Abstract
AIDS is often associated with growth retardation in children and wasting in adults. The dissociated envelope protein of the HIV (HIV-1), gp120, can be found in significant concentrations in the parenchyma and cerebrospinal fluid of brains in infected individuals, even in the earliest stages of HIV-1 disease. On the basis of this and the fact that we observed pentapeptide sequence homology between GH-releasing hormone (GHRH) and the V2 receptor-binding region of gp120, we initiated experiments to determine whether gp120 could affect GH secretion and growth in vivo and/or interact with anterior pituitary GHRH receptors in vitro. Although acute IV administration of gp120 in conscious rats had no effect on plasma GH levels, acute administration of gp120 (400 ng) into the brain significantly suppressed pulsatile GH release over a 6-h period compared with saline-injected controls. Furthermore, the putative gp120 antagonist, Peptide T (DAPTA), prevented the suppression of GH by gp120. In support of these in vivo findings, gp120 also significantly (P < 0.05) suppressed GHRH-stimulated GH release in static cultures of dispersed pituitary cells and from cells undergoing perifusion with the peptides. DAPTA prevented the GH suppression by gp120 in both of the pituitary cell paradigms. Furthermore, chronic administration of gp120 into the third ventricle significantly reduced body weight in juvenile rats, compared with saline-injected controls. Thus, gp120 appears to act both at the hypothalamus and pituitary to suppress GH release, and its action at these two locations is associated with a significant loss in body weight in chronically treated young animals. These findings may suggest a specific mechanism for the pathogenesis of wasting in HIV-1 patients that involves blockade of endogenous GHRH receptors by gp120.
Keywords: HIV-1, growth hormone, Peptide T
Several hormonal and metabolic disturbances have been described in HIV-1 infection, one of the most important of which is the wasting syndrome (1–12), in which adults experience weight loss and children exhibit developmental retardation. Depletion of lean body mass is most profound in the acutely ill patient with active secondary infection (7–10). The course of illness in a pediatric setting differs from that in the adult and strongly tends toward developmental retardation and failure of somatic growth (1–5) with occasional immunosuppression (4). Subclinical endocrine insufficiency in asymptomatic patients occurs with much greater frequency and is evident very early in the course of infection (13–15).
Initial studies (1–4) investigated the effects of HIV-1 on growth and hormonal profiles of infected children and found low circulating levels of growth hormone (GH) (both basal and stimulated) and insulin-like growth factor I (IGF-I). A recent study of growth and development in hemophiliac males shows that diminished growth velocity and growth hormone levels are common in HIV-infected boys, that these diminutions occur early, and that they precede immunological compromise (16). GH deficiency has also been observed in HIV-1-infected adults (5–7). Deficiencies in GH and IGF-I levels have been seen at all clinical and immunologic stages of disease, but are most prominent in advanced cases (4). Some studies report increases in GH levels (17) in adult hypogonadal subjects with weight loss, an adaptation that may be related to reduced caloric intake. Early HIV infection therefore may cause abnormal endocrine function characterized by dysregulated GH secretion and androgen production, which then lead to lean tissue loss.
Controlled clinical trials in AIDS patients have demonstrated that GH, or IGF-I, can increase lean body mass and improve quality of life (12, 18–22). Larger trials have also documented weight gains with recombinant human (rh) GH plus rhIGF-I over a 6-week administration (21) or GH only for 12 weeks (12). The evidence that GH levels are suppressed with HIV-1 infection coupled with the reports that exogenous GH ameliorated AIDS-associated wasting suggest that HIV infection or viral proteins disrupt endocrine function leading to diminished growth hormone and androgen production, which then contribute to pediatric developmental retardation or wasting in adults.
The pathophysiological mechanisms of AIDS wasting in adults, and retardation of somatic growth in children with HIV-1 disease are presently unknown (10, 23). Obvious virus-induced abnormalities or infection of endocrine tissues seems rare (25, 26), although cytomegalovirus, toxoplasmosis, and cytokine release have been proposed (27) as agents.
Interestingly, HIV gp120 infused into rat brain causes release of the cytokines interleukin 1 or tumor necrosis factor (28), which have been shown to suppress GH and its releasing factor, GHRH, when elevated in the brain (29). The envelope protein gp120 has been found to cause death of neurons at low concentrations by indirect mechanisms (30–32), and gp120 slows or prevents the appearance of developmental milestones when administered subcutaneously to neonatal rats (33).
A previously identified homology in the V2 region of gp120 between vasoactive intestinal peptide (VIP) and its homolog, GHRH, the major peptide releasing factor for GH, have been noted, and the structural features have been discussed previously (34–36) (Table 1). Short peptides from this region of gp120 act as antagonists to gp120 binding, infectivity, and neurotoxicity (32–36). Based on the sequence homology between gp120 and GHRH, we reasoned that gp120 might interact with VIP/GHRH hypothalamic and/or pituitary receptors to suppress directly the endogenous GHRH-mediated signaling events that drive GH secretion.
Table 1.
Pentapeptide sequences shared by GP120, GHRH, and VIP
| Peptides | Sequence |
|---|---|
| gp120 Isolates | |
| IIIB | Thr Thr Ser Tyr Thr |
| WMJ1 | Ser Ser Thr Tyr Arg |
| SF-2 | Thr Thr Asn Tyr Thr |
| GHRH(7-11) | Thr Asn Ser Tyr Arg |
| VIP(7-11) | |
| (Human, porcine, rat) | Thr Asp Asn Tyr Thr |
| (Chicken, guinea pig) | Thr Asp Thr Tyr Thr |
| DAPTA (D-Ala-Peptide T-amide) | d-Ala Ser Thr Thr Thr Asn Tyr Thr-NH2 |
Therefore, we investigated whether intracerebroventricular (ICV) administration of gp120 could suppress GH release in vivo in rats and whether this suppression was associated with concomitant weight loss. We also characterized the effects of gp120 on the ability of GHRH to release GH in dissociated pituitary cell cultures. To clarify the receptor mechanisms of gp120 action we also determined whether the small V2 region-derived gp120 antagonist DAPTA (D-Ala-peptide T amide) would block gp120’s actions to suppress GH secretion.
METHODS
Effects of Central gp120 on Circulating Growth Hormone.
Young adult Sprague–Dawley rats (12–14 weeks of age, Harlan, Indianapolis) were housed individually in an approved animal facility with environmentally controlled rooms and a 12-h light cycle (0700–1900 h). Animals received tap water and food ad libitum. All protocols were approved by the Georgetown University Animal Care and Use Committee. Animals were anesthetized with tribromoethanol (2.5%, 1 ml/100 g body weight), and 23-gauge stainless steel guide cannulae were implanted stereotaxically into the third ventricle (3V) of the hypothalamus, 1 week before administration of drugs. The morning before the experiments, animals were anesthetized under ether and Silastic catheters were placed in the animals’ jugular veins for blood sampling, and patency was maintained with a 500 units/ml sodium heparin block. The following day the jugular lines were opened and attached to PE-50 tubing, so blood sampling would not disturb the conscious animals. At 0715 h, saline vehicle, gp120 (400 ng), DAPTA (300 ng), or gp120 (400 ng) + DAPTA (300 ng) was administered intraventricularly with a Hamilton syringe (Reno, NV) in a volume of 4 μl. This dose of gp120 was similar to concentrations of GHRH that have been injected ICV in previous in vivo GH studies (37). The injection was given over a period of 60 sec through a 30-gauge inner infusion cannula. As a control for the specificity of action, multiple freeze-thawed (4×), inactivated gp120 was injected ICV at the same dose, and plasma GH profiles were obtained over a 6-h period. To determine whether the same concentration of gp120 had peripheral effects on pituitary GH release, gp120 (400 ng) was administered i.v., instead of into the 3V, to a separate group of animals. Serial blood samples (0.2 ml) were obtained every 15 min from 0730–1345 h from all groups of animals. Packed red blood cells resuspended in saline were reinjected every other blood draw to maintain blood volume and hematocrit. Plasma was frozen at −80°C until growth hormone assay. Basal and end levels of corticosterone were also analyzed in the different groups. The rhgp120 SF-2 (lot 167A) used was provided by the National Institutes of Health AIDS Reagent program and Chiron Corporation, Emeryville, CA.
Effects of gp120 on GH Release from Pituitary Cells.
Cell culture protocol. Anterior pituitary glands were removed from donor male rats after decapitation, minced with a sterile razor blade, and placed in dispersal medium [DMEM (GIBCO) containing 0.1% BSA, 0.1% trypsin, 20 mM Hepes, and 1 ml/10 ml penicillin-streptomycin]. Cells were dispersed in a spinner flask at 37°C with gentle mechanical disruption. Monodispersed cells were centrifuged to remove dispersal medium (10 min, 600 × g) and resuspended in overnight culture medium (Medium 199, 10% horse serum, 20 mM Hepes, 1 ml/10 ml antibiotic/antimycotic). Incubations (static and dynamic perifusion) were performed as previously described (38, 39) and as characterized below.
Static incubations.
The effects of gp120 in the presence or absence of GHRH on pituitary cell growth hormone secretion were first examined in static cultures. For the overnight incubation, cells were placed in 12 × 75-mm polystyrene tubes at a density of approximately 300,000 cells per ml medium. The next day, the tubes were centrifuged (10 min, 600 × g), medium was aspirated, and the cells were resuspended in medium (Medium 199, 1% BSA, 20 mM Hepes, 1 ml/100 ml penicillin-streptomycin, 0.06 M ascorbic acid, 0.02 M bacitracin). The cells were incubated with GHRH alone (0.1–10.0 pM); gp120 alone (1.0–100 pM); and GHRH in the presence of a range of gp120 concentrations (1.0–100 pM). Incubations (30 min) were conducted at 37°C in room air and terminated by centrifugation (10 min × 600 g), and medium was aspirated for determination of hormone content. Samples were stored at −80°C until growth hormone assay. All samples were tested in duplicate, at two dilutions, in two separate assays. The entire cell culture protocol was repeated in three individual cell harvests with similar results.
Dynamic perifusions.
For overnight incubation, the dispersed cells were plated in 60-cm cell culture dishes at a density of approximately 3 × 106 cells per dish in 8 ml of medium. After an 18-h incubation, cells were scraped from the dishes and centrifuged (10 min × 600 g), and overnight incubation medium was removed. The cells were resuspended in incubation medium (Medium 199, 1% BSA, 20 mM Hepes, 1 ml/100 ml penicillin-streptomycin, 0.06 M ascorbic acid, 0.02 M bacitracin), loaded onto Bio-Gel p-2 columns (0.4 × 1.5 cm) (Bio-Rad), and perifused at a flow rate of 0.5 ml/min. A stabilization period of 120 min preceded all testing. Testing consisted of intervals of consecutive exposure to control medium and medium containing test substances in various combinations (at concentrations of 1.0 nM GHRH, 1.0 nM gp120, and 5.0 nM DAPTA). Four columns were run in parallel for each cell dispersion. To determine the specificity of the hormonal response to gp120, a second series of experiments was performed examining the effects of lutenizing hormone-releasing hormone (LHRH, 1 nM) and various combinations of gp120 (1.0 nM) and DAPTA (5 nM) on LH secretion. Perfusates from all experiments were collected automatically at 5-min intervals, and medium was stored at −80°C until hormone assay. Samples were assayed in duplicate or triplicate, as described above.
Effects of Chronic gp120 Administration on Somatic Growth in Juvenile Rats.
Central administration. To examine the effects of central gp120 on somatic growth, cannulae were implanted in the 3V of juvenile (5-week-old) male Sprague–Dawley rats. Animals were housed separately, weighed, and handled daily for acclimation. When all animals had recovered their preoperative body weights and were growing (usually by day 5 postcannulation), they received intracerebroventricular injections of saline (1 μl) or gp120 (2 ng, in 1 μl saline) twice daily (at 0800 and 1300 h) for 5 days. Body weights were measured daily, and organ weights were obtained at the end of the experiment. All animals ate comparable amounts of food and water throughout the experimental periods.
Peripheral administration.
To determine whether peripherally administered gp120 alters somatic growth, juvenile (4- to 5-week-old) male Sprague–Dawley rats were given gp120 i.p. (100 ng, two or three times daily) in Alzet minipumps (200 ng over 3 days) or by intravenous injections through chronic jugular catheters (100 ng, twice daily; or 1 mg, three times daily). Other animals were given saline vehicle i.p. by minipump or through IV injections and served as controls. Daily body weights were recorded and, at the end of the experiments, organs were removed and weighed.
Analysis.
Plasma and media GH and LH levels were measured by the RIA kits supplied by the National Institute of Diabetes and Digestive and Kidney Diseases Hormone and Pituitary Program. Results are expressed in terms of the RP-6 reference preparation for rat GH and RP-3 standard for the rat LH. The inter- and intraassay coefficients of variation for all assays were below 10%. The minimum detectable levels of GH and LH were 0.5 ng/ml. All GH and LH concentrations were determined from duplicate aliquots. Plasma corticosterone levels were determined by using a commercial kit from Nichols Diagnostics.
Statistical analysis.
Multiple comparisons (between three or more groups) were analyzed by using one-way ANOVA and Student–Newman–Keuls posthoc tests. Statistical comparisons were made within and between two groups by paired and unpaired Student’s t test, respectively. The growth rates of juvenile rats were equated with the slopes of the growth curves for the individual animals by using a linear regression analysis. Individual slopes of pre- and posttreated animals within the same groups were assessed by paired Student’s t tests; comparisons between saline- and gp120-treated animals were performed by using unpaired Student’s t tests. Significance was assigned at the P < 0.05 level.
RESULTS
Effects of Acute Central gp120 on Circulating Growth Hormone.
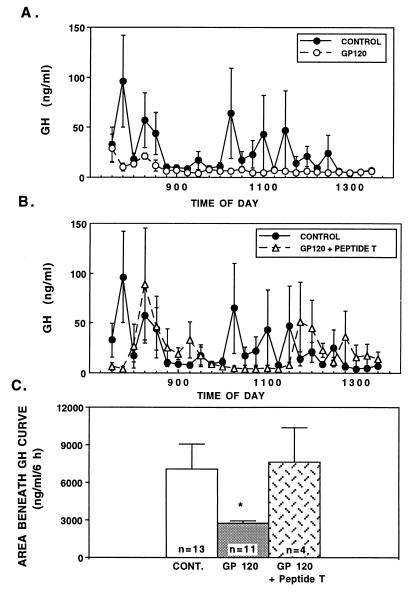
Injection of nonpyrogenic saline into the third ventricle of conscious adult rats resulted in a typical pulsatile secretion of GH over 6 h (Fig. 1A, solid circles). In contrast, a single injection of gp120 (400 ng) suppressed the pulsatile release of GH over the entire experimental period (Fig. 1A, open circles). This was evident from both the lack of peaks (Fig. 1A) as well as significantly reduced area under the curve for GH (P < 0.05) over the 6-h period (Fig. 1C). Concomitant 3V administration of gp120 (400 ng) + DAPTA (300 ng) prevented the suppression of GH observed with gp120 alone (Fig. 1 B and C). DAPTA alone had no effect on GH secretion (data not shown). Furthermore, the inactivated preparations of gp120 failed to alter the normal patterns of GH secretion or total amount of GH secreted over the experimental period (data not shown), thereby indicating a specific action of intact gp120 on GH release, rather than a nonspecific response to a foreign protein. Also, the immediate suppression of GH release in gp120-treated animals suggests a specific central action of gp120 on the GHRH–GH axis, rather than through an alternate pathway, which would typically take longer to initiate. Basal corticosterone levels in the gp120 group were not significantly different from saline controls, either at the beginning [99.5 ± 37.4 vs. 56.5 ± 33.1 ng/ml, respectively; not significant (n.s.)] or end (258.5 ± 12.7 vs. 290.2 ± 22.1 ng/ml in controls, n.s.) of the blood sampling period, thereby arguing against a glucocorticoid-mediated suppression of GH.
Figure 1.
(A) Effects of administration of gp120 (400 ng ICV) on circulating GH levels in conscious adult rats. (B) Effects of ICV administration of DAPTA (Peptide T) in the presence of gp120 on circulating GH levels in conscious adult rats. (C) Area beneath the curve of GH in control (saline), gp120, and gp120 + DAPTA-treated adult rats. ∗, P < 0.05 vs. saline controls.
Effects of gp120 on GH Release from Pituitary Cells.
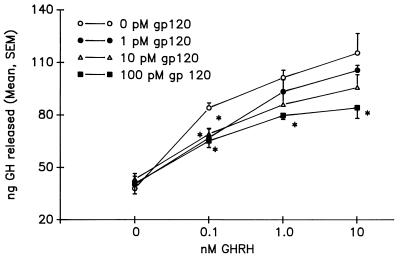
Static incubations. As expected, GHRH stimulated a release of GH in a dose-related fashion, from dispersed anterior pituitary cells in static incubation. In the presence of gp120, however, there was significant inhibition of GH secretion from the cell cultures. Fig. 2 illustrates the dose-dependent inhibition attained by using three doses of gp120: 1.0, 10, and 100 pM. Interestingly, in the presence of 0.1 nM GHRH, all doses of gp120 produced a significant 40% inhibition (P < 0.05) of GH release, indicating the potency of gp120. The highest dose of gp120 (100 pM) consistently reduced GH secretion by more than 40% in response to increasing GHRH concentrations (P < 0.0001).
Figure 2.
Effects of gp120 (0.01 and 0.1 nM) on GHRH-mediated GH secretion from static, dispersed pituitary cell cultures. The inhibitory effects of both doses of gp120 are significant at 0.1 nM GHRH (P < 0.05) and 1.0 nM GHRH (P < 0.0001).
Dynamic perfusions.
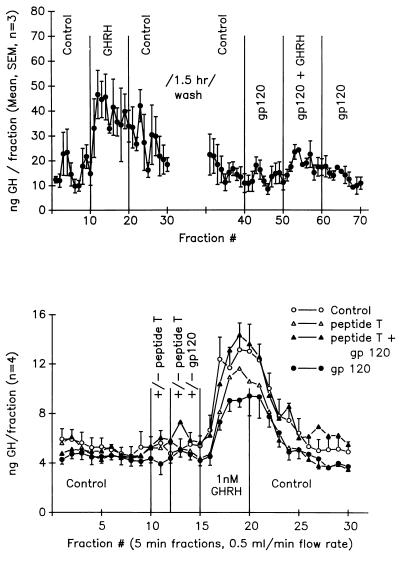
Fig. 3 Upper depicts GH secretion from samples collected every 5 min from the perifusion of dispersed pituitary cells. Addition of GHRH (1.0 nM) to the perfusate significantly (P < 0.001) increased GH release in the collections (fractions 10–20). Gp120 alone had no effect on basal GH release (fractions 40–50), but when combined with GHRH (1.0 nM), gp120 (1 nM) suppressed the normal GHRH-mediated GH release by 75% (P < 0.01) (fractions 50–60).
Figure 3.
(Upper) Effects of gp120 on GHRH-mediated GH secretion from dynamic perifusion of pituitary cell cultures. Fractions were taken every 5 min under basal perifusion conditions and in the presence of GHRH, gp120, or GHRH + gp120. (Lower) Combined effects of gp120 and DAPTA (Peptide T) on GHRH-stimulated GH release from dynamic perifusion of pituitary cell cultures. Fractions were taken every 5 min under basal perifusion conditions and in the presence of GHRH, gp120, DAPTA, or GHRH + gp120 + DAPTA.
In contrast to the results in Fig. 3 Upper, the addition of DAPTA (peptide T) to the gp120 + GHRH being perifused through the cells restored the GHRH-mediated GH release (Fig. 3 Lower, fractions 16–20, closed triangles). This response was the same magnitude observed with GHRH alone (Fig. 3 Lower, fractions 16–20, open circles) and was significantly greater than the 47% decrease in GH release observed in the gp120 + GHRH group (closed circles). DAPTA alone did not affect basal GH levels (fractions 10–12) or the GHRH-mediated increase in GH (fractions 16–20). Again, gp120 alone had no significant effect on basal GH levels (fractions (13–15). Cells challenged with GHRH at the end of the experiments demonstrated a robust increase in GH secretion, indicating the viability of the cells at the end of the perifusion periods (data not shown). In addition, gp120 and DAPTA, either alone or in combination, failed to alter either baseline or LHRH-stimulated LH secretion (Δ in LH from baseline: controls, 67%; gp120, 71%; DAPTA, 70%; gp120 + DAPTA, 70%).
Effects of Chronic gp120 Administration on Somatic Growth in Juvenile Rats.
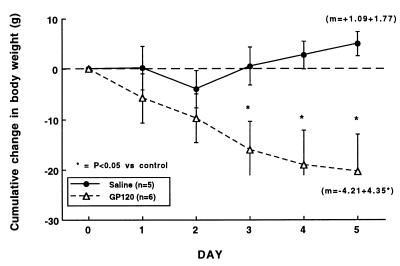
Central administration. After 3V cannulation, animals were randomly separated into two groups with similar growth rates over the 5 days of recovery (growth slopes of 4.39 ± 1.34 and 4.91 ± 0.94, n.s.). Chronic 3V injections of saline reduced the normal rapid rate of growth in the juvenile rats, although they maintained a positive growth rate (slope of +1.09 ± 1.77) (Fig. 4, closed circles, n = 5). In contrast, administration of gp120 resulted in a dramatic reduction in body weight over the experimental period, which was significantly different (P < 0.05) from saline controls by day 3 (Fig. 4, open triangles, n = 6). The growth rate, as indicated by the slope of the change in cumulative body weight line (m), was significantly reduced in the animals treated with gp120 (−4.21 ± 4.35, P < 0.01, vs. saline controls). Organ weights and organ/body weight ratios for these animals after 5 days of treatment are shown in Table 2. Although most of the organs analyzed were comparable in weight to the controls, both the thymus and spleen of the gp120-injected animals weighed significantly less than their age-matched, saline-injected controls. Interestingly, when comparing the organ/body weight ratios as a measure of proportionality, the thymus remained significantly smaller than would be predicted (0.12 ± 0.1 vs. 0.17 ± 0.02% in controls, P < 0.05), indicating actual regression of thymic weight.
Figure 4.
Effects of chronic central injections of saline vehicle or gp120 (4 ng in 1 μl, twice daily) on cumulative whole body weight gain in conscious juvenile rats. The dotted horizontal line is the zero identity line. ∗, P < 0.05 vs. saline controls.
Table 2.
Central (3V) injections
| Group | Brain | Thymus | Spleen | Heart | Kidneys | Testes |
|---|---|---|---|---|---|---|
| Organ weights, g | ||||||
| Saline (n = 5) | 0.52 ± 0.03 | 0.35 ± 0.04 | 0.64 ± 0.03 | 0.74 ± 0.03 | 1.52 ± 0.03 | 3.31 ± 0.19 |
| gp120 (n = 6) | 1.44 ± 0.05 | 0.22 ± 0.03* | 0.51 ± 0.05* | 0.76 ± 0.02 | 1.49 ± 0.04 | 2.96 ± 0.06 |
| Organ weight/body weight ratio, % | ||||||
| Saline (n = 5) | 0.74 ± 0.02 | 0.17 ± 0.02 | 0.31 ± 0.02 | 0.36 ± 0.01 | 0.74 ± 0.01 | 1.60 ± 0.08 |
| gp120 (n = 6) | 0.81 ± 0.05 | 0.12 ± 0.01* | 0.28 ± 0.02 | 0.42 ± 0.02 | 0.83 ± 0.03 | 1.67 ± 0.13 |
Numbers are means ± SEM.
P < 0.05 vs. saline, by Student’s t-test.
Peripheral administration.
Several different methods were employed to assess whether peripherally circulating gp120 has effects on somatic growth in young, growing rats. Intraperitoneal injections, minipump infusion, and i.v. administration at varying doses of gp120 had no effect on somatic growth in the young animals (body weight gain over 9 days: i.v. saline controls, 55 ± 3 g; gp120 (1 ng i.v.), 55 ± 2 g; gp120 (100 ng i.v.), 54 ± 3 g, n.s.). Organ weights were also unchanged by systemic gp120 administration (data not shown).
DISCUSSION
There is close sequence homology between a 5-aa epitope of GHRH (and the homologous peptides VIP and PACAP) and peptides derived from the V2 region of the gp120 molecule (34) (Table 1). This suggests that gp120 could compete for GHRH and VIP receptors, potentially contributing to GH suppression and the wasting syndrome observed with AIDS. The present studies support this notion by determining that central injection of gp120 was able to significantly and substantially suppress the normal pulsatile release of GH in conscious rats. Furthermore, the gp120 antagonist DAPTA, when coadministered with gp120, was able to prevent the GH suppression, suggesting, in view of the sequence homology, that the effects of gp120 at the level of the hypothalamus may occur through suppression of VIP receptor-mediated GHRH release.
Because VIP can stimulate GHRH release from the hypothalamus (40), it is also conceivable that gp120 blockade of GHRH and/or VIP on their pituitary receptors could further account for the inhibition of GH secretion. Pituitary cell cultures were employed to determine whether gp120 has a pituitary site of action when exposed directly to these cells, as might occur with circulating gp120 in actual AIDS patients. In support of our hypothesis, and consistent with the in vivo findings of suppressed GH by central gp120 injection, GHRH-mediated release of GH in static pituitary cultures was blocked in the presence of low concentrations of gp120 (Fig. 2). Dynamic pituitary perifusion studies confirmed the static incubation results, again illustrating the ability of gp120 to suppress GHRH-induced GH secretion (Fig. 3). Concomitant administration of DAPTA, a gp120 antagonist (32–35), to the gp120/GHRH perifusate restores the GHRH-mediated GH secretion. These in vivo and in vitro results indicate that both hypothalamic and pituitary GHRH and VIP receptors may be targets for gp120 action, which would ultimately account for the reduction of pulsatile GH release in the whole animal. In addition to the effects of gp120 on the central somatotropic axis, binding of gp120 to VIP/GHRH receptors at other central or peripheral sites may have specific deleterious manifestations.
Because the essential feature of AIDS wasting disease is the loss of body mass in juveniles and adults [the actual cause of death (10)], our focus was to determine whether chronic gp120-induced suppression of pituitary GH secretion affected somatic growth in juvenile animals. Although peripheral administration of gp120 had no effect on growth (possibly because the doses of gp120 were insufficient, or it did not reach critical sites), central (3V) administration resulted in significant weight loss after only 3 days of administration. This weight loss in the typically rapidly growing animal was associated with significantly reduced spleen and thymus weights. Indeed, the thymic weight was actually proportionately lower than predicted by the organ/body weight ratios, suggesting actual regression of the tissue. GH has been shown to be an important hormone in thymic lymphocyte development (41), and because there is involution and degeneration of immune tissues in AIDS (42), we would hypothesize that the chronic suppression of GH by central administration of gp120 may also be affecting immune cell function. Thus, our findings indicate that gp120 suppresses GH release by blocking the action of GHRH at the pituitary, as well as possibly by interacting with (VIP) receptors at the level of the hypothalamus. The suppression of circulating GH levels through central hypothalamic and pituitary mechanisms in animals treated with gp120 causes weight loss and may directly or indirectly have negative effects on immune tissues, specifically the thymus and spleen.
The model, subject to further verification, appears to re-create certain aspects of the wasting pathology of AIDS. It is recognized that wasting disease is caused by many pathologic processes and is not simply accounted for by suppressed GH levels (11). However, the current findings implicate gp120 as a potential agent for GH suppression and weight loss and suggest specific GHRH receptor pathways by which gp120 acts. The studies indicate that DAPTA, an antagonist of gp120 action, blocks the suppression of GH by gp120, in vitro and in vivo, thereby indicating that gp120 does not cause a nonspecific or toxic inhibition of GH secretion. Interestingly, although patients with asymptomatic HIV infection appear to have no change in GH levels (43), some cases of HIV-wasting have reported elevated GH levels (17); however, the latter may actually be the result of a compensatory response to starvation (44, 45) and may represent yet another variation in AIDS wasting.
The receptor pathways for these effects are of interest, as they suggest specific targets for therapeutic intervention. Our findings that gp120 acts on isolated pituitary cells to block GHRH-stimulated GH release suggests that gp120 can act through GHRH receptors. This appears to be specific, because the release of pituitary LH (through LH-releasing hormone receptors) was not affected by gp120. The concept that gp120 can act through GHRH receptors is further supported by the fact that DAPTA, an antagonist of gp120 binding and activity (32, 37), which shares a homology with GHRH (Table 1), blocks the suppression of pulsatile GH in vivo, as well as in both static and perifused pituitary cultures. This would be in keeping with pharmacological theory that antagonist activity is mediated through competition with agonists at receptor sites. However, definitive proof of this hypothesis awaits future demonstration of displacement of radio-labeled GHRH binding by DAPTA and gp120 in direct binding experiments. Additionally, both GHRH and VIP neurons synapse on somatostatin (SRIF) neurons in the brain (46, 47), and GHRH normally stimulates SRIF release centrally (48, 49). This action of GHRH might be suppressed by central gp120. However, because gp120 may also act at VIP receptors, and VIP inhibits SRIF and stimulates GHRH from the hypothalamus, it is possible that gp120 would block these actions of VIP, leading to an overall increased SRIF output and decreased GHRH secretion. This would not be unexpected, because VIP and VIP receptors are more prevalent in the brain than are GHRH and GHRH receptors. Thus, this central action, in addition to the effects of gp120 on pituitary GHRH receptors, would have a net effect of suppressing GH levels, which is consistent with our hypothesis and observations.
In conclusion, the present in vivo and in vitro results indicate that gp120 blocks the GHRH-mediated GH release, most likely through both hypothalamic and pituitary mechanisms by using homologous VIP and GHRH receptors. The GH-suppressing effects of gp120 can be prevented by a small peptide antagonist of gp120 infectivity and neurotoxicity that shares sequence homology to GHRH. These findings support a receptor-active mechanism for gp120 action and suggest that the V2 epitope of HIV is responsible for the neuroendocrine-related receptor binding of gp120. The relationship of hypothalamic and pituitary receptors that bind gp120, suggested here, to other G-protein-coupled gp120 coreceptors (51–54) is currently under investigation. Drugs that block the GH-suppressing actions of gp120 (like DAPTA) may now be considered for their potential therapeutic efficacy in the setting of HIV-1 weight loss, pediatric development, and wasting by their ability to normalize endogenous GH levels.
Acknowledgments
The authors thank Melissa Morales, Reem Saadih, Bernardo Issel, and Craig Woda for their excellent assistance. We would also like to thank Dr. Barbara Bayer for analysis of corticosterone levels. This research was funded by Advanced Peptides and Biotechnology Sciences, Stony Brook, NY (M.D.L., C.B.P., M.R.R.), National Institutes of Health Grant R01-NS-23036 (M.D.L.), and National Science Foundation Grant IBN 9511677 (S.E.M.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: IGF-I, insulin-like growth factor I; GH, growth hormone; GHRH, GH-releasing hormone; n.s., not significant; VIP, vasoactive intestinal peptide; 3V, third ventricle; ICV, intracerebroventricular; DAPTA, D-Ala-Peptide T-amide.
References
- 1.Kaufman F R, Gomperts E D. Am J Pediatr Hematol Oncol. 1989;11(3):292–294. [PubMed] [Google Scholar]
- 2.Jospe N, Powell K R. Pediatrics. 1990;86:309–312. [PubMed] [Google Scholar]
- 3.Lepage P, Van de Perre P, Van Vliet G, Nsengumuremyi F, Van Goethem C, Kestelyn P, Msellati P, Hitimana D G. Am J Dis Child. 1991;145:1248–1251. doi: 10.1001/archpedi.1991.02160110040017. [DOI] [PubMed] [Google Scholar]
- 4.Laue L, Pizzo P A, Butler K, Cutler G B., Jr J Pediatr. 1990;117:541–545. doi: 10.1016/s0022-3476(05)80685-7. [DOI] [PubMed] [Google Scholar]
- 5.Ng T T, O’Connell I P, Wilkins E G. Clin Endocrinol (Oxford) 1994;41:689–693. doi: 10.1111/j.1365-2265.1994.tb01838.x. ; discussion, 693–704. [DOI] [PubMed] [Google Scholar]
- 6.Krentz A J, Koster F T, Crist D M, Finn K, Johnson L Z, Boyle P J, Schade D S. J Acquired Immune Defic Syndr. 1993;6:245–251. [PubMed] [Google Scholar]
- 7.Kotler, D. P. & Grunfeld, C. (1995–96) AIDS Clin. Rev. 229–275. [PubMed]
- 8.Weinroth S E, Parenti D M, Simon G L. Infect Agents Dis. 1995;4:76–94. [PubMed] [Google Scholar]
- 9.Macallan D C, Noble C, Baldwin C, Jebb S A, Prentice A M, Coward W A, Sawyer M B, McManus T J, Griffin G E. N Engl J Med. 1995;333:83–88. doi: 10.1056/NEJM199507133330202. [DOI] [PubMed] [Google Scholar]
- 10.Grunfeld C, Feingold K R. N Engl J Med. 1992;327:329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- 11.Frost R A, Furhrer J, Steigbigel R, Mariuz P, Lang C H, Gelato M C. Clin Endocrinol. 1996;44:501–514. doi: 10.1046/j.1365-2265.1996.705526.x. [DOI] [PubMed] [Google Scholar]
- 12.Grinspoon S K, Bilezikian J P. N Engl J Med. 1992;327:1360–1375. doi: 10.1056/NEJM199211053271906. [DOI] [PubMed] [Google Scholar]
- 13.Merenich J. Baillieres Clin Endocrinol Metab. 1994;4:757–767. doi: 10.1016/s0950-351x(05)80298-8. [DOI] [PubMed] [Google Scholar]
- 14.Schlienger J L, Lang J M. Pathol Biol (Paris) 1989;8:921–926. [PubMed] [Google Scholar]
- 15.Merenich J A, McDermott M T, Asp A A, Harrison S M, Kidd G S. J Clin Endocrinol Metab. 1990;3:566–571. doi: 10.1210/jcem-70-3-566. [DOI] [PubMed] [Google Scholar]
- 16.Ratner Kaufman F, Gertner J M, Sleeper L A, Donfield S M. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;15:137–144. doi: 10.1097/00042560-199706010-00007. [DOI] [PubMed] [Google Scholar]
- 17.Grinspoon S, Corcoran C, Lee K, Burrows B, Katznelson L, Walsh M, Guccione A, Cannan J, Heller H, Basgoz N, Klibanski A. J Clin Endocrinol Metab. 1996;81:4051–4058. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- 18.Mulligan K, Grunfeld C, Hellerstein M K, Neese R A, Schambelan M. J Clin Endocrinol Metab. 1993;4:956–962. doi: 10.1210/jcem.77.4.8408471. [DOI] [PubMed] [Google Scholar]
- 19.Schambelan M, LaMarca A, Mulligan K, Grunfeld C, Kennedy S, Breitmeyer J, Daar E. Int Conf AIDS. 1994;10:35. , 432B. [Google Scholar]
- 20.Lieberman S A, Butterfield G E, Harrison D, Hoffman A R. J Pediatrics. 1994;117:541–545. [Google Scholar]
- 21.Waters D, Danska J, Hardy K, Koster F, Qualls C, Nickell D, Nightingale S, Gesundheit N, Watson D, Schade D. Ann Int Med. 1996;125:865–872. doi: 10.7326/0003-4819-125-11-199612010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Schambelan M, Mulligan K, Grunfeld C, Daar E, LaMarca A, Kotler D P, Wang J, Bozzette S A, Breitmeyer J B. Ann Int Med. 1996;125:873–882. doi: 10.7326/0003-4819-125-11-199612010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lee P D, Pivarnik J M, Bukar J G, Muurahainen N, Berry P S, Skolnik P R, Nerad J L, Kudsk K A, Jackson L, Ellis K J, Gesundheit N. J Clin Endocrinol Metab. 1996;81:2968–2975. doi: 10.1210/jcem.81.8.8768860. [DOI] [PubMed] [Google Scholar]
- 24.Marks J B. Am J Med Sci. 1991;302:110–117. doi: 10.1097/00000441-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Ferreiro J, Vinters H V. Pathology. 1988;20(3):211–215. doi: 10.3109/00313028809059495. [DOI] [PubMed] [Google Scholar]
- 26.Ho D D, Rota T R, Schooley R T. N Engl J Med. 1985;313:1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- 27.Verges B, Chavanet P, Desgres J, Kisterman J P, Waldner A, Vaillant G, Portier H, Brun J M, Putelat R. Presse Med. 1990;19:1267–1270. [PubMed] [Google Scholar]
- 28.Sundar S K, Cierpial M A, Kamaraju L S, Long S, Hsieh S, Lorenz C, Aaron M, Richie J C, Weiss J M. Proc Natl Acad Sci USA. 1991;88:11246–11250. doi: 10.1073/pnas.88.24.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peisen J N, McDonnell K J, Mulroney S E, Lumpkin M D. Endocrinology. 1995;136:3378–3390. doi: 10.1210/endo.136.8.7628373. [DOI] [PubMed] [Google Scholar]
- 30.Brenneman D E, Westbrook G L, Fitzgerald S P, Ennist D L, Elkins K L, Ruff M R, Pert C B. Nature (London) 1988;355:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- 31.Dryer E B, Kaiser P K, Offermann J T, Lipton S A. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 32.Brenneman D E, Buzy M R, Ruff M R, Pert C B. Drug Dev Res. 1988;15:361–369. [Google Scholar]
- 33.Hill J M, Mervis R F, Avidor R, Moody T W, Brenneman D E. Brain Res. 1993;603:222–233. doi: 10.1016/0006-8993(93)91241-j. [DOI] [PubMed] [Google Scholar]
- 34.Pert C B, Hill J M, Ruff M R, Berman R M, Robey W G, Arthur L O, Ruscetti F W, Farrar W L. Proc Natl Acad Sci USA. 1986;83:9254–9258. doi: 10.1073/pnas.83.23.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruff M R, Martin B M, Ginns E I, Farrar W L, Wahl S M, Pert C B. FEBS Lett. 1987;211:17–22. doi: 10.1016/0014-5793(87)81265-6. [DOI] [PubMed] [Google Scholar]
- 36.Ruff M R, Hallberg P L, Hill J M, Pert C P. Lancet. 1987;2:751. doi: 10.1016/s0140-6736(87)91120-2. [DOI] [PubMed] [Google Scholar]
- 37.Lumpkin M D, McDonald J K. Endocrinology. 1989;124:1522–1527. doi: 10.1210/endo-124-3-1522. [DOI] [PubMed] [Google Scholar]
- 38.Samson W K, Said S I, Snyder G, McCann S M. Peptides. 1980;1:325–353. doi: 10.1016/0196-9781(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 39.Samson W K, Lumpkin M D, McCann S M. Endocrinology. 1986;119:554–560. doi: 10.1210/endo-119-2-554. [DOI] [PubMed] [Google Scholar]
- 40.Harvey S. In: Growth Hormone. Harvey S, Scanes C G, Daughaday W H, editors. Boca Raton, FL: CRC Press; 1995. , Ch. 9, pp. 131–162. [Google Scholar]
- 41.Murphy L J, Durum S K, Anver M R, Longo D L. J Immunol. 1992;148:3799–3805. [PubMed] [Google Scholar]
- 42.Muro-Cacho C A, Pantaleo G, Fauci A S. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 43.Heijligenberg R, Sauerwein H P, Brabant G, Endert E, Hommer M J, Romijn J A. J Clin Endo Metab. 1996;81:4028–4032. doi: 10.1210/jcem.81.11.8923855. [DOI] [PubMed] [Google Scholar]
- 44.Ho K Y, Veldhuis J S, Johnson M L, Furlanetto R, Evans W S, Alberti K G, Thorner M O. J Clin Invest. 1988;181:968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rojdmark S. Clin Endocrinol. 1986;25:721–728. doi: 10.1111/j.1365-2265.1986.tb03628.x. [DOI] [PubMed] [Google Scholar]
- 46.Willoughby J O, Brogan M, Kapoor R. Neuroendocrinology. 1989;50:584–591. doi: 10.1159/000125285. [DOI] [PubMed] [Google Scholar]
- 47.Horvath S, Palkovits M, Gorcs T, Arimura A. Brain Res. 1989;481:8–15. doi: 10.1016/0006-8993(89)90479-4. [DOI] [PubMed] [Google Scholar]
- 48.Aguila M C, McCann S M. Endocrinology. 1985;117:762–765. doi: 10.1210/endo-117-2-762. [DOI] [PubMed] [Google Scholar]
- 49.Mitsugi N, Arita J, Kimura F. Neuroendocrinology. 1990;51:93–96. doi: 10.1159/000125322. [DOI] [PubMed] [Google Scholar]
- 50.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 51.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 52.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 53.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 54.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]