Abstract
Insulin-like growth factors I and II (IGF-I and IGF-II) play an important role in normal growth and brain development and protect brain cells from several forms of injury. The effects of IGFs are mediated by type-I and type-II receptors and modulated by potentially six specific binding proteins that form high-affinity complexes with IGFs in blood and cerebrospinal fluid (CSF) and under most circumstances inactivate them. Because brain injury is commonly associated with increases in IGFs and their associated binding proteins, we hypothesized that displacement of this large “pool” of endogenous IGF from the binding proteins would elevate “free” IGF levels to elicit neuroprotective effects comparable to those produced by administration of exogenous IGF. A human IGF-I analog [(Leu24, 59, 60, Ala31)hIGF-I] with high affinity to IGF-binding proteins (Ki = 0.3–3.9 nM) and no biological activity at the IGF receptors (Ki = >10,000 nM) increased the levels of “free, bioavailable” IGF-I in the CSF. Intracerebroventricular administration of this analog up to 1h after an ischemic insult to the rat brain had a potent neuroprotective action comparable to IGF-I. This novel strategy for increasing “free” IGF levels in the brain may be useful for the treatment of stroke and other neurodegenerative diseases.
Insulin-like growth factors I and II (IGF-I and IGF-II) are multifunctional peptides essential for normal growth and development (1). Their biological actions are mediated by the type-I IGF receptor (2) and possibly the type-II IGF receptor, which is identical to the cation-independent mannose 6-phosphate receptor (3). In the circulation and interstitial fluids, including the cerebrospinal fluid (CSF), IGFs are almost entirely associated with one or more of at least six IGF-binding proteins (IGFBPs) that bind IGFs with high affinity, thus limiting their interaction with receptors, and potentially providing a “reservoir” of biologically inactive IGF (1). IGFs undoubtedly play an important role in brain development and may also be important after injury. IGF treatment protects the developing or adult brain from hypoxic-ischemic injury (4–7) and forebrain ischemia (8), induces myelination (9–11), and reduces neuronal death in vitro caused by diverse forms of injury (12–16). Paradoxically, injury to the developing or adult brain is commonly associated with increases in brain IGFs as well as their associated binding proteins (4, 17–26). Consequently, even though IGFs are elevated, they may be complexed with their binding proteins and unavailable to provide neuroprotection. The IGF system, therefore, provides a rather unique opportunity for utilizing an endogenous neuroprotective factor. We hypothesized that displacement of the large “pool” of IGF from the IGFBPs in the brain would elevate “free” IGF levels, increasing receptor activation to elicit similar actions to administration of IGF-I itself.
In the present studies, we examined the role of brain IGFs and IGFBPs in neuroprotection by comparing the effects of hIGF-I with the selective, high-affinity IGFBP ligand inhibitor, [Leu24,59,60, Ala31]hIGF-I in in vitro studies of release of “free” bioactive IGF-I from rat cerebrospinal fluid and in in vivo studies to evaluate their neuroprotective effects in a rat model of focal ischemia. Data suggest that IGFBPs, by neutralizing IGFs, may serve to limit the actions of the peptides under both physiological and pathological conditions. Furthermore, the results demonstrating potent neuroprotective effects of the IGFBP ligand inhibitor comparable to IGF-I suggest that this strategy for increasing “free” IGF levels in the brain may be useful for the treatment of stroke and other neurodegenerative diseases.
MATERIALS AND METHODS
Synthesis and Purification of Peptides.
hIGF-I and hIGF-II were obtained from Sigma. [Nle29]hIGF-I and [Leu24,59,60, Ala31]hIGF-I were synthesized by a solid-phase peptide synthesis procedure as described previously (27) by using a t-butoxycarbonyl-Ala-(oxymethyl)-phenylacetamidomethyl (PAM) resin on a Beckman 990 peptide synthesizer. Derivatized amino acids and resin used in the synthesis were purchased from Bachem. After the last residue was coupled onto the growing peptide chain, the protected peptide resin was treated with the low–high hydrogen fluoride cleavage procedure (28) to remove the peptide from the resin anchor and deprotect the side-chain functional groups. The crude peptide was extracted with 5 M guanidine HCl in 0.1 M NH4OAc, and the pH of the extract was maintained at 5 with HOAc. After filtering off the resin, the solution was diluted with 0.1 M NH4OAc to 2 M guanidine HCl to a peptide concentration of ≈1 mg/ml. The peptide was cyclized by air oxidation by stirring at room temperature for 24 h while maintaining the pH at 8.4 with 10% concentrated NH4OH. After oxidation, the pH was adjusted to 5 and the solution was dialyzed against 0.1 M acetic acid at room temperature to remove the guanidine salt. The recovered dialysate was lyophilized and the crude product was purified by gel filtration on Sephadex G-50F, followed by carboxymethyl cellulose cation-exchange chromatography and preparative HPLC on a KP-100 Gradient HPLC system with a Vydac C18 cartridge (Biotage, Charlottesville, VA). The purified product was verified by mass spectrometric analysis on a SCIEX/AP1 LC/MS system equipped with an ion-spray source (Perkin–Elmer).
Radioligand Binding Assay.
Human IGFBP-1, BP-4, and BP-5 were expressed in the BaculoGold Expression System (PharMingen) in Sf9 insect cells and purified by affinity chromatography on a hIGF-I-coupled Affi-Gel 10 column, followed by reverse-phase HPLC. Human IGFBP-2 and BP-3 were isolated from outdated plasma as described previously (29). The binding assay was performed at room temperature in duplicate in 0.02% Nonidet P-40/PBS buffer, pH 7.2. Two hundred microliters of a 2.5 nM IGFBP solution (0.5 pmol) was added to a 12 × 75-mm glass test tube. The reaction was started by the addition of 100 μl buffer, hIGF-I, hIGF-II, or [Leu24,59,60, Ala31]hIGF-I solution, followed by 100 μl of [125I]hIGF-I (30,000 cpm, specific activity ≈2,200 Ci/mmol; New England Nuclear). After incubation for 2 h, 100 μl of 20% BSA and 500 μl of 20% PEG-8000 in the PBS buffer were added and the mixture was vortexed and then centrifuged for 30 min at 3,000 rpm. The supernatant was carefully removed by suction and the pellet was counted in a γ-counter.
Radioligand and Western Blot Analysis of the IGF-Binding Proteins.
Twenty microliters of rat CSF was fractionated by SDS/PAGE and blotted onto nitrocellulose paper, and the blot was then incubated with [125I]hIGF-I and examined by autoradiography according to the procedure described previously (30). Western blot analysis of the IGFBPs was performed by electrophoresing 20 μl of rat CSF per lane on SDS/PAGE, followed by blotting of the gel onto nitrocellulose paper, according to the published procedure (30). The nitrocellulose paper was then cut into replicate strips, and one strip was incubated with IGFBP-2 antiserum (Upstate Biotechnology, Lake Placid, NY) whereas the other was incubated with IGFBP-5 antiserum raised in a rabbit with a synthetic peptide fragment as described previously (30). The stained bands were revealed by incubation with peroxidase-conjugated goat anti-rabbit IgG, followed by chemiluminescence detection with a commercial kit (Pierce).
Gel Filtration Analysis of Dissociated IGF-I from the IGF-I/IGF-Binding Protein Complex.
Five hundred microliters of rat CSF was incubated with [125I]hIGF-I at 37°C for 1 h to incorporate the radioiodinated peptide into the complex, and the incubated fluid was divided into 100-μl aliquots. To each aliquot was added buffer (control), IGF-I, or [Leu24,59,60, Ala31]hIGF-I, and the mixture was incubated for 1 h at 37°C, followed by storage on ice. For gel-filtration analysis, each aliquot was diluted with 400 μl 0.02% NaN3/0.1% BSA/PBS buffer and the diluted sample was loaded onto a 1 × 50 cm Sephadex G-50F column; the column was developed with the same buffer at a flow rate of 0.5 ml/min at room temperature. The collected fractions were counted in a γ-counter.
Fibroblast Proliferation Assay.
Biological activities of hIGF-I, [Nle59]hIGF-I, and the IGFBP ligand inhibitor [Leu24,59,60, Ala31]hIGF-I were tested in a BALB/c 3T3 fibroblast assay (31). The ability of the peptides to induce proliferation was measured by counting the amount of [3H]thymidine incorporated by the cells. Cells were aliquoted to 96-well microtiter plates (180 μl per well). After a 48-h incubation at 37°C and 5% CO2, the plates were washed twice with 0.1% calf serum/DMEM and incubated for an additional 24 h. Twenty microliters of sample and 1 μCi [3H]thymidine (New England Nuclear) were added to each well, and the plates were incubated for a further 24 h. After incubation, the medium was removed and the cells were fixed by the addition of 200 μl of a 25% acetic acid/75% ethanol solution per well. After removal of the fixing solution, the plates were washed three times with cold 10% trichloroacetic acid and the cells were lysed in 200 μl 0.2 M NaOH. The entire 200 μl of lysate solution was transferred into a scintillation vial; 2.5 ml of scintillation liquid was added and the vials were counted in a γ-counter.
Rat Middle Cerebral Artery Occlusion Model of Focal Ischemia.
Male Sprague–Dawley rats (Charles River) were housed in a 12-h light/12-h dark cycle and allowed food and water ad libitum. The experiment protocol was approved by the Institutional Animal Care and Use Committee in accordance with National Institutes of Health guidelines. Rats weighing 160–190 g were anesthetized with isoflurane (4% induction, 2.2% maintenance) in O2, and indwelling guide cannulae were stereotaxically implanted in the right, lateral ventricle [coordinates in mm: lateral (+1.5); anteroposterior (−0.8); dorsoventral (−3.0), relative to Bregma] to permit subsequent injections into the CSF. Ten days later, rats were reanesthetized with halothane (4% induction, 2–2.5% maintenance) in O2, and focal cerebral ischemia was induced by permanent occlusion of the left, middle cerebral artery (MCAo) proximal to the lenticulostriate branch, by electrocoagulation. Throughout surgery and recovery from anesthesia, animals were maintained normothermic by means of a heated blanket. Peptides or vehicle (sterile water) were injected into the lateral ventricle (in a volume of 5 μl) over 2–3 min, either concurrent with or 1 h after MCAo. One day after MCAo, animals were killed and the brains were removed. Delineation of the lesion was determined on fresh 500-μm coronal brain sections incubated in 2% tri-phenyl-tetrazolium chloride (TTC; Sigma) by using an indirect approach thus “correcting” for any swelling. Lesion volume was calculated for each brain by integration of the areas of infarct in each section. These procedures are described in more detail elsewhere (32). Peptide-treated groups were compared with vehicle-treated animals by using Student’s unpaired t test.
RESULTS AND DISCUSSION
Before this study, Bayne et al. (33) had reported that an hIGF-I analog, [Leu24,60, Ala31]hIGF-I, has a >1,200-fold loss in affinity to the type-I IGF receptor and little measurable affinity for the type-II receptor. To facilitate the synthesis and stability of this analog, the endogenous methionine at position 59 was replaced with leucine. The resulting compound, [Leu24,59,60, Ala31]hIGF-I, was tested with hIGF-I and hIGF-II for displacement of [125I]hIGF-I binding to human IGFBP-1, -2, -3, -4, and -5, and biological activity in BALB/c 3T3 fibroblast cells, which proliferate in response to IGFs. As shown in Table 1, hIGF-I and hIGF-II have comparable or somewhat higher affinities for IGFBP-1, -2, -3, -4, and -5 (Ki values = 0.01–0.22 nM) than for their homologous type-I and type-II receptors (Ki values = 1.5 and 0.2 nM, respectively). In contrast to the relative lack of selectivity of IGF-I and IGF-II between the IGFBPs and IGF receptors, [Leu24,59,60, Ala31]hIGF-I has high affinity for IGFBP-1, -2, -3, -4, and -5 (Ki values = 0.28–3.91 nM) and is inactive at IGF receptors (Ki values = >10,000 nM) (Table 1). Furthermore, in contrast to hIGF-I, which dose-dependently stimulated DNA synthesis in 3T3 fibroblasts with an IC50 of 5–10 nM, [Leu24,59,60, Ala31]hIGF-I had no activity in the assay at concentrations of up to 8 μM, indicating a lack of interaction with the IGF receptors in this functional assay.
Table 1.
Relative affinity and selectivity of IGF-I, IGF-II, and IGFBP ligand inhibitor (IGFBP-LI) for IGF-binding proteins and the type-I and type-II IGF receptors
| Protein or receptor | IGF-I | Ki, nM
|
|
|---|---|---|---|
| IGF-II | IGFBP-LI | ||
| IGFBP-1 | 0.12 ± 0.03 | 0.051 ± 0.008 | 1.91 ± 0.10 |
| IGFBP-2 | 0.06 ± 0.01 | 0.010 ± 0.005 | 1.92 ± 0.80 |
| IGFBP-3 | 0.21 ± 0.04 | 0.023 ± 0.005 | 1.80 ± 0.20 |
| IGFBP-4 | 0.10 ± 0.03 | 0.032 ± 0.004 | 0.28 ± 0.10 |
| IGFBP-5 | 0.22 ± 0.04 | 0.040 ± 0.004 | 3.91 ± 2.40 |
| Type-I receptor | 1.5 | 3.0 | >10,000 |
| Type-II receptor | 400 | 0.2 | >10,000 |
The relative affinities of hIGF-I, hIGF-II, and the IGFBP-LI, [Leu24,59,60, Ala31]hIGF-I for the various IGFBPs were determined in radioligand-binding assays as described in Materials and Methods. Data represent the mean ± SEM of three separate determinations. The affinity constants of the IGF-I and IGF-II for the type-I and type-II receptors were taken from ref. 34 and 35, respectively, whereas the affinity constants of the IGFBP ligand inhibitor for the IGF receptors were taken from ref. 33.
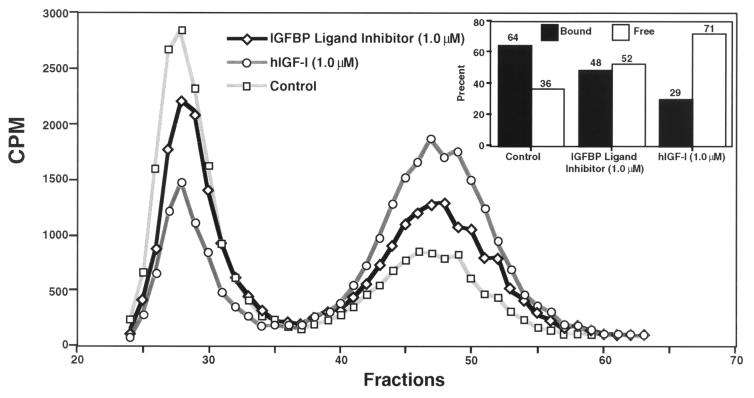
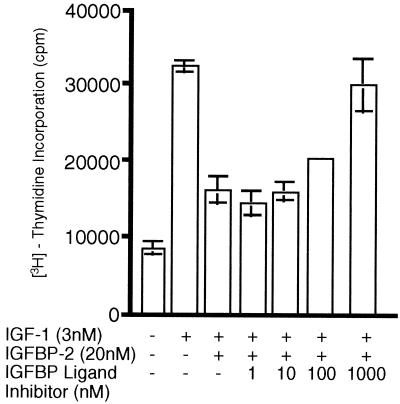
We evaluated the ability of the IGFBP ligand inhibitor, [Leu24,59,60, Ala31]hIGF-I, to displace the bound IGF-I and elevate “free” biologically active levels of the peptide in rat CSF and in the BALB/c 3T3 fibroblast proliferation assays. In agreement with previous reports (36, 37), using ligand and Western blot analyses, we determined that the most abundant IGFBP in rat CSF is BP-2 (Fig. 1 A and B, respectively). Gel filtration analysis of rat CSF that had been preincubated with trace quantities of [125I]hIGF-I demonstrated that ≈64% of [125I]hIGF-I eluted as a higher molecular mass complex (presumably bound to IGFBP-2) and ≈36% eluted at a molecular mass corresponding to “free” [125I]hIGF-I (Fig. 2). Incubation of the [125I]hIGF-I-incorporated CSF with either IGF-I (0.1 μM) or [Leu24,59,60, Ala31]hIGF-I (1 μM) resulted in a decrease in the proportion of IGF-I/IGFBP complex and a corresponding increase in “free” [125I]hIGF-I levels (Fig. 2). The higher concentration of the IGFBP ligand inhibitor than IGF-I required to increase “free” IGF-I levels is in keeping with the ≈10- to 20-fold lower affinity of the IGFBP ligand inhibitor for IGFBPs than IGF-I itself (Table 1). The ability of the IGFBP ligand inhibitor to release bioactive IGF-I was further evaluated in the 3T3 fibroblast assay. Human IGF-I (3 nM) produced robust proliferation of 3T3 fibroblasts as reflected by increased [3H]thymidine incorporation; the hIGF-I-induced proliferation was substantially blocked by addition of 20 nM IGFBP-2 (Fig. 3). The addition of [Leu24,59,60, Ala31]hIGF-I dose-dependently reversed the neutralizing effect of IGFBP-2 on IGF-I (ED50 = 200 nM), demonstrating the ability of the IGFBP ligand inhibitor to displace hIGF-I bound to IGFBP-2 (Fig. 3). Overall, these in vitro data clearly demonstrate that the IGFBP ligand inhibitor is capable of interacting with the binding protein in a specific manner to displace complexed IGF-I and release “free” bioactive peptide.
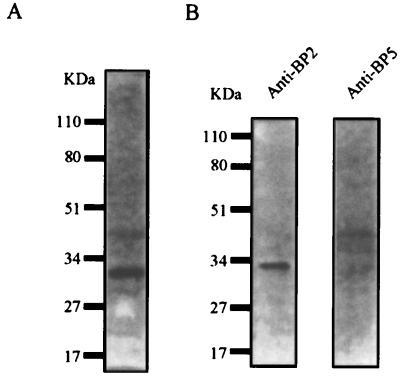
Figure 1.
Identification of IGF-binding proteins in rat cerebrospinal fluid. (A) Radioligand blot of IGF-binding proteins present in rat cerebrospinal fluid. Note that the major radiolabeled band detected has a molecular mass of ≈32 kDa, which corresponds to the molecular mass of IGFBP-2 or IGFBP-5. (B) Western blot identifying the major IGFBP in rat CSF as BP-2.
Figure 2.
Gel-filtration analysis of rat CSF showing the relative proportions of bound and “free” [125I]hIGF-I dissociated by IGF-I or the IGFBP ligand inhibitor. The Sephadex G-50F gel-filtration profiles of rat CSF in the absence (Control) or presence of hIGF-I [hIGF-I (0.1 μM)] or 1.0 μM of [Leu24,59,60, Ala31]hIGF-I [IGFBP Ligand Inhibitor (1.0 μM)] are shown. The quantified data representing the relative proportions of the hIGF-I/IGFBP complex (Bound) and “free” hIGF-I (Free) are presented in the Inset.
Figure 3.
Reversal of IGFBP-2 inhibition of hIGF-I-stimulated fibroblast proliferation by [Leu24,59,60, Ala31]hIGF-I in vitro. Human IGF-I dose-dependently stimulated DNA synthesis with an ED50 of 5–10 nM. In contrast, [Leu24,59,60, Ala31]hIGF-I did not induce DNA synthesis in 3T3 cells at any of the doses tested (0.1–8,000 nM). IGFBP-2 (20 nM) substantially inhibited the proliferative effect of 3 nM hIGF-I. Addition of IGFBP ligand inhibitor dose-dependently reversed this inhibition with an ED50 of 200 nM.
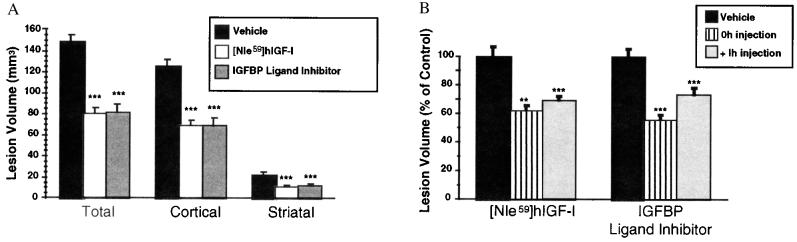
In view of the potent neuroprotective and regenerative effects of IGFs (4–16), we tested the hypothesis that displacement of IGF from its BPs in the brain could confer neuroprotection in a clinically relevant model of stroke in the rat. Although the models of ischemia [hypoxic-ischemia (4–7) and forebrain ischemia (8)] previously used to evaluate the effects of IGF-I provide important information on the effects of ischemia on the brain, they are not considered as models of stroke. It is generally accepted that occlusion of a single intracranial artery (the middle cerebral artery, MCA) provides the best model to study stroke (38). Because of the relatively large quantities of IGF-I required for in vivo studies, synthetic [Nle59]hIGF-I was employed. In [Nle59]hIGF-I the endogenous methionine at position 59 is replaced by the isosteric norleucine to eliminate the possibility of oxidizing the methionine to methionine sulfoxide during cyclization of the three disulfide bonds by air oxidation in the synthesis of the molecule. Bioassay of [Nle59]hIGF-I and hIGF-I in BALB/c 3T3 fibroblasts showed no difference in the proliferative potency between the two compounds. Adult male rats previously implanted with lateral cerebral ventricular guide cannula were subjected to ischemia by permanent occlusion of the MCA (MCAo), and the resulting brain lesion was visualized and quantified 24 h later. Animals that had received a single intracerebroventricular (i.c.v.) injection of synthetic [Nle59]hIGF-I (50 μg) or the IGFBP ligand inhibitor (50 μg) at the time of MCAo had much smaller total lesion volumes than those injected with vehicle, primarily because of a reduction of the cerebral cortical infarct volume, although some protection was also evident in the striatum (Fig. 4A). The extent of neuroprotection (40–50%) was comparable for [Nle59]hIGF-I and the IGFBP ligand inhibitor and is in keeping with that seen after treatment with NMDA receptor antagonists (39, 40). Remarkably, the extent of protection was similar whether [Nle59]hIGF-I or [Leu24,59,60, Ala31]hIGF-I was administered concurrent with (0 h) or 1 h after occlusion of the artery (Fig. 4B), providing a therapeutic window for the treatment as is available in this rat model of ischemia. In the more slowly developing hypoxic-ischemia model, IGFs confer neuroprotective effects when administered up to 2 h after the insult (4–7). These observations taken together with previous data indicating that i.c.v. injection of 50 μg hIGF-I has no impact on plasma glucose levels or body temperature of ischemic rats (7) suggest that IGFs protect neurons by interfering with the pathological pathways that are initiated after ischemia.
Figure 4.
The protective effects of [Nle59]hIGF-I and the IGFBP ligand inhibitor on ischemic brain damage. (A) In the first series of experiments, the effect of concurrent administration of [Nle59]hIGF-I or [Leu24,59,60, Ala31]hIGF-I was determined. Data are presented as mean lesion volume ± SEM. Animals injected at the time of MCAo with [Nle59]hIGF-I (50 μg, n = 7, open bar) or the IGFBP ligand inhibitor, [Leu24,59,60, Ala31]hIGF-I (50 μg, n = 6, gray bar) had dramatically and statistically reduced lesion volumes compared with animals injected with vehicle (n = 6, solid bar). Protection was observed in cerebral cortical and striatal tissue. ∗∗∗, P < 0.001. (B) The effects of delaying administration of [Nle59]hIGF-I or the IGFBP ligand inhibitor [Leu24,59,60, Ala31]hIGF-I were determined in a separate series of experiments. Data are presented as the percentage of the mean lesion size of the respective vehicle-treated group (mean ± SEM). As observed in the previous experiment, animals injected at the time of MCAo with [Nle59]hIGF-I (50 μg, n = 7, striped bar) or the IGFBP ligand inhibitor (50 μg, n = 6, striped bar) had dramatically and statistically reduced lesion volumes compared with animals injected with vehicle (n = 6, solid bar). When administration of the peptide was delayed to 1 h after MCAo, protection with [Nle59]hIGF-I (50 μg, n = 7, gray bar) was remarkably similar, and protection with IGFBP ligand inhibitor (50 μg, n = 8, gray bar) was only slightly less than observed with concurrent administration. ∗∗, P < 0.01; ∗∗∗, P < 0.001.
The mechanisms through which the IGFBP ligand inhibitor and IGFs produce their neuroprotective effects are at present unclear. Ischemic neuronal damage has been attributed, in part, to the extracellular accumulation of excitatory amino acids; in preliminary studies done in our laboratory, the IGFBP ligand inhibitor [Leu24,59,60, Ala31]hIGF-I attenuated the loss of pyramidal neurons in the hippocampus after intrahippocampal administration of quinolinic acid. In addition to producing their neuroprotective effects by interfering with endogenous mediators of ischemia such as glutamate, IGFs have the distinct advantage of also having the ability to act as regenerative growth factors. The IGFBP ligand inhibitor is capable of increasing the release of not only IGF-I but also IGF-II, which also has neuroprotective effects (13). The release of IGF-II in addition to IGF-I by the IGFBP ligand inhibitor may represent a therapeutic advantage over IGF-I treatment whose selectivity may be limited to actions at the type-I IGF receptor. Because neurodegeneration may be associated with lower levels of “free” bioactive IGFs, in part, because of increased brain expression of IGFBPs (4, 17–26), displacement of this “pool” of endogenous IGFs from their binding proteins with ligand inhibitors seems appropriate. The increased expression of brain IGFBPs (4, 17–26) may also serve to limit the actions of exogenously administered IGFs and provides strong support for the therapeutic relevance of IGFBP ligand inhibitors for the treatment of neurodegeneration. In addition, because the IGFBP ligand inhibitor approach achieves its effect by elevating local endogenous levels of “free” IGFs, a ceiling effect is reached when all the IGFs are released from IGFBPs, thus limiting the side effects that may occur after global activation of IGF receptors by exogenously administered IGF-I. This advantage is evident in other systems such as the corticotropin-releasing factor (CRF) family of peptides, in which the actions of the endogenous peptide(s) are limited by a CRF-binding protein (41). For example, CRF-binding protein ligand inhibitors, like CRF-receptor agonists, enhance learning and memory (42, 43) and blunt excessive weight gain (44) in a variety of rodent models. However, in marked contrast to the effects of a CRF-receptor agonist, CRF-binding protein ligand inhibitors do not induce anxiety (42), stimulate adrenocorticotropic hormone secretion, or elevate heart rate and blood pressure (44). A further advantage in targeting IGFBPs is that it may be possible to identify nonpeptide small molecules that act as IGFBP ligand inhibitors, with the potential for good blood–brain barrier penetration and oral activity.
In summary, our data demonstrate that pharmacological elevation of “free” endogenous IGFs in the brain confers protection in a clinically relevant model of stroke. Because of the dramatic protection observed with this strategy, even when treatment is delayed for 1 h after occlusion of the artery, these data suggest that displacement of IGFs from IGFBPs in the brain is a potential treatment for stroke. Moreover, in view of the potent actions of IGFs on survival of neurons and glial cells as well as the widespread protective effects against a variety of brain insults, IGFBP ligand inhibitors may have broader utility for the treatment of various neurodegenerative disorders as well as traumatic brain and spinal cord injury.
Acknowledgments
We thank Yan Gao, Mila Lagman, Lan Yang, and Joann Xie for their excellent technical assistance. W.W.V. has equity in and is a Member of the Board of Directors and Chairman of the Scientific Advisory Board of Neurocrine Biosciences, Inc.
ABBREVIATIONS
- IGF
insulin-like growth factor
- IGFBP
IGF binding protein
- CSF
cerebrospinal fluid
References
- 1.Jones J I, Clemmons D R. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Ullrich A, Gray A, Tam A W, Yang-Fang T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, et al. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan D O, Edman J C, Standring D N, Fried V A, Smith M C, Roth R A, Rutter W. Nature (London) 1987;329:301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. Biochem Biophys Res Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 5.Johnston B, Mallard E, Williams C, Gluckman P. J Clin Invest. 1996;97:300–308. doi: 10.1172/JCI118416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan J, Williams C E, Skinner S J, Mallard E C, Gluckman P D. Endocrinology. 1996;137:893–898. doi: 10.1210/endo.137.3.8603600. [DOI] [PubMed] [Google Scholar]
- 7.Guan J, Williams C, Gunning M, Mallard C, Gluckman P. J Cereb Blood Flow Metab. 1993;13:609–616. doi: 10.1038/jcbfm.1993.79. [DOI] [PubMed] [Google Scholar]
- 8.Zhu C Z, Auer R N. J Cereb Blood Flow Metab. 1994;14:237–242. doi: 10.1038/jcbfm.1994.30. [DOI] [PubMed] [Google Scholar]
- 9.McMorris F A, Mozell R L, Carson M J, Shinar Y, Meyer R D, Marchetti N. Ann N Y Acad Sci. 1993;692:321–334. doi: 10.1111/j.1749-6632.1993.tb26247.x. [DOI] [PubMed] [Google Scholar]
- 10.Ye P, Carson J, D’Ercole A J. J Neurosci. 1995;15:7344–7356. doi: 10.1523/JNEUROSCI.15-11-07344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth G A, Spada V, Hamill K, Bornstein M B. Dev Brain Res. 1995;88:102–108. doi: 10.1016/0165-3806(95)00088-u. [DOI] [PubMed] [Google Scholar]
- 12.Tagami M, Yamagata K, Nara Y, Fujino H, Kubota A, Numano F, Yamori Y. Lab Invest. 1997;76:603–612. [PubMed] [Google Scholar]
- 13.Cheng B, Mattson M P. J Neurosci. 1992;12:1558–1566. doi: 10.1523/JNEUROSCI.12-04-01558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galli C, Meucci O, Scorziello A, Werge T M, Calissano P, Schettini G. J Neurosci. 1995;15:1172–1179. doi: 10.1523/JNEUROSCI.15-02-01172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sortino M A, Canonico P L. Endocrinology. 1996;137:1418–1422. doi: 10.1210/endo.137.4.8625919. [DOI] [PubMed] [Google Scholar]
- 16.Dore S, Kar S, Quirion R. Proc Natl Acad Sci USA. 1997;94:4772–4777. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klempt N D, Klempt M, Gunn A J, Singh K, Gluckman P D. Mol Brain Res. 1992;15:55–61. doi: 10.1016/0169-328x(92)90151-z. [DOI] [PubMed] [Google Scholar]
- 18.Lee W-H, Clemens J A, Bondy C A. Mol Cell Neurosci. 1992;3:36–43. doi: 10.1016/1044-7431(92)90006-n. [DOI] [PubMed] [Google Scholar]
- 19.Beilharz E J, Klempt N D, Klempt M, Sirimanne E, Dragunow M, Gluckman P D. Mol Brain Res. 1993;18:209–215. doi: 10.1016/0169-328x(93)90191-q. [DOI] [PubMed] [Google Scholar]
- 20.Bergstedt K, Wieloch T. J Cereb Blood Flow Metab. 1993;13:895–898. doi: 10.1038/jcbfm.1993.112. [DOI] [PubMed] [Google Scholar]
- 21.Klempt, M., Klempt, N. D. & Gluckman, P. D. (1993) Mol. Brain Res. 17(3-4), 347–350. [DOI] [PubMed]
- 22.Beilharz E J, Bassett N S, Sirimanne E S, Williams C E, Gluckman P D. Mol Brain Res. 1995;29:81–91. doi: 10.1016/0169-328x(94)00232-4. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson D, Rash K, Clemens J. J Cereb Blood Flow Metab. 1995;15:1022–1031. doi: 10.1038/jcbfm.1995.128. [DOI] [PubMed] [Google Scholar]
- 24.Breese C R, D’Costa A, Rollins Y D, Adams C, Booze R M, Sonntag W E, Leonard S. J Comp Neurol. 1996;369:388–404. doi: 10.1002/(SICI)1096-9861(19960603)369:3<388::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee W H, Wang G M, Seaman L B, Vannucci S J. J Cereb Blood Flow Metab. 1996;16:227–236. doi: 10.1097/00004647-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Sandberg Nordqvist A C, von Holst H, Holmin S, Sara V R, Bellander B M, Schalling M. Mol Brain Res. 1996;38:285–293. doi: 10.1016/0169-328x(95)00346-t. [DOI] [PubMed] [Google Scholar]
- 27.Ling N, Esch F, Bohlen P, Brazeau P, Wehrenberg W B, Guillemin R. Proc Natl Acad Sci USA. 1984;81:4302–4306. doi: 10.1073/pnas.81.14.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam J P, Heath W F, Merrifield R B. J Am Chem Soc. 1983;105:6442–6455. [Google Scholar]
- 29.Shimasaki S, Shimonaka M, Zhang H, Ling N. J Biol Chem. 1991;266:10646–10653. [PubMed] [Google Scholar]
- 30.Liu X J, Malkowshi M, Guo Y, Erickson G F, Shimasaki S, Ling N. Endocrinology. 1993;132:1176–1183. doi: 10.1210/endo.132.3.7679972. [DOI] [PubMed] [Google Scholar]
- 31.Maciag T, Cerundolo J, Ilesley S, Kelly P R, Forand R. Proc Natl Acad Sci USA. 1979;76:5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loddick S A, Rothwell N J. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Bayne M L, Applebaum J, Chicchi G G, Miller R E, Cascieri M A. J Biol Chem. 1990;265:15648–15652. [PubMed] [Google Scholar]
- 34.Steele-Perkins G, Turner J, Edman J C, Hari J, Pierce S B, Stover C, Rutter W J, Roth R A. J Biol Chem. 1988;263:11486–11492. [PubMed] [Google Scholar]
- 35.Tong P Y, Tollefsen S E, Kornfeld S. J Biol Chem. 1988;263:2585–2588. [PubMed] [Google Scholar]
- 36.Ocrant I, Fay C T, Parmelee J T. Endocrinology. 1990;127:1260–1267. doi: 10.1210/endo-127-3-1260. [DOI] [PubMed] [Google Scholar]
- 37.Tseng L Y-H, Brown A L, Yang Y W, Romanus J A, Orlowshi C C, Taylor T, Rechler M M. Mol Endocrinol. 1989;3:1559–1568. doi: 10.1210/mend-3-10-1559. [DOI] [PubMed] [Google Scholar]
- 38.Garcia J H. Stroke. 1984;15:5–14. doi: 10.1161/01.str.15.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Minematsu K, Fisher M. Cerebrovasc Dis. 1993;3:99–104. [Google Scholar]
- 40.Hasegawa Y, Fisher M, Baron B, Metcalf G. Stroke. 1994;25:1241–1246. doi: 10.1161/01.str.25.6.1241. [DOI] [PubMed] [Google Scholar]
- 41.Potter E, Behan D P, Linton E A, Lowry P J, Sawchenko P E, Vale W W. Proc Natl Acad Sci USA. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behan D P, Heinrichs S C, Troncoso J C, Liu X-J, Ling N, De Souza E B. Nature (London) 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 43.Heinrichs S C, Vale E A, Lapsansky J, Behan D P, McLure L V, Ling N, De Souza E B, Schulteis G. Peptides. 1997;18:215–224. doi: 10.1016/s0196-9781(97)00120-4. [DOI] [PubMed] [Google Scholar]
- 44.Heinrichs S C, Lapsansky J, Behan D P, Chan R K W, Sawchenko P E, Lorang M, Ling N, Vale W W, De Souza E B. Proc Natl Acad Sci USA. 1996;93:15475–15480. doi: 10.1073/pnas.93.26.15475. [DOI] [PMC free article] [PubMed] [Google Scholar]