Abstract
There are well known mechanistic similarities in human physiology between adaptations for endurance performance and hypoxia tolerance. By using background principles arising from recent studies of the evolution of the diving response in marine mammals, here we analyze human responses to hypobaric hypoxia based on studies with several different low and high altitude human lineages. As in the evolution of the diving response in pinnipeds, we found “conservative” and “adaptable” physiological characters involved in human responses to hypoxia. Because the analysis concerns traits within a single species, conservative characters dominate the picture (they define basic human physiology and largely are independent of environmental parameters). Most notably, we also found evidence for adaptable characters forming the foundations for a fairly unique physiological phenotype—a low capacity version favored under hypobaric hypoxia and a high capacity one favored for endurance performance. Because current evidence implies that the human species arose under conditions that were getting colder, drier, and higher (situations in which these traits would have been advantageous), we hypothesize that this physiology is our “ancestral” condition.
Keywords: high altitude, Quechua, Sherpa, evolutionary physiology
Numerous recent studies on origins and evolution of hominids imply that the human species arose in environments that were getting drier and higher under which endurance performance capacities and hypoxia tolerance would have been favored (1). This idea is interesting to us because biomedical researchers have long known that there are quite a few mechanistic similarities in human physiology between adaptations for endurance performance and for hypoxia tolerance (2–5). How physiological systems for hypoxia tolerance or endurance performance might have evolved within our phylogeny has remained unknown and uninvestigated in part because, until recently, there were few if any guidelines for tracing the evolutionary pathways of complex physiological systems in humans or in animals. At least, initial guidelines have arisen from recent quantitative analyses of the variability of the diving response in pinnipeds. These studies (6) led to three principles of evolution of the diving response that may be generally applicable to the evolution of complex physiological systems: (i) Some physiological/biochemical characters considered necessary in diving animals are conserved in all pinnipeds; these traits, presumably maintained largely by negative selection (any mutations affecting them not surviving), include diving apnea, bradycardia, tissue hypoperfusion, and hypometabolism of hypoperfused tissues. At this stage in our understanding of diving physiology and biochemistry, we are unable to detect any correlation between these characters and diving capacity; (ii) a few other physiological/biochemical characters are more malleable and are correlated with long duration diving and prolonged foraging at sea. These characters include spleen weight, blood volume, and red blood cell (RBC) mass. The larger these are, the greater the diving capacity (defined as diving duration). Because the relationships between diving capacity and any of these traits are evident even when corrected for body weight, it is reasonable to conclude that these three traits—large spleens, large blood volume, and large RBC mass—extend diving duration. That is, in contrast to conserved traits such as bradycardia, these kinds of characters have evolved presumably by positive selection to enable prolonged dive times; and (iii) the evolutionary physiology of the diving response thus can be described in terms of the degree of development of adaptable vs. conservative categories of diving characters, i.e., in terms of how these patterns change through time and how the patterns are lineage-specific.
Time and Adaptation.
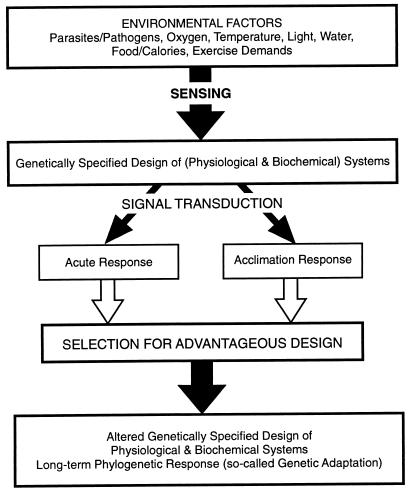
With these studies of the evolution of the diving response in marine mammals (6) as background, we decided to analyze human responses to hypobaric hypoxia based on studies (7–16) with several different low and high altitude human lineages. To appreciate our approach, it is important to understand that the strategies used for dealing effectively with environmental or other selectively significant parameters depend on the time available for the response. Traditionally, the timeline for response is divided into three categories: acute, acclimatory, and genetic or phylogenetic (17). The formal relationship among these three timelines of responses is shown diagrammatically in Fig. 1 and involve several key components. First, initiating the whole cascade are sensing mechanisms, which tell the organism when the problem arises and perhaps how serious it is. Second, this information must be transduced at various levels of organization into the appropriate functional responses. Third, a specific set of signal transduction pathways is involved in acute responses to the stress. Fourth, either the same or different sets of signal transduction pathways may be used to orchestrate more complex acclimatory responses. And, fifth, all of the above—the sensing step, the signal transduction pathways, the acute response, and the acclimatory responses—may be changed gradually through evolutionary time.
Figure 1.
Diagrammatic summary of the formally defined relationships between time and physiological responses to environmental change. Acute responses are those that occur essentially instantaneously with the environmental change. Adjustments requiring some fraction of the organism’s lifetime (from minutes, to hours, to days to reach a new steady state) are termed “acclimatory responses” or “acclimations”. In the North American literature, the response is termed an “acclimatization” if it occurs naturally (in which parameters other than the one of interest cannot be controlled fully). Only acute and acclimatory responses are possible within a given generation. However, all components of the cascade (from sensing and signal transduction to acclimatory response) can change through evolutionary time, a process defined in the literature as “phylogenetic adaptation” (17, 35, 42).
Acute and Acclimation Responses to Hypoxia—Lowlanders.
In acute high altitude exposure in lowlanders, hypoxia defenses are initiated by several O2-sensing, signal transduction pathways. In brief, we can describe these as five general hypoxia response systems each initiated by a different hypoxia-sensing mechanism: (i) carotid body O2 sensors (18) initiate the hypoxic ventilatory response (HVR) (19) that, despite risk of alkalosis (20), helps to compensate for O2 shortage; (ii) pulmonary vasculature O2 sensors (21) initiate regulation of the hypoxic pulmonary vasoconstrictor response and, thus, adjustments in lung perfusion (22, 23) and in ventilation–perfusion matching (22); (iii) O2 sensors in the vasculature of other tissues activate the expression of vascular endothelial growth factor 1 (24) with its receptor (25) and thus initiate angiogenesis especially in the heart (26, 27) and probably in the brain (28); (iv) O2 sensors in kidney and liver activate expression of erythropoietin and so begin the process of up-regulating RBC mass (29–32); and (v) tissue-specific O2-sensing and signal transduction pathways lead to metabolic reorganization (9, 10, 33) at least in part by altering expression rates of hypoxia-sensitive genes for metabolic enzymes and metabolite transporters (32, 34, 35).
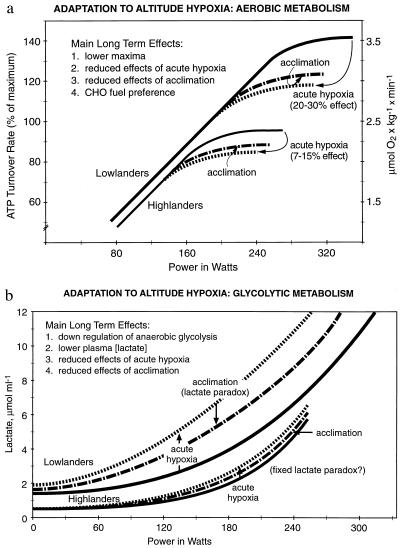
Although there is no sharp line separating acute and acclimatory phases of hypoxia exposure (17, 35), it is clear that most hypoxia response systems do not have time to go to completion during acute hypoxia. Thus, despite these adjustments being activated, the effects of acute hypoxia exposure are considerable. This result can be illustrated by considering an exercise protocol under acute hypoxia (equivalent to ≈4,200 m altitude), in which a relatively large (20–35%) decline in V̇O2 max in lowlanders is observed (Fig. 2a). Furthermore, metabolic attempts to make up the energy deficit caused by O2 lack [or to redress the balance between lactate appearance and disappearance (2)] are expressed as large increases in lactate accumulation in the blood during exercise (Fig. 2b).
Figure 2.
(a) Effect of acute and acclimatory exposure to hypoxia on aerobic metabolism during exercise in two human lineages (lowlanders compared with Andean and Himalayan natives). Diagrammatic summary based on lowlander and Quechua data from Hochachka et al. (7). Main long term effects shown in Inset refer to the metabolic responses in Quechuas (7). ATP turnover rates during exercise standardized to Quechua data (7). (b) Effect of acute and acclimatory exposure to hypoxia on plasma lactate response during exercise in two human lineages (lowlanders compared with Andean and Himalayan natives). Diagrammatic summary based on lowlander and Quechua data from Hochachka et al. (7). Main long term effects shown in Inset refer to metabolic responses in Quechuas (7).
With continued exposure of lowlanders to hypoxia, acclimation processes: (i) increase the hypoxia sensitivity of the HVR [at the biochemical level, this process may require increasing the O2 affinity of the O2 sensor (33, 34)], and for a given hypoxic stimulus, the ventilatory response is exaggerated (19); (ii) extend the hypoxic pulmonary vasoconstrictor response sometimes causing hypertension (22); (iii) maintain angiogenesis (22, 28); (iv) maintain erythropoiesis and up-regulation of RBC mass (7, 36), which, if prolonged through an individual’s lifetime, may lead to Monge’s disease (36); and (v) allow gene-based metabolic reorganization, one expression of which is an increased carbohydrate preference during exercise (37). These acclimations typically require days to weeks to reach new steady states.
In terms of compensating for the O2 deficit of hypoxia, acclimations are effective more than are acute adjustments. This can be illustrated as above with a whole body exercise protocol. After acclimation in lowlanders, the V̇O2 max is still affected by hypoxia but to a lesser degree than previously (Fig. 2a). There is less deficit caused by O2 lack and less lactate accumulation (Fig. 2b). The attenuation of lacate accumulation despite maintained hypoxia has been perplexing to physiologists. Because it was noted that the higher the altitude for acclimation, the lower the blood [lactate] during a given exercise protocol, the attenuation became known as the lactate paradox (2, 7). In the context of this analysis, the key insight is that essentially all acute hypoxia response systems can be adjusted further during acclimation (22, 33, 36).
Highlander Acute and Acclimation Responses.
To assess how these traits change through generational time, we compared the above acute and acclimatory patterns of lowlanders to those found in indigenous highlanders. We used a framework derived from earlier studies of the diving response in pinnipeds that identified two categories of (conservative vs. adaptable) physiological characters used in orchestrating the evolution of this complex physiological system (6). As in the pinniped example, current evidence indicates that “conservative” and “adaptable” physiological characters are involved in human responses to hypoxia. Because now we are dealing with traits within a single species, conservative characters clearly are dominant and are too numerous to outline in detail; three examples are Hb O2 affinity and regulation (36), muscle organization into different fiber types (11, 38, 39), and the region-specific organization of brain metabolism with the brain’s almost exclusive preference for glucose as a fuel (13, 14). These kinds of physiological traits—which in sum make up most of our physiology—appear to be conserved through phylogenetic time by negative selection, and the ways they are used on hypoxia exposure appear common in humans no matter what the O2 content of the inspired air in the normal environment.
Phenotype for Hypoxia Tolerance.
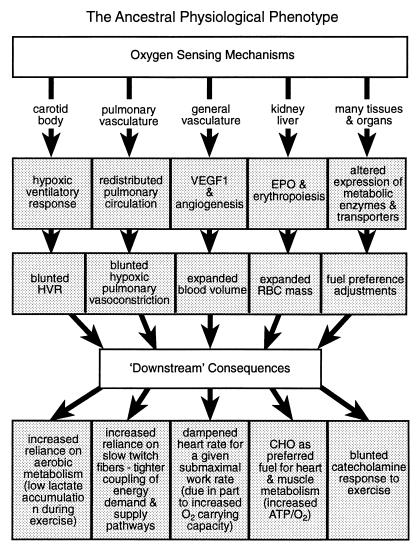
Most notably, we also found evidence for adaptable characters that were similar in Quechuas and Sherpas. Such traits appeared at all levels of organization examined and can be summarized as adjustments in the above five loosely linked response systems that seem to be a key to the complex physiology of hypoxia tolerance (Fig. 3): (i) blunted HVR, mediated by the carotid body O2 sensor and serving to counteract potential alkalosis arising from hyperventilation (20, 40, 41); (ii) blunted hypoxic pulmonary vasoconstrictor response mediated by pulmonary vasculature O2 sensors and functioning to minimize risks of pulmonary hypertension (23); (iii) up-regulated expression of vascular endothelial growth factor 1 (vascular O2 sensors), angiogenesis, and thus increased blood volume (36); (iv) up-regulated erythropoietin expression (kidney O2 sensors), erythropoiesis, and thus increased RBC mass and O2-carrying capacity (36); and (v) regulatory adjustments of metabolic pathways to alter fuel preferences and, in skeletal muscle, to attenuate concentrations of enzymes especially in aerobic metabolic pathways and thus alter ratios of glycolytic/mitochondrial metabolism (5, 9, 10, 15). These in turn lead to several fundamental downstream consequences (Fig. 3). For example, we find that in Andean and Himalayan natives maximum aerobic and anaerobic exercise capacities (Fig. 2 a and b) are down-regulated (7–10). The acute effects of hypoxia (expressed as imbalances between lactate appearance and disappearance rates) expected from lowlanders are blunted (2, 7, 10, 33, 36), and metabolic acclimation effects (7, 8, 36) also are attenuated (Fig. 2 a and b). The in vivo biochemical properties of skeletal muscles, formed predominantly of slow twitch fibers (38, 39), are consistent with up-regulation of oxidative vs. glycolytic contributions to energy supply, thus improved yield of ATP per mole of carbon fuel used (42). Of interest, the relative ratios of fiber types do not change with acclimation of highlanders to low altitudes (39). The relative preponderance of slow fibers helps to explain improved coupling between ATP demand and ATP supply pathways [lesser perturbation of phosphate metabolite pools during rest–exercise transitions (43)], lower lactate accumulation (7), and improved endurance especially under submaximal conditions (8). In fact, low lactate accumulation during exercise is traditionally one of the most characteristic metabolic hallmarks of indigenous highlanders (7, 36, 42). Kenyans native to medium altitude environments, although not as intensively studied (44, 45), show similar—if higher capacity—biochemical and physiological properties [adjustments at least in part based on preponderance of slow twitch fibers in skeletal muscles (44, 45)]; this result is not evident in Africans originating from lowland regions of west Africa, showing a much higher preponderance of fast twitch fibers in their muscles (46). Heart adaptations also seem to rely on stoichiometric efficiency adjustments (15, 16), improving the yield of ATP per mole of O2 consumed [as in muscle (37, 47) by increased preference for carbohydrate as a carbon and energy source (15)]. Together with increased blood volume and RBC mass (i.e., increased whole body O2-carrying capacity), these adaptations imply dampened heart work requirements at any given altitude for a similar submaximal level of whole body exercise. Finally, a blunted catecholamine response to hypoxia in indigenous highlanders indicates reduced hypoxic sensitivity of sympathadrenergic control (48–52), below the normally expected desensitization on exposure of human cells to hypoxia (53) and is indicative of efficacy of above metabolic and physiological adjustments. Compared with acute or acclimatory adjustments, these longer term (phylogenetic) adaptations appear to compensate pretty well for O2 deficits caused by hypoxia, but this advantage appears to be gained at a cost: an attenuation of maximum aerobic and anaerobic metabolic capacities (Fig. 2 a and b). In short, a high altitude physiological phenotype (Fig. 3) based on numerous similar traits mainly in Quechua and Sherpas natives is the picture emerging to this point.
Figure 3.
A diagrammatic summary of the proposed ancestral physiological phenotype as a phylogenetic adaptation to hypobaric hypoxia. The summary is based largely on studies of individuals indigenous to the Andes (Quechuas and Aymara) and the Himalayas (Sherpas and Tibetans). Essentially, all of the characteristics summarized here also are expressed in individuals well adapted for endurance performance. In the latter, the main modification involves an up-regulation of mitochondrial volume densities at the working tissues (altered expression of mitochondrial metabolic enzymes and metabolite transporters above), which is why this is referred to as a “high capacity” version of the lower capacity high altitude phenotype. See text for further details.
As an aside, we should emphasize that, although overall hypoxia responses appear to involve fine tuning each of the above sensing and signal transduction pathway cascades, the hypoxia “defense” adjustments of Quechuas and Himalayan natives are not always exactly the same. Of the above O2 sensor-linked response systems, for example, the blunting of HVR in Quechuas (40) and Sherpas (41) and the increase in RBC mass in Andeans (7, 14–16) are more robust than in Tibetans (40). Similarily, glucose metabolic rates are down-regulated mildy in the central nervous system in Quechuas, hypometabolism seemingly being used as an hypoxia defense strategy (13), but this trait is not expressed in Sherpas (14). Such differences in some physiological characters are not unexpected, given the length of time these lineages have been evolving separately (see below), and they do not alter our impression of a high altitude physiological phenotype based on many similar traits in Quechuas and Sherpas.
Similar Phenotypes for Hypoxia Tolerance and for Endurance Performance.
The possibility that the adjusted hypoxia response systems in Fig. 3 are “linked” at least loosely is indicated by the fact that most of them are found also in humans adapted for endurance performance. Frequently, this includes a blunted HVR and hypoxic pulmonary vasoconstrictor response, expanded blood volume, altered expression of metabolic enzymes and metabolite transporters, fuel preference adjustments, enhanced ratio of aerobic/anaerobic contributions to exercise (2–5, 7–12, 43–45), and enhanced endurance (2, 8). In endurance-adapted athletes, who display much higher maximum aerobic capacities than do altitude natives (2, 7, 33, 36, 43–45), many of these series of traits appear as high performance versions of those found in high altitude natives. Whereas up-regulation of mitochondrial O2 flux capacities at working muscles (2–5, 7–12, 33, 42–47) may be the main change in the physiological phenotype summarized in Fig. 3, in theory, some regulatory adjustments may well be required at other steps in the path of O2 from air to mitochondria (54). Nevertheless, the Fig. 2 a and b comparisons of lowlanders and highlanders under normoxia are qualitatively good descriptions of the difference between individuals who are well adapted for endurance vs. those who are not. Low plasma [lactate] values in the exercise protocols shown in Fig. 2 are as characteristic of endurance performers as those of highlanders.
Put another way, the biochemical and physiological organizations of both indigenous highlanders and individuals adapted for endurance performance are similar to each other, but both differ strikingly from the homologous organization in “burst performance” individuals (2, 5, 7, 8, 30, 36, 46). Similar differences emerge when (either untrained or trained and elite) individuals from east Africa [medium altitude origins (44, 45)] are compared with individuals of west African (lowland) origins, in whom fast twitch fibers form a much larger percentage of skeletal muscle (46). In the latter, exercise-induced lactate concentrations can reach very high levels and cardiovascular adjustments play as important a role in recovery from performance as they do during performance per se (2). As in highlanders, fiber-type compositions in muscles of individuals adapted for either endurance or power performance are relatively refractory to training or acclimation (2, 33).
A key point to emphasize is that environmental vs. genetic contributions to the above kinds of physiological character traits are hard to quantify. Most physiological studies of indigenous highlanders, including those involving prolonged (up to a generation of) deacclimation (8, 39, 47, 55, 56), actually are not designed properly to evaluate this issue (sample numbers are small, genotype is usually unknown, or genetic effects are lost in the “signal/noise”). Preferred approaches to this question require parallel studies of identical twins (46, 55, 57) or large scale statistical sampling of family trees (40). Although these two approaches are not usually possible, from available data, it appears that genetic factors account for ≈50% or more of the variance of these kinds of physiological systems (40, 46, 55, 57) and that the genetic contribution to any given trait may vary in different lineages. For example, the HVR is higher in Tibetans than in Andean natives (40) and higher in Tibetans compared with Sherpas (41). It is “a given,” of course, that natural selection can act only on components that are under genetic influence (57).
Phylogenetic Pathways and Timelines.
If the Fig. 3 hypoxia response adjustments compromise the bulk of our species’ “solution” to the “problems/requirements” of hypobaric hypoxia and/or endurance performance, the question arises of whether the same solution has arisen more than once in our species history—such independent origins would be good evidence for selection and evolutionary adaptation. A search for such evidence requires insight into the evolutionary pathways of our species. To this end, we constructed a simplified “phylogenetic tree” for the human species (Fig. 4) from an indepth summary of human genetics and evolution by Cavalli-Sforza et al. (58). It is important to note that influences on the root of this sort of phylogeny, on the origins of our species, go back a long way. During a time span of ≈3 million years, from 4 to 1 million years ago, several different Australopithecines lived along the east African Rift Valley system under challenging environmental conditions (59, 60). A topographical profile along the equator from the west (Atlantic) to the east coast approaches 2,000 m crossing the central Rift Valley regions (1), an altitude easily high enough—and possibly ideal from the athletic training point of view (3)—for physiological response and adaptation (3, 36). Recent geological data give evidence that this Rift Valley system was lifted up by a fluctuating movement with an increasing speed from Miocene to Pleistocene, the fastest movement occurring during the last 2 million years [Gregory Rift system (61)]. It was this lifting and opening of the Rift System during the late Cenozoic that led to diversification of local climates (colder, drier, and higher) and of primate/hominid lineages (1, 62). Even in the Hadar Region, which actually belongs to the lower part of the Rift Valley system, Australopithecines lived for ≈1.5 million years, from 4 million to 2.5 million years ago, at a moderate altitude of ≈1,000 m (60). In addition, it should be emphasized that at least nine important glaciations during the last 0.7 million years are believed to have influenced the Rift Valley region; these cold oscillations are clearly documented by paleontological data (63), and in fact, they seem to have been as pronounced between 2 million years and 0.7 million years ago as subsequently (59). In addition, there is increasing evidence that seasonal variations and food resource patchiness during the last several million years in the Rift Valley region are consistent with increasing endurance performance requirements for food foraging (64). Even improved cognitive capabilities for dealing with environmental variations may have been under selection (64). Be that as it may, the early phases of hominid evolution in the east African Rift evidently can be characterized as occurring under conditions of mild altitude hypoxia. These conditions prevailed for the ancestors of our species, they prevailed at the origins of our species, and indeed, they prevail in east Africa today. The question is: Could these conditions have influenced the evolution of human physiology and led to the appearance of the hypoxia tolerance/endurance performance phenotype in the first place?
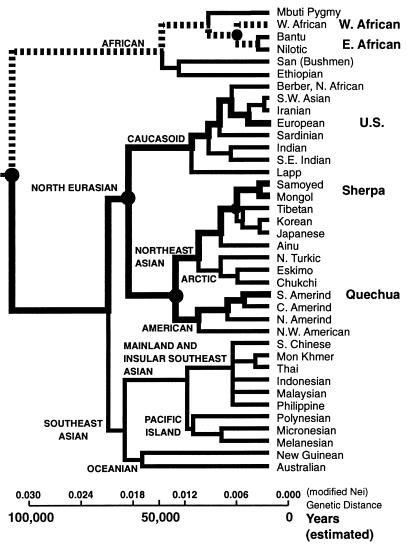
Figure 4.
Simplified phylogenetic tree of the human species as summarized by Cavalli-Sforza et al. (58), with an estimated species age of 100,000 years included for temporal reference. This species age estimate is controversial, but as explained in the text, the actual age is not critical to the main argument presented. The four main groups for which detailed data are used for this analysis are shown on the right. The pathways tracing each of these lineages back in time are shown in thick, stippled lines. Dashed lines are used for African lineages, for which much fewer data are available. Filled in circles identify nodes from which different lineages diverged: Sherpas vs. Tibetans; Quechuas vs. Himalayan natives; Caucasians vs. Sherpas and Quechuas; west African vs. east African; and African lineages vs. all other lineages. Such phylogenetic analysis shows that the last time Caucasians shared common ancestors with Sherpas and Quechuas was >50,000 years ago, approximately one-half the age of our species. Similarily, Sherpas and Tibetans last shared ancestors with Quechuas and Aymara some 30,000 years ago, approximately one-third the age of our species. When east Africans are considered, common ancestry with Himalayan and Andean lineages goes even farther back in phylogeny. Despite such distant divergences, all five high altitude groups (Quechua, Aymara, Sherpa, Tibetan, and Kenyan) show numerous similarities in physiological hypoxia defense mechanisms (Fig. 3). See text for further details.
The main groups whose physiological responses to hypobaric hypoxia to date have been extensively studied are: (i) lowland Caucasians and Asians; (ii) Sherpas and Tibetans of the Himalayan plateau; and (iii) Quechuas and Aymara of the Andean range (7, 12, 36, 40). A few new studies of west Africans contrasted with east Africans indigenous to medium altitudes recently also have become available (40, 45). If we assume that our species age is ≈100,000 years [this is controversial, but if our species is even older (1, 60), the arguments below will be even stronger], then a close examination of Fig. 4 is highly instructive. First, it suggests that the last time Caucasians, Sherpas, and Quechuas shared common ancestors was over one-half the age of our species. Second, the last time the Himalayan highlanders (Sherpas and Tibetans) and the Andean highlanders (Quechuas and Aymaras) shared common ancestors was in the range of 30,000 years ago—a time equivalent to approximately one-third of our species’ history. Third, divergence times between these groups and east Africans from medium altitude environments are even greater. Despite the distant divergences of the latter three—the Andean, the Himalayan, and the east African—lineages, many of their metabolic and physiological responses to hypobaric hypoxia are similar. And, fourth, numerous other lineages (including intermediate branches in the Fig. 3 phylogenetic tree) are known absolutely not to show these characteristics. These phylogenetic data are consistent with two possible scenarios.
Hypotheses.
One plausible hypothesis (i) is that, with only modest differences, the same metabolic and physiological solution arose independently by positive natural selection in the two high altitude lineages (Andean and Himalayan) for which we have the most data and possibly in a third east African lineage for which the data are not as extensive. If so, these comparisons satisfy at least one of the criteria of evolutionary biology and support the conclusion that the above suite of physiological characters are defense adaptations against hypobaric hypoxia that arose by positive selection. Whereas this was our thinking initially, the “low vs. high capacity phenotype” observations noted above are not readily compatible with this alternative.
A second hypothesis (ii) is that the above suite of physiological and metabolic traits represents the “ancestral” condition, arising as above by positive natural selection as our species’ response to a world growing colder, drier, and higher. In this hypothesis, which is consistent with geological and historical evidence on the origin of our species (1, 59–63), common descent with positive selection fine-tuning specific physiological traits in specific environmental conditions is the main explanation for the occurrence of a similar phenotype in disparate lineages (rather than independent and novel reinvention for each lineage). In this view, over some 5,000 or more generations of our species’ history, a down-regulated low capacity form of the ancestral phenotype was favored by positive selection in high altitude groups (Sherpas, Tibetans, Quechuas, and Aymaras) whereas an up-regulated high capacity form was favored in groups selected mainly for endurance performance (including Kenyan highlanders, who continue to thrive on the same plateau that served as the colder, drier, and higher birthplace of our species). In both cases, the overall physiological pattern remained similar presumably because similar (overlapping) physiological solutions “worked” for extending endurance or hypoxia tolerance. In high altitude populations, the ancestral physiological condition was fine-tuned for “life in the slow lane” under hypobaric hypoxia (7–16, 33, 36, 42). In groups selected for endurance performance, the ancestral physiological condition in relative terms involved the high capacity energy supply pathways being fine-tuned for the high capacity energy demand of sustained physical activity. In situations such as the moderate hypobaric hypoxia of the east African rise, selection pressures for both hypoxia tolerance and endurance performance may well have been applied simultaneously (44). In any event, hypothesis (ii) predicts that the ancestral organization of our physiology (Fig. 3) was inherently very dependent on efficient physiological O2 delivery systems and on “aerobic” metabolic pathways, with relatively minor development of, or reliance on, anaerobic metabolic systems to sustain whole body performance. Finally, because common descent of an ancestral physiological framework is a key mechanism leading to similar physiology in rather disparate (east African, Andean, and Himalayan) lineages, this phenotype should be distributed widely within different branches of our species phylogeny, as indeed seems to be the case (2, 3, 33, 44, 45), although this question has not been examined exhaustively and systematically . Indeed, some representatives of this phenotype may be expected in essentially all branches of our phylogeny because populations often differ in percentages of alleles rather than in the total absence (or complete fixation of) specific alleles (57). Be that as it may, in the beginning, apparently most of us were naturally good endurance performers.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council (Canada).
ABBREVIATIONS
- RBC
red blood cell
- HVR
hypoxic ventilatory response
References
- 1.Vrba E S, Denton G H, Partridge T C, Burckle L H, editors. Paleoclimate and Evolution with Emphasis on Human Origins. New Haven, CT: Yale Univ. Press; 1994. pp. 1–547. [Google Scholar]
- 2.Brooks G A, Fahey T D, White T P. Exercise Physiology–Human Bioenergetics and Its Applications. London: Mayfield; 1996. pp. 1–750. [Google Scholar]
- 3.Levine B D, Stray-Gunderson J. J Appl Physiol. 1996;13:S209–S212. [Google Scholar]
- 4.Terrados N. Int J Sports Med. 1992;13:S206–S209. doi: 10.1055/s-2007-1024641. [DOI] [PubMed] [Google Scholar]
- 5.Green H G. Int J Sports Med. 1992;13:S163–S165. doi: 10.1055/s-2007-1024627. [DOI] [PubMed] [Google Scholar]
- 6.Hochachka P W, Mottishaw P D. Soc Exp Biol Semin Ser. 1998;66:391–431. [Google Scholar]
- 7.Hochachka P W, Stanley C, Matheson G O, McKenzie D C, Allen P S, Parkhouse W S. J Appl Physiol. 1991;70:1720–1730. doi: 10.1152/jappl.1991.70.4.1720. [DOI] [PubMed] [Google Scholar]
- 8.Matheson G O, Allen P S, Ellinger D C, Hanstock C C, Gheorghiu D, McKenzie D C, Stanley C, Parkhouse W S, Hochachka P W. J Appl Physiol. 1991;70:1963–1976. doi: 10.1152/jappl.1991.70.5.1963. [DOI] [PubMed] [Google Scholar]
- 9.Hochachka P W, Stanley C, McKenzie D C, Villena A, Monge C. Int J Sport Med. 1992;13:S119–S123. doi: 10.1055/s-2007-1024613. [DOI] [PubMed] [Google Scholar]
- 10.Hochachka P W. Int J Sport Med. 1992;13:S89–S91. doi: 10.1055/s-2007-1024606. [DOI] [PubMed] [Google Scholar]
- 11.Kayser B, Hoppeler H, Classsen H, Cerretelli P. J Appl Physiol. 1991;70:1938–1942. doi: 10.1152/jappl.1991.70.5.1938. [DOI] [PubMed] [Google Scholar]
- 12.Moore L G, Curran-Everett L, Droma T S, Groves B M, McCullough R E, Sun S F, Sutton J R, Zamudio S, Zhuang J G. Int J Sports Med. 1992;13:S86–S88. doi: 10.1055/s-2007-1024605. [DOI] [PubMed] [Google Scholar]
- 13.Hochachka P W, Clark C M, Brown W D, Stanley C, Stone C K, Nickles R J, Zhu G G, Allen P S, Holden J E. J Cereb Blood Flow Metab. 1994;14:671–679. doi: 10.1038/jcbfm.1994.84. [DOI] [PubMed] [Google Scholar]
- 14.Hochachka P W, Clark C M, Monge C, Stanley C, Brown W D, Stone C K, Nickles R J, Holden J E. J Appl Physiol. 1996;81:1355–1361. doi: 10.1152/jappl.1996.81.3.1355. [DOI] [PubMed] [Google Scholar]
- 15.Hochachka P W, Clark C M, Holden J E, Stanley C, Ugurbil K, Menon R S. Proc Natl Acad Sci USA. 1996;93:1215–1220. doi: 10.1073/pnas.93.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holden J E, Stone C K, Clark C M, Brown W D, Nickles R J, Stanley C, Hochachka P W. J Appl Physiol. 1995;79:222–228. doi: 10.1152/jappl.1995.79.1.222. [DOI] [PubMed] [Google Scholar]
- 17.Hochachka P W, Somero G N. Biochemical Adaptation. Princeton: Princeton Univ. Press; 1984. pp. 1–557. [Google Scholar]
- 18.Acker H, Xue D. News Physiol Sci. 1995;10:211–216. [Google Scholar]
- 19.Lahiri S. Handb Physiol, Sect 4: Env Physiol. 1996;2:1183–1206. [Google Scholar]
- 20.Samaja M, Mariani C, Prestini A, Cerretelli P. Acta Physiol Scand. 1997;159:249–256. doi: 10.1046/j.1365-201X.1997.574342000.x. [DOI] [PubMed] [Google Scholar]
- 21.Weir E K, Archer S L. Fed Proc. 1955;9:183–189. [Google Scholar]
- 22.Heath D, William D R. Man at High Altitude. London: Churchill Livingstone; 1981. pp. 3–23. [Google Scholar]
- 23.Groves B M, Droma T, Sutton J R, McCullough RG, McCullough R E, Zhuang J, Rapmund G, Sun S, Janes C, Moore L G. J Appl Physiol. 1993;74:312–318. doi: 10.1152/jappl.1993.74.1.312. [DOI] [PubMed] [Google Scholar]
- 24.Forsythe J S, Hang B H, Iyer N V, Agani F, Leung S W, Koos R D, Semenza G L. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Detmar M, Brown L F, Berse B, Jackman R W, Elicker B M, Dvorak H F, Claffey K P. J Invest Dermatol. 1996;108:263–268. doi: 10.1111/1523-1747.ep12286453. [DOI] [PubMed] [Google Scholar]
- 26.Ogita H E, Nakaoka T, Matsuoka R, Takao A, Kira Y. Am J Physiol. 1994;267:H1948–H1954. doi: 10.1152/ajpheart.1994.267.5.H1948. [DOI] [PubMed] [Google Scholar]
- 27.Ladoux A, Felin C. Biochem Biophys Res Commun. 1993;195:1005–1010. doi: 10.1006/bbrc.1993.2144. [DOI] [PubMed] [Google Scholar]
- 28.Harik N, Harik S I, Kuo N T, Sakai K, Przybylski R J, LaManna J C. Brain Res. 1996;737:335–338. doi: 10.1016/0006-8993(96)00965-1. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg M A, Dunning S P, Bunn H F. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell P H, Pugh C W, Ratcliffe P J. Proc Natl Acad Sci USA. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G L, Jian B H, Rue E A, Semenza G L. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenger R H, Gassman M. Biol Chem Hoppe-Seyler. 1997;378:609–616. [Google Scholar]
- 33.Hochachka P W. Muscles as Molecular and Metabolic Machines. Boca Raton, FL: CRC; 1994. pp. 1–158. [Google Scholar]
- 34.Bunn H F, Poyton R O. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 35.Hochachka P W, Buck L T, Doll C, Land S C. Proc Natl Acad Sci USA. 1996;93:9493–9499. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winslow R M, Monge C. Hypoxia, Polycythemia, and Chronic Mountain Sickness. Baltimore: Johns Hopkins Univ. Press; 1987. pp. 1–255. [Google Scholar]
- 37.Brooks, G. A. (1998) Comp. Biochem. Physiol. B, in press.
- 38.Rosser B W C, Hochachka P W. Eur J Appl Physiol. 1994;67:513–517. doi: 10.1007/BF00241647. [DOI] [PubMed] [Google Scholar]
- 39.Kayser B, Hoppeler H, Desplanches D, Marconi C, Broers B, Cerretelli P. J Appl Physiol. 1996;80:632–637. doi: 10.1152/jappl.1996.81.1.419. [DOI] [PubMed] [Google Scholar]
- 40.Strohl K P, Beall C M. In: Women at Altitude. Houston C, editor. Burlington, VT: Queen City Printers; 1997. pp. 154–165. [Google Scholar]
- 41.Lahiri S, Edelman N H, Cherniack N S, Fishman A P. Fed Proc. 1969;28:1289–1295. [PubMed] [Google Scholar]
- 42.Hochachka P W. Handb Physiol, Sect 4: Adapt Environ. 1996;2:1115–1124. [Google Scholar]
- 43.Allen P S, Matheson G O, Zhu G, Gheorgiu D, Dunlop R S, Falconer T, Stanley C, Hochachka P W. Am J Physiol. 1997;273:R999–R1007. doi: 10.1152/ajpregu.1997.273.3.R999. [DOI] [PubMed] [Google Scholar]
- 44.Saltin B, Larsen H, Torrados N, Bangsbo J, Bak T, Kim C K, Svedenhag J, Rolf C J. Scand J Med Sci Sports. 1995;5:209–221. doi: 10.1111/j.1600-0838.1995.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 45.Saltin B, Kim C K, Terrados N, Larsen H, Svedenhag J, Rolf C J. Scand J Med Sci Sports. 1995;5:222–230. doi: 10.1111/j.1600-0838.1995.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 46.Ama P H M, Simoneau J A, Boulay M R, Serresse C W, Theriault G, Bouchard C. J Appl Physiol. 1986;61:1758–1761. doi: 10.1152/jappl.1986.61.5.1758. [DOI] [PubMed] [Google Scholar]
- 47.Brooks G A, Butterfield G E, Wolfe R R, Groves B M, Mazzeo R S, Sutton J R, Wolfel E E, Reeves J T. J Appl Physiol. 1991;70:919–927. doi: 10.1152/jappl.1991.70.2.919. [DOI] [PubMed] [Google Scholar]
- 48.Antenzana A M, Richalet J P, Antenzana G, Spielvogel H, Kacini R. Int J Sports Med. 1992;13:S92–S95. doi: 10.1055/s-2007-1024607. [DOI] [PubMed] [Google Scholar]
- 49.Antenzana A M, Richalet J P, Noriega I, Galarza M, Antenzana G. J Appl Physiol. 1995;79:795–800. doi: 10.1152/jappl.1995.79.3.795. [DOI] [PubMed] [Google Scholar]
- 50.Mazzeo R S, Bender P R, Brooks G A, Butterfield G E, Groves B M, Sutton J R, Wolfel E E, Reeves J T. Am J Physiol. 1991;261:E419–E424. doi: 10.1152/ajpendo.1991.261.4.E419. [DOI] [PubMed] [Google Scholar]
- 51.Colice G L, Lawrason J, Munsef A, Bittle P, Dietz J, Ramirez G. Aviat Space Environ Med. 1993;64:512–516. [PubMed] [Google Scholar]
- 52.Favier R, Desplanches D, Hoppeler H, Caceres E, Grunenfelder A, Koubi H, Leuenberger M, Sempore B, Tuscher L, Spielvogel H. J Appl Physiol. 1996;80:632–637. doi: 10.1152/jappl.1996.80.2.632. [DOI] [PubMed] [Google Scholar]
- 53.Resink T, Buravkova L, Mirzapoyazova T, Kohler E, Erne P, Tkachuk V. Biochem Biophys Res Commun. 1996;222:753–758. doi: 10.1006/bbrc.1996.0816. [DOI] [PubMed] [Google Scholar]
- 54.Jones, J. H. (1998) Comp. Biochem. Physiol. B, in press.
- 55.Fagard R, Bielen E, Amery A. J Appl Physiol. 1991;70:357–362. doi: 10.1152/jappl.1991.70.1.357. [DOI] [PubMed] [Google Scholar]
- 56.Desplanches D, Hoppeler H, Tuscher L, Mayet M H, Spielvogel H, Ferretti G, Kayser B, Leuenberger M, Grunenfelder A, Favier R. J Appl Physiol. 1996;81:1946–1951. doi: 10.1152/jappl.1996.81.5.1946. [DOI] [PubMed] [Google Scholar]
- 57.Li W-H, Graur D. Fundamentals of Molecular Evolution. Sunderland, MA: Sinauer; 1991. pp. 1–284. [Google Scholar]
- 58.Cavalli-Sforza L L, Menozzi P, Piazza A. The History and Geography of Human Genes. Princeton: Princeton Univ. Press; 1994. pp. 1–535. [Google Scholar]
- 59.Hamilton A C. Environmental History of East Africa. London: Academic; 1982. pp. 1–328. [Google Scholar]
- 60.Jones S, Martin R, Pilbeam D, editors. Human Evolution. Cambridge, U. K.: Cambridge Univ. Press; 1995. pp. 1–506. [Google Scholar]
- 61.Bishop W W. Geological Background to Fossil Man. London: Scottish Academic Press; 1978. pp. 1–585. [Google Scholar]
- 62.Maglio V C, Cooke H B, editors. Evolution of African Mammals. Cambridge, MA: Harvard Univ. Press; 1978. pp. 1–641. [Google Scholar]
- 63.Coppens Y, Howell F C, Isaac G L, Leakey R E F, editors. Earliest Man and Environments in the Lake Rudolf Basin. Chicago: Univ. of Chicago Press; 1976. pp. 1–615. [Google Scholar]
- 64.Schrenk F. Die Fruehzeit des Menschen. Munich, Germany: C. H. Beck; 1977. pp. 1–128. [Google Scholar]