Abstract
We have studied the involvement of proteolytic pathways in the regulation of the Na/Pi cotransporter type II by parathyroid hormone (PTH) in opossum kidney cells. Inhibition of lysosomal degradation (by leupeptin, ammonium chloride, methylamine, chloroquine, l-methionine methyl ester) prevented the PTH-mediated degradation of the transporter, whereas inhibition of the proteasomal pathway (by lactacystin) did not. Moreover it was found (i) that whereas lysosomal inhibitors prevented the PTH-mediated degradation of the transporter they did not prevent the PTH-mediated inhibition of the Na/Pi cotransport and (ii) that treating opossum kidney cells with lysosomal inhibitors led to an increased expression of the transporter without any concomitant increase in the Na/Pi cotransport. Further analysis by subcellular fractionation and morphological techniques showed (i) that the Na/Pi cotransporter is constitutively transported to and degraded within late endosomes/lysosomes and (ii) that PTH leads to the increased degradation of the transporter in late endosomes/lysosomes.
Molecular mechanisms involved in rapid increases or decreases in membrane transport fall into two categories: alteration in transport activities (i.e., transporter kinetics) of membrane resident proteins and alterations in the number of transport proteins in the plasma membrane (1). Alterations in the number of transport proteins in the plasma membrane may be achieved by exocytic insertion of preexisting transport proteins from an intracellular vesicular pool into the plasma membrane or by endocytic retrieval of transport proteins back into intracellular storage vesicles [“shuttle” mechanism (2–4)], thereby ensuring a rapid and reversible regulation of membrane transport. Also the regulation of sodium-dependent reabsorption of phosphate (Pi) in renal proximal tubules involves alterations in the number of Na/Pi cotransporters (Na/Pi cotransporter type II) expressed in the apical membrane. Parathyroid hormone (PTH)-induced phosphaturia is related to an endocytic retrieval of type II Na/Pi cotransporters from the brush border membrane (5). A similar mechanism explains the reduced Pi reabsorption as observed after Pi refeeding of Pi-deprived rats (6). These two phenomena can also be observed in opossum kidney cells [OK cells (7, 8)], a frequently used “in vitro” system to analyze cellular mechanisms involved in the regulation of proximal tubular transport functions (9). In this cell system PTH leads to a time-dependent inhibition of apical Na/Pi cotransport activity (7). Surprisingly, recovery of the Na/Pi cotransport from PTH inhibition required de novo protein synthesis (10). Furthermore, PTH treatment of OK cells led to a time-dependent decrease of the type II Na/Pi cotransporter protein detectable on Western blots and to a parallel decrease in the transporter-related immunofluorescence at the apical surface (9). These findings suggested that PTH leads to the degradation of the type II Na/Pi cotransporter.
In the present study we have examined the possible involvement of proteolytic pathways in the PTH-mediated regulation of this transporter. Two major proteolytic systems involved in different aspects of protein breakdown are existing in mammalian cells, the lysosomal and the nonlysosomal system (11). Degradation of plasma membrane proteins as well as of proteins entering the cell by receptor-mediated endocytosis or by pinocytosis takes place in lysosomes (12–14). An important nonlysosomal proteolytic pathway is the ubiquitin-proteasome pathway (11, 15, 16) in which soluble nuclear and cytoplasmic proteins are degraded. Also integral membrane proteins that fail to fold or oligomerize correctly were found to be degraded by cytoplasmic proteasomes after they have been exported from the endoplasmic reticulum (17–22).
In the present report we demonstrate that PTH leads to the endocytosis and degradation of the renal type II Na/Pi cotransporter via the lysosomal proteolytic pathway and not to its endocytic retrieval into intracellular storage vesicles.
EXPERIMENTAL PROCEDURES
Materials.
The following reagents were purchased from Sigma: ammonium chloride, chloroquine, leupeptin, l-methionine methyl ester, methylamine. Lactacystin was obtained from Dr. E. J. Corey (Harvard University).
Cells.
All cell culture supplies were obtained from GIBCO/BRL (Basel, Switzerland). OK cells (clone 3B/2) (24) were maintained in DMEM/Ham’s F12 medium (1:1) supplemented with 10% fetal calf serum/22 mM NaHCO3/20 mM Hepes/2 mM l-glutamine in a humidified atmosphere of 5% CO2/95% air at 37°C.
Cell-Surface Biotinylation.
Biotinylation was performed similarly as previously described (23). All the steps were carried out at 4°C. 100-mm cell culture dishes (Corning) were washed 5 times with ice-cold PBS containing 1 mM MgCl2/0.1 mM CaCl2 (PBS-C/M) and gently agitated for 30 min at 4°C. Sulfo-N-hydroxysuccinimide-LC-biotin stock solutions (200 mg/ml in dimethyl sulfoxide) were stored frozen at −20°C and thawed just before use. Sulfo-N-hydroxysuccinimide-LC-biotin (Pierce) was diluted to a final concentration of 0.125 mg/ml in ice-cold PBS-C/M and used immediately. To each 100-mm dish 15 ml of this sulfo-N-hydroxysuccinimide-biotin solution was added. After 30 min of gentle agitation at 4°C the dishes were washed 5 times with ice-cold PBS.
Percoll Gradient.
Subcellular fractionation by Percoll (Pharmacia) gradients was performed similarly as previously described (25). All the steps were carried out at 4°C. After cell-surface biotinylation OK cell monolayers were washed twice with 0.9% (wt/vol) NaCl. Cells were scraped off from the dishes and suspended in 2 ml/100-mm dish buffer A (1 mM phenylmethylsulfonyl fluoride, 1 μM pepstatin, 1 μg/ml leupeptin, 250 mM sucrose, 1 mM EDTA, 10 mM triethanolamine/acetic acid, pH 6.5). Cells from 4 dishes were pooled and homogenized by 12 strokes with a tight fitting pestle in a Kontes metal Dounce tissue grinder (maximal volume, 15 ml). The resulting homogenates were pooled and centrifuged for 10 min at 1,600 rpm (400 × gav) in a Sorvall RT6000 centrifuge. The supernatant was brought to exactly 34.4 ml with buffer A and 9.15 ml of isoosmotic Percoll (density of Percoll, 1.12; initial density, 1.061). The Percoll gradient was centrifuged for 45 min at 20,000 rpm (36,000 × gav) in a Sorvall RC 5C centrifuge by using an SS34 rotor. For routine analyses the gradient was divided into three equal fractions. To eliminate Percoll 5 ml of each fraction was layered over a 3-ml 2.5 M sucrose cushion and centrifuged for 1 h at 49,000 rpm (180,000 × gav) in a Sorvall ultracentrifuge with a T875 rotor. The membrane was recovered from the interphase, diluted with buffer A to a total volume of 3 ml, and centrifuged for 1.5 h at 67,000 rpm (180,000 × gav) in a Sorvall RC M120EX centrifuge with an RP100-AT4 rotor. The supernatant was sucked off, and the membrane adhering to the Percoll pellet was resuspended in 100 μl of buffer A with a 1-ml syringe connected to a 25-gauge needle. Lysosomal marker enzyme activity was measured as previously described (25). Distribution of biotinylated cell-surface membranes in the gradient was assayed by dot blotting of individual gradient fractions onto a nitrocellulose membrane and probing the nitrocellulose with streptavidin–horseradish peroxidase.
SDS–Polyacrylamide Gel Electrophoresis and Immunoblotting.
Gradient samples as well as samples corresponding to a total cellular homogenate were analyzed by SDS-PAGE and immunoblotting. Preparation of the gradient samples was as described under Percoll Gradient. For preparation of total cellular homogenates cells were grown to confluency on 6-cm Petri dishes (Corning), washed twice with PBS, and scraped into 2 ml of PBS. The scraped-off cells were centrifuged for 10 min at 3,000 rpm in an Eppendorf centrifuge at 4°C. The supernatant was sucked off, and 250 μl of 50 mM mannitol/10 mM Hepes–Tris, pH 7.2 was added to each sample. The samples were homogenized 10 times with a 1-ml syringe connected to a 25-G needle. Total protein (25 μg) of cell homogenates was used for SDS-PAGE (9%) and subsequent transfer to nitrocellulose (Schleicher & Schuell, 0.2 μm). Immunodetection of NaPi-4 protein has been described previously (9).
Immunofluorescence.
3B/2 OK cells were grown to confluency on coverslips. These cells were processed for immunofluorescence as described previously (9).
Phosphate Uptake Measurements.
Sodium-dependent uptake of phosphate was measured in cells grown to confluency on 35-mm plastic dishes (Nunc) as described previously (26).
Incubation of Cells with PTH and Cycloheximide.
Incubation of OK cells with PTH has been described previously (27), and treatment of OK cells with cycloheximide to prevent protein synthesis also has been described previously (10).
Assay of 20 S Proteasome Activity in Crude Cell Extracts.
The 20 S proteasome activity was measured as recently described (28, 29). Cells grown to confluency in 6-cm Petri dishes (Corning) were treated for 30 min to 4.5 h with different concentrations of lactacystin. The cells were washed twice with ice-cold PBS and were scraped into 2 ml of cold 5 mM Hepes, pH 7.5, with 1 mM DTT. The scraped-off cells were homogenized with 12 strokes of a tight-fitting pestle in a Kontes metal Dounce tissue grinder. The samples were centrifuged for 10 min at 16,000 × g in the cold, and the supernatants were collected. The total soluble protein was measured by using the Bio-Rad protein assay. Treating OK cells with 10–50 μM lactacystin for 4.5 h was found to lead to an increase of the protein amount in the cytosolic fraction by about 10%, whereas treating OK cells with 1 μM lactacystin for 4.5 h and with 10–50 μM lactacystin for 30 min did not lead to a significant increase in the protein amount in the cytosolic fraction. To measure 20 S proteasome peptidase activity 2 ml of assay buffer (20 mM Hepes/0.035% (wt/vol) SDS/0.5 mM EDTA, pH 8.0) and 80 μg of the total cytosolic protein were added to a 2-ml quartz cuvette. After 5 min succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin in dimethyl sulfoxide was added into the cuvette to a final concentration of 10 μM. The substrate hydrolysis was measured with a Shimadzu RF-510 spectrofluorophotometer at 37°C by monitoring the fluorescence increase (λex = 380 nm; λem = 440 nm) because of the liberation of 7-amino-4-methylcoumarin. An initial linear increase in the fluorescence intensity was used to calculate percent inhibition of the proteasomal activity. The proteasomal peptidase activity could be completely blocked in vitro by addition of lactacystin to a final concentration of 10 μM (28, 29). For this purpose 80 μg of total cytosolic protein was preincubated for 5 min in 2 ml of assay buffer containing 10 μM lactacystin before addition of the substrate.
Presentation of the Results.
All experiments were repeated at least twice, and one representative experiment was chosen for presentation. Statistical results are expressed as means ± SE for three dishes. Significance was accepted at P < 0.05.
RESULTS
Two major cellular protein degradation systems are known to date, the lysosomal and the proteasomal system. To determine the importance of these degradation pathways for the PTH-dependent degradation of the type II Na/Pi cotransporter we used specific inhibitor(s) for each degradation system.
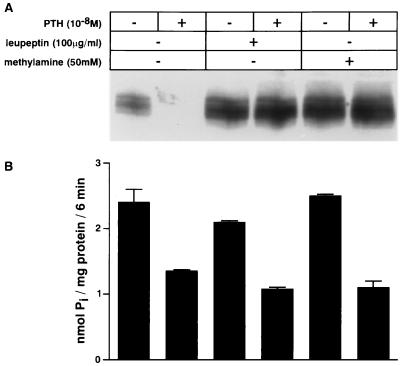
Degradation by the lysosomal pathway was blocked by the use of the following inhibitors: leupeptin, l-methionine methyl ester, and the lysosomotropic agents ammonium chloride, methylamine, and chloroquine (30–32). Cells were pretreated for 30 min with the inhibitors, followed by an incubation for 4 h with or without PTH (10−8 M) in the continued presence or absence of the inhibitor. Fig. 1A shows that leupeptin (100 μg/ml) and methylamine (50 mM) inhibited the PTH-mediated degradation of the transporter completely, whereas the PTH-mediated inhibition of the Na/Pi cotransport was unaffected (Fig. 1B). Furthermore, compared with untreated cells it is seen that treating cells with leupeptin or methylamine leads to an accumulation of the transporter detectable on Western blots, without a concomitant increase in transport activity (Fig. 1 A and B). Densitometric analysis revealed that leupeptin and methylamine led to an increase in the amount of the transporter by about 100% in Fig. 1A, whereas total cellular protein content increased only slightly by about 10% (data not shown). The same results as shown in Fig. 1 for leupeptin and methylamine were also obtained for l-methionine methyl ester (20 mM) as well as for ammonium chloride (50 mM) and chloroquine (0.2 mM) (data not shown). These results suggest that lysosomal degradation participates in a high turnover of the type II Na/Pi cotransporter under control conditions as well as in its PTH-dependent degradation. In addition Fig. 1 A and B shows that PTH alone leads to the almost complete degradation of the transporter but only to a 50% decrease of the Na/Pi cotransport. As described previously (9), the residual Na/Pi cotransport activity cannot be related to the type II Na/Pi cotransporter and suggests that at least two Na/Pi cotransport systems are expressed at the apical membrane of OK cells.
Figure 1.
Inhibitors of lysosomal degradation lead to an accumulation of the type II Na/Pi cotransporter and prevent its PTH-mediated degradation. OK cells were pretreated with leupeptin (100 μg/ml) or methylamine (50 mM) for 30 min. After the pretreatment cells were incubated for 4 h with or without PTH (10−8 M) in the continued presence or absence of the corresponding lysosomal inhibitor. The effects of the lysosomal inhibitors were investigated by immunodetection of the Na/Pi cotransporter on Western blot (A) as well as by measuring Na/Pi cotransport (B).
Lactacystin is a potent inhibitor of proteasomal degradation (33, 34). To block the proteasomal pathway cells were pretreated for 30 min with lactacystin before incubation for 4 h with or without PTH in the continued presence of the proteasomal inhibitor. Inhibition of the proteasomal activity by lactacystin was assayed after preincubation with lactacystin as well as at the end of the PTH treatment. To this end a cytosolic fraction was isolated, and the proteolysis of a fluorogenic proteasomal substrate was measured (Table 1) (ref. 28; see also Experimental Procedures). Fig. 2 and Table 1 show that inhibition of the proteasomal activity did not prevent the PTH-mediated degradation of the transporter. In parallel experiments we measured the effect of the lactacystin treatment on the inhibition of the sodium-dependent phosphate transport by PTH. In agreement with the data shown in Fig. 2 it was found that the PTH-mediated inhibition of the Na/Pi cotransport was not prevented by this treatment (data not shown). In Fig. 2 it is furthermore seen that incubating cells with increasing concentrations of lactacystin led to an increased expression of the transporter in cells not treated with PTH. Whether this increased expression of the transporter is a direct or indirect consequence of the inhibition of the proteasomal pathway is unknown. As the proteasome is involved both in the normal turnover of cellular proteins (35) as well as in the processing and degradation of regulatory proteins controlling cell growth and metabolism (36, 37), proteasomal inhibitors can obviously have profound biological consequences. Together the results presented in Figs. 1 and 2 show that the PTH-mediated degradation of the Na/Pi cotransporter can be prevented by inhibitors of the lysosomal pathway but not by inhibition of the proteasomal pathway.
Table 1.
Inhibition of the proteasomal activity by lactacystin in OK cells
| Lactacystin treatment | Inhibition (%) |
|---|---|
| 0 μM (30 min) | 0 |
| 1 μM (30 min) | 64 |
| 10 μM (30 min) | 83 |
| 50 μM (30 min) | 97 |
| 0 μM (4.5 h) | 0 |
| 1 μM (4.5 h) | 82 |
| 10 μM (4.5 h) | >99 |
| 50 μM (4.5 h) | >99 |
Cells were incubated for 30 min or 4.5 h with different concentrations of the proteasomal inhibitor lactacystin. The cells were homogenized and centrifuged for 10 min at 16,000 × g in the cold, and the supernatants were assayed for proteasomal activity. Proteasomal peptidase activity was measured by monitoring the proteolysis of the fluorogenic proteasomal substrate succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (28). The percent inhibition by each treatment (relative to no treatment) is shown. For the details refer to Experimental Procedures.
Figure 2.
Inhibition of proteasomal activity by lactacystin does not prevent the PTH-mediated degradation of the type II Na/Pi cotransporter. OK cells were pretreated with different concentrations of lactacystin for 30 min. After the pretreatment cells were incubated for 4 h with or without PTH (10−8 M) in the continued presence of the corresponding lactacystin concentration. Of each sample equal amounts of protein were analyzed by SDS-PAGE and immunoblotting.
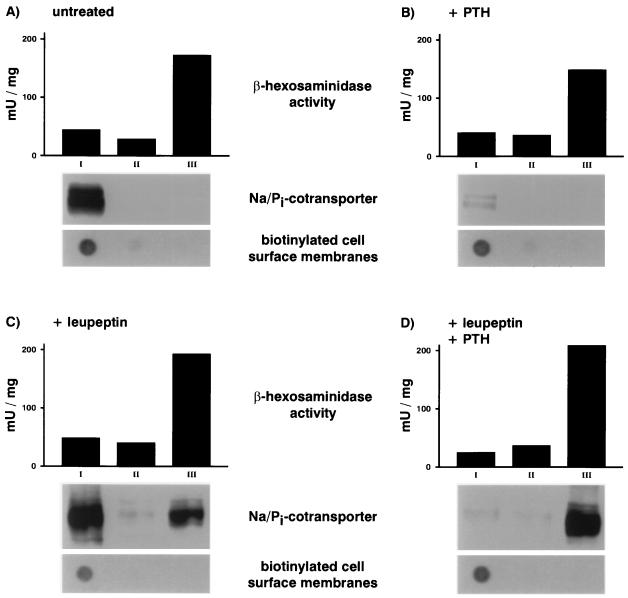
Next we performed subcellular fractionation studies on Percoll gradients. The lysosomal degradation in these experiments was blocked by the use of leupeptin. To obtain a reliable marker for cell-surface membranes, cells were surface-biotinylated in the cold before their homogenization. After centrifugation the gradient was fractionated for routine analysis into three fractions of equal volume: fraction I containing low density membranes, fraction II containing membranes of intermediate density, and fraction III containing high density membranes. Each of these fractions was analyzed for the activity of the lysosomal enzyme marker β-hexosaminidase (upper panels in Fig. 3 A–D), the presence of biotinylated cell-surface membranes (lower panels in Fig. 3 A-D), and the expression of the Na/Pi cotransporter (middle panels in Fig. 3 A-D). To determine the subcellular distribution of the transporter equal volumes of the gradient fractions were analyzed by SDS-PAGE and immunoblotting. In parallel, equal volumes of the same fractions were dot-blotted and probed with streptavidin–horseradish peroxidase to detect biotinylated cell-surface membranes by chemiluminescence (see Experimental Procedures). Fractionation of untreated cells showed that the transporter is contained exclusively in fraction I, which is highly enriched in biotinylated cell-surface membranes (Fig. 3A). After treating OK cells for 4 h with PTH (10−8 M) the transporter is barely detectable in any of the three gradient fractions (Fig. 3B), consistent with the almost complete degradation of the transporter. After treating cells for 4.5 h with leupeptin (100 μg/ml) the transporter is contained not only in the lightest gradient fraction but also in the heaviest gradient fraction, which is enriched for the lysosomal enzyme marker β-hexosaminidase (Fig. 3C), suggesting that in the absence of PTH the transporter is constitutively transported to and degraded in late endosomes/lysosomes. Fig. 3D depicts the results from fractionation of cells pretreated for 30 min with leupeptin and then treated for 4 h with PTH (10−8 M) in the continued presence of leupeptin. Comparing Fig. 3D with Fig. 3C it is seen that PTH leads to the disappearance of the transporter in fraction I, which is paralleled by an increase in the amount of transporter detectable in fraction III with no changes occurring in the distribution of the lysosomal enzyme marker activity and of the biotinylated cell-surface membranes. Thus PTH leads to a shift in the subcellular distribution of the transporter from a fraction enriched for biotinylated cell-surface membranes to the heaviest gradient fraction enriched for the lysosomal enzyme marker β-hexosaminidase.
Figure 3.
Subcellular distribution of the type II Na/Pi cotransporter in untreated OK cells and in OK cells treated with PTH and/or leupeptin. OK cells were pretreated with nothing or leupeptin (100 μg/ml) for 30 min. After the pretreatment cells were incubated for 4 h with or without PTH (10−8 M) in the continued presence or absence of the lysosomal inhibitor. Before homogenization cells were surface-labeled with biotin. After centrifugation the Percoll density gradients were fractionated into three fractions of equal volume: fraction I containing low density membranes, fraction II containing membranes of an intermediate density, and fraction III containing high density membranes. Each of these fractions was analyzed for the activity of the lysosomal enzyme marker β-hexosaminidase, the presence of biotinylated cell-surface membranes, and the expression of the Na/Pi cotransporter. To determine the subcellular distribution of the transporter equal volumes of the gradient fractions were analyzed by SDS-PAGE and immunoblotting. In parallel, equal volumes of the same fractions were dot-blotted and probed with streptavidin–horseradish peroxidase to detect biotinylated cell-surface membranes. Depicted above are the distribution of the β-hexosaminidase, the Na/Pi cotransporter, and the biotinylated cell-surface membranes in untreated cells (A), in cells treated for 4 h with PTH (10−8 M) (B), in cells treated for 4.5 h with leupeptin (100 μg/ml) (C), and in cells pretreated for 30 min with leupeptin and treated thereafter for 4 h with PTH in the presence of the lysosomal inhibitor (D).
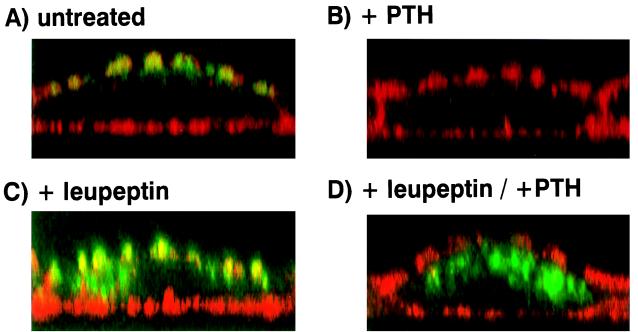
To complement the above data we investigated the effect of leupeptin and other lysosomal inhibitors on the PTH-mediated regulation of the transporter by confocal microscopy. Fig. 4 depicts immunohistochemical double stainings of β-actin (red) and the Na/Pi cotransporter (green) in OK cells. In the absence of PTH and any lysosomal inhibitor the transporter is localized exclusively at the apical membrane (Fig. 4A), whereas 4 h of PTH treatment led to its almost complete disappearance at the apical membrane, without any intracellular appearance (Fig. 4B). In cells treated for 4.5 h with leupeptin the transporter did not only localize to the apical membrane but is also seen in intracellular structures (Fig. 4C). PTH in the continued presence of leupeptin led to a retrieval of the transporter from the apical surface (Fig. 4D). Because of accumulation of intracellular transporters in the presence of leupeptin alone an increase in intracellular staining cannot be observed after PTH treatment. Similar results were obtained with cells treated with the lysosomotropic agents ammonium chloride (50 mM), methylamine (50 mM), and chloroquine (0.2 mM) (data not shown). These immunofluorescence experiments are in complete agreement with above subcellular fractionation studies showing that blocking lysosomal degradation by leupeptin leads to the accumulation of the transporter in a heavy gradient fraction enriched for the lysosomal enzyme marker β-hexosaminidase. Thus a late endosomal/lysosomal degradation system is involved in PTH control as well as in “normal” turnover of the type II Na/Pi cotransporter.
Figure 4.
Subcellular localization of the type II Na/Pi cotransporter by confocal microscopy in untreated OK cells and in OK cells treated with PTH and/or leupeptin. OK cells grown to confluency on glass coverslips were pretreated with nothing or leupeptin (100 μg/ml) for 30 min. After the pretreatment cells were incubated for 4 h with or without PTH (10−8 M) in the continued presence or absence of the lysosomal inhibitor. Depicted above are immunohistochemical double stainings of β-actin (red) and the Na/Pi cotransporter (green) in untreated OK cells (A), in cells treated for 4 h with PTH (10−8 M) (B), in cells treated for 4.5 h with leupeptin (100 μg/ml) (C), and in cells pretreated for 30 min with leupeptin and treated thereafter for 4 h with PTH in the presence of the lysosomal inhibitor (D).
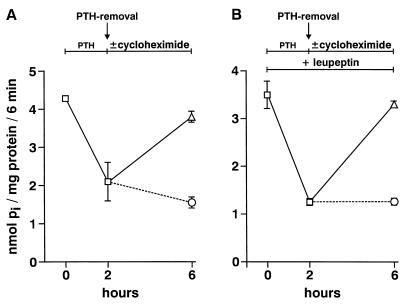
Finally we tested whether in cells treated with leupeptin and PTH the intracellularly accumulated Na/Pi cotransporters could be reutilized after PTH withdrawal (Fig. 5). For this purpose cells have been preincubated for 30 min with or without leupeptin. After preincubation cells were treated for 2 h with PTH (10−8 M) in the continued presence or absence of leupeptin. PTH was removed by washing the cells twice with normal medium, and the cells were incubated for 4 h in normal medium with or without cycloheximide (100 μM) again in the continued presence or absence of leupeptin. Fig. 5 shows that de novo synthesis is needed for the recovery of the Na/Pi cotransport from PTH inhibition. This suggests that de novo synthesis of transporters is needed for recovery in the presence as well as in the absence of leupeptin, i.e., the transporters accumulated intracellularly do not recycle back to the plasma membrane.
Figure 5.
Requirement of de novo synthesis for the recovery of the Na/Pi cotransport from PTH inhibition. OK cells have been preincubated for 30 min with (B) or without (A) leupeptin (100 μg/ml) followed by an incubation for 2 h with PTH (10−8 M) in the continued presence (B) or absence (A) of leupeptin. Thereafter cells were washed twice with normal medium and incubated for 4 h with (○) or without (▵) cycloheximide (100 μM) in the continued presence (B) or absence (A) of leupeptin.
DISCUSSION
Rapid increases or decreases in the number of transport proteins in the plasma membrane can be achieved by either recruiting preexisting transporters from an intracellular vesicular pool or by endocytic retrieval of transporters into intracellular storage vesicles (“shuttle” mechanism). Well characterized examples of such a regulation are provided by the insulin-regulatable glucose transporter GLUT-4 (2, 3) and the water channel aquaporin-2 (4). Also the regulation of the Na/Pi reabsorption in renal proximal tubules occurs via alterations in the number of Na/Pi cotransporters (Na/Pi cotransporter type II) expressed in the apical membrane of these tubules (5, 6). However, regulation of this transporter might involve degradation and consequently also resynthesis of the transporter (9).
By using specific inhibitors for the lysosomal pathway (30–32) we found in the present study that PTH-mediated degradation of the transporter is almost completely inhibited, whereas inhibition of proteasomal degradation by lactacystin (33, 34) did not significantly inhibit the PTH-mediated degradation of this transporter. Moreover it was found (i) that whereas lysosomal inhibitors prevented the PTH-mediated degradation of the transporter they did not prevent the PTH-mediated internalization of the Na/Pi cotransport and (ii) that treating OK cells with lysosomal inhibitors led to an increased expression of the transporter without any concomitant increase in the Na/Pi cotransport. By further analysis of these findings with the use of subcellular fractionation and morphological techniques we show (i) that the Na/Pi cotransporter is constitutively transported to and degraded within late endosomes/lysosomes and (ii) that PTH led to the increased degradation of the transporter in late endosomes/lysosomes. Furthermore, results presented in this report suggest that not only the lysosomal degradative pathway is involved in the normal turnover but also the proteasomal pathway.
Down-regulation by lysosomal degradation is a well known mechanism leading to the desensitization of cell-surface hormone receptors. The similarities between the down-regulation of certain hormone receptors (e.g., the epidermal growth factor receptor) and the Na/Pi cotransporter type II are striking. (i) Exposure of fibroblast cells to epidermal growth factor leads to the almost complete degradation of the epidermal growth factor receptor within a few hours (38); also exposure of OK cells to PTH leads to the almost complete degradation of the Na/Pi cotransporter in lysosomes within a few hours (9). (ii) Removal of the growth factor leads to the recovery of the level of cell-surface hormone receptors in fibroblasts but only after 8–9 h (39); also removal of PTH leads to the recovery of the level of Na/Pi cotransport and Na/Pi cotransporter in OK cells but only after 8–10 h (7, 9). (iii) Either recovery process is known to be inhibited by cycloheximide, i.e., synthesis of new receptors or transporters is needed to replace the degraded ones (10, 39). (iv) Also a seemingly rather high turnover (40) of the Na/Pi cotransporter is in common with many cell-surface receptors. These similarities between the regulation of certain cell-surface receptors and the Na/Pi cotransporter type II suggest that similar mechanisms underlie their regulation. Comparing the regulation of cell-surface receptors to the regulation of plasma membrane transport proteins in general shows that they occur by similar ways. Rapid desensitization of receptors within a few minutes occurs by two means (40). Receptors may remain on the cell surface, but they are uncoupled from the normal intracellular signal transduction, or they may be internalized into intracellular vesicles so that they can no longer interact with their agonists. Similarly rapid inhibition of transport within a few minutes occurs by changing the kinetics of membrane resident transport proteins or by endocytic retrieval of the transport proteins into intracellular storage vesicles (1–4). Slow desensitization of receptors is achieved by endocytic retrieval and degradation of the receptors in lysosomes (down-regulation). This process occurs with half-lives in the region of hours and can result in a 10-fold reduction in receptor level (40). In the present report we show for the first time that inhibition of solute transport can occur in analogy to the down-regulation of cell-surface receptors by endocytic retrieval of the transporter followed by its lysosomal degradation; PTH leads to the endocytosis of the Na/Pi cotransporter type II followed by its degradation in late endosomes/lysosomes. As it is the case for the down-regulation of cell-surface hormone receptors, also the down-regulation of Na/Pi cotransport (27) by endocytosis and degradation of the transport protein has been found to be a rather slow process compared with inhibition of transporters by other means (2–4, 41).
In conclusion, PTH leads to the internalization and late endosomal/lysosomal degradation of the renal Na/Pi cotransporter type II in OK cells. Although this PTH-mediated degradation of the transporter cannot be prevented by the inhibition of the proteasomal degradation pathway we provide evidence that the proteasomal pathway participates together with the lysosomal pathway in the turnover of the type II Na/Pi cotransporter under control conditions.
Acknowledgments
We thank C. Gasser for professional assistance in preparing the figures. This work was supported by Swiss National Science Foundation Grant 31.46523 (to H.M.).
ABBREVIATIONS
- PTH
parathyroid hormone
- OK cells
opossum kidney cells
References
- 1.Bradbury N A, Bridges R J. Am J Physiol. 1994;267:C1–C24. doi: 10.1152/ajpcell.1994.267.1.C1. [DOI] [PubMed] [Google Scholar]
- 2.James D E, Strube M, Mueckler M. Nature (London) 1989;338:83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- 3.James D E, Brown R, Navarro J, Pilch P F. Nature (London) 1988;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 4.Knepper M A, Wade J B, Terris J, Ecelbarger C A, Marples D, Mandon B, Chou C C, Kishore B K, Nilsen S. Kidney Int. 1996;49:1712–1717. doi: 10.1038/ki.1996.253. [DOI] [PubMed] [Google Scholar]
- 5.Kempson S A, Lötscher M, Kaissling B, Biber J, Murer H, Levi M. Am J Physiol. 1995;268:F784–F791. doi: 10.1152/ajprenal.1995.268.4.F784. [DOI] [PubMed] [Google Scholar]
- 6.Levi M, Lötscher M, Sorribas V, Custer M, Arar M, Kaissling B, Murer H, Biber J. Am J Physiol. 1994;267:F900–F908. doi: 10.1152/ajprenal.1994.267.5.F900. [DOI] [PubMed] [Google Scholar]
- 7.Malmström K, Murer H. Am J Physiol. 1986;251:C23–C31. doi: 10.1152/ajpcell.1986.251.1.C23. [DOI] [PubMed] [Google Scholar]
- 8.Biber J, Forgo J, Murer H. Am J Physiol. 1988;255:C155–C161. doi: 10.1152/ajpcell.1988.255.2.C155. [DOI] [PubMed] [Google Scholar]
- 9.Pfister M F, Lederer E, Forgo J, Ziegler U, Lötscher M, Quabius E S, Biber J, Murer H. J Biol Chem. 1997;272:20125–20130. doi: 10.1074/jbc.272.32.20125. [DOI] [PubMed] [Google Scholar]
- 10.Malmström K, Murer H. FEBS Lett. 1987;216:257–260. doi: 10.1016/0014-5793(87)80701-9. [DOI] [PubMed] [Google Scholar]
- 11.Ciechanover A. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 12.Hare J F. Biochim Biophys Acta. 1990;1031:71–90. doi: 10.1016/0304-4157(90)90003-u. [DOI] [PubMed] [Google Scholar]
- 13.Lamaze C, Schmid S L. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 14.Mellman I. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 15.Hilt W, Wolf D H. Trends Biochem Sci. 1996;21:96–102. [PubMed] [Google Scholar]
- 16.Hochstrasser M. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 17.Jensen T J, Loo M A, Pind S, Williams D B, Goldberg A L, Riordan J R. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 18.Ward C L, Omura S, Kopito R R. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 19.Biederer T, Volkwein C, Sommer T. EMBO J. 1996;15:2069–2076. [PMC free article] [PubMed] [Google Scholar]
- 20.Wiertz E J H J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 21.Wiertz E J H J, Tortorella D, Bogyo M, Yu J, Mothes W, Jones T R, Rapoport T A, Ploegh H L. Nature (London) 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 22.Kopito R R. Cell. 1997;88:427–430. doi: 10.1016/s0092-8674(00)81881-4. [DOI] [PubMed] [Google Scholar]
- 23.Sargiacomo M, Lisanti M, Graeve L, Le Bivic A, Rodriguez-Boulan E. J Membr Biol. 1989;107:277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- 24.Sorribas V, Markovich D, Hayes G, Stange G, Forgo J, Biber J, Murer H. J Biol Chem. 1994;269:6615–6621. [PubMed] [Google Scholar]
- 25.Matter K, Stieger B, Klumperman J, Ginsel L, Hauri H-P. J Biol Chem. 1990;265:3503–3512. [PubMed] [Google Scholar]
- 26.Reshkin S J, Forgo J, Murer H. Pflügers Arch. 1990;416:554–560. doi: 10.1007/BF00382689. [DOI] [PubMed] [Google Scholar]
- 27.Reshkin S J, Forgo J, Murer H. Pflügers Arch. 1990;416:624–631. doi: 10.1007/BF00370606. [DOI] [PubMed] [Google Scholar]
- 28.Dick R L, Cruikshank A A, Destree A T, Grenier L, McCormack T A, Melandri F D, Nunes S L, Palombella V J, Parent L A, Plamondon L, Stein R L. J Biol Chem. 1997;272:182–188. doi: 10.1074/jbc.272.1.182. [DOI] [PubMed] [Google Scholar]
- 29.Dick R L, Cruikshank A A, Grenier T A, Melandri F D, Nunes S L, Stein R L. J Biol Chem. 1996;271:7273–7276. doi: 10.1074/jbc.271.13.7273. [DOI] [PubMed] [Google Scholar]
- 30.Poole B, Ohkuma S. J Cell Biol. 1981;90:665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libby P, Goldberg A L. Science. 1978;199:534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- 32.Reeves J P, Decker R S, Crie J S, Wildenthal K. Proc Natl Acad Sci USA. 1981;78:4426–4429. doi: 10.1073/pnas.78.7.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenteany G, Standaert R F, Reichard G A, Corey E J, Schreiber S L. Proc Natl Acad Sci USA. 1994;91:3358–3362. doi: 10.1073/pnas.91.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 35.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 36.Palombella V J, Rando O J, Goldberg A L, Maniatis T. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 37.Treier M, Staszewski L M, Bohmann D. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 38.Stoscheck C M, Carpenter G. J Cell Biol. 1984;98:1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter G, Cohen S. J Cell Biol. 1976;71:159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohse M J. Biochim Biophys Acta. 1993;1179:171–188. doi: 10.1016/0167-4889(93)90139-g. [DOI] [PubMed] [Google Scholar]
- 41.Helmle-Kolb C, Montrose M H, Stange G, Murer H. Pflügers Arch. 1989;415:461–470. doi: 10.1007/BF00373624. [DOI] [PubMed] [Google Scholar]