Acknowledgments
We thank Drs. Gal Richter-Levine and Dana Hevroni for help with LTP experiments, Rafael Malach for helpful discussions, and Sara Lavi for technical assistance. This work was supported by the German-Israel Foundation, the Israel Basic Research Fund, the Leo and Julia Forchheimer Center for Molecular Genetics, the Israel Ministry of Health, and the Israel Institute for Psychobiology.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- CPP
3-[(RS)-2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid
- DG
dentate gyrus
- EPSP
excitatory postsynaptic potential
- HFS
high-frequency stimulation
- KA
kainic acid
- LTP
long term potentiation
- NDF
Neu differentiation factor
- NMDA
N-methyl-d-aspartate
References
- 1.Isackson P J, Huntsman M M, Murray K D, Gall C M. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 2.Zafra F, Castren E, Thonen H, Lindholm D. Proc Natl Acad Sci USA. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castren E, Zafra F, Thoenen H, Lindholm D. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gall C. J Neurosci. 1988;8:1852–1862. doi: 10.1523/JNEUROSCI.08-06-01852.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen D, Peles E, Cupples R, Suggs S V, Bacus S S, Luo Y, Trail G, Hu S, Silbiger S M, Ben-Levy R, et al. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 6.Holmes W E, Sliwkowski M X, Akita R W, Henzel W J, Lee J, Park J W, Yansura D, Abadi N, Raab H, Lewis G D, et al. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 7.Falls D L, Rosen K M, Corfas G, Lane W S, Fischbach G D. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 8.Marchionni M A, Goodearl A D J, Chen M S, Bermingham-McDonogh O, Kirk C, Hendricks M, Denehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Nature (London) 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 9.Burden S, Yarden Y. Neuron. 1997;18:847–855. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 10.Orr-Urtreger A, Trakhtenbrot L, Ben-Levi R, Wen D, Rechavi G, Lonai P, Yarden Y. Proc Natl Acad Sci USA. 1993;90:1867–1871. doi: 10.1073/pnas.90.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carraway K L, Sliwkowski M X, Akita R, Platko J V, Guy P M, Nuijens A, Diamonti A J, Vandlen R L, Cantley C L, Cerione R A. J Biol Chem. 1994;269:14303–14306. [PubMed] [Google Scholar]
- 12.Tzahar E, Levkowitz G, Karunagaran D, Yi L, Peles E, Lavi S, Chang D, Liu N, Yayon A, Wen D, Yarden Y. J Biol Chem. 1994;269:25226–25233. [PubMed] [Google Scholar]
- 13.Plowman G D, Green J M, Culouscou J M, Carlton G W, Rothwell V M, Buckley S. Nature (London) 1993;366:473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- 14.Guy P M, Platko J V, Cantley L C, Cerione R A, Carraway K L. Proc Natl Acad Sci USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riese D J, van Raaij T M, Plowman G D, Andrews G C, Stern D F. Mol Cell Biol. 1995;15:5770–5776. doi: 10.1128/mcb.15.10.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin B, Sela M, Yarden Y. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer D, Birchmeier C. Proc Natl Acad Sci USA. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinkas-Kramarski R, Eilam R, Spiegler O, Lavi S, Liu N, Chang D, Wen D, Schwartz M, Yarden Y. Proc Natl Acad Sci USA. 1994;91:9387–9391. doi: 10.1073/pnas.91.20.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vartanian T, Corfas G, Li Y, Fischbach G D, Stefansson K. Proc Natl Acad Sci USA. 1994;91:11626–11630. doi: 10.1073/pnas.91.24.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah N M, Marchionni M A, Isaacs I, Stroobant P, Anderson D J. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 21.Pinkas-Kramarski R, Eilam R, Alroy I, Levkowitz G, Lonai P, Yarden Y. Oncogene. 1997;15:2803–2815. doi: 10.1038/sj.onc.1201466. [DOI] [PubMed] [Google Scholar]
- 22.Corfas G, Rosen K M, Aratake H, Krauss R, Fischbach G D. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 23.Wen D, Suggs S V, Karunagaran D, Liu N, Cupples R L, Luo Y, Jansen A M, Ben-Baruch N, Trollinger D B, Jacobson V L, et al. Mol Cell Biol. 1994;14:1909–1919. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eilam R, Malach R, Bergmann F, Segal M. J Neurosci. 1991;11:401–411. doi: 10.1523/JNEUROSCI.11-02-00401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan B, Constantini F, Lacy E. Manipulating the Mouse Embryo. Cold Spring, New York: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 26.Maniatis T, Fritsch E F, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring, New York: Cold Spring Harbor Lab. Press; 1982. [Google Scholar]
- 27.Plowman G D, Whitney G S, Neubauer M G, Green J M, McDonald V I, Todaro G J, Shoyab M. Proc Natl Acad Sci USA. 1990;87:4905–4909. doi: 10.1073/pnas.87.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plowman G D, Culouscou J M, Whitney G S, Green J M, Carlton G W, Foy L, Neubauer M G, Shoyab M. Proc Natl Acad Sci USA. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo D C. Current Op Neurobiol. 1992;2:336–340. doi: 10.1016/0959-4388(92)90125-5. [DOI] [PubMed] [Google Scholar]
- 30.Collingridge G L, Wolf S. Trends Pharmacol Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- 31.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Nature (London) 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 32.Vanderwolf C. Electroencephalog Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 33.Bliss T V P, Collinridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 34.Dragunow M, Beilharz E, Sirimanne E, Lawlor P, Williams C, Bravo R, Gluckman P. Brain Res Mol Brain Res. 1994;25:19–33. doi: 10.1016/0169-328x(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 35.Schnepp B, Grumbling G, Donaldson T, Simcox A. Genes Dev. 1996;15:2302–2313. doi: 10.1101/gad.10.18.2302. [DOI] [PubMed] [Google Scholar]
- 36.Shatz C J. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 37.Kandel E R, O’Dell T J. Science. 1992;258:243–245. doi: 10.1126/science.1411522. [DOI] [PubMed] [Google Scholar]
- 38.Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Nature (London) 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 39.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey Y, Kuhl D. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyford G L, Yamagata K, Kaufmann W E, Barnes C, Sanders L K, Copeland N G, Gilbert D J, Jenkins N A, Lanahan A A, Worlley P F. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 41.Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- 42.Neeper S A, Gomez-Pinilla F, Choi J, Cotman C W. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 43.Merlio J-P, Ernfors P, Kokaia Z, Middlemas D S, Bengzon J, Kokaia M, Smith M-L, Siesjo B K, Hunter T, Lindvall O, Persson H. Neuron. 1993;10:151–164. doi: 10.1016/0896-6273(93)90307-d. [DOI] [PubMed] [Google Scholar]
- 44.Rio C, Rieff H I, Qi P, Corfas G. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 45.Choi D W. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben-Ari Y. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 47.Fischbach G D, Rosen K M. Annu Rev Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- 48.Widmer H R, Knusel B, Hefti F. Neuroreport. 1993;4:363–366. doi: 10.1097/00001756-199304000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Bermingham-McDonogh O, McCabe K L, Reh T A. Development. 1996;122:1427–1438. doi: 10.1242/dev.122.5.1427. [DOI] [PubMed] [Google Scholar]
- 50.Syroid D E, Maycox P R, Burrola G P, Liu N, We D, Lee K-F, Lemke G, Kilpatrick T J. Proc Natl Acad Sci USA. 1996;93:9229–9234. doi: 10.1073/pnas.93.17.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carraway K L, III, Weber J L, Unger M J, Ledesma J, Yu N, Gassman M. Nature (London) 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]