Abstract
We have used Arabidopsis calmodulin (CaM) covalently coupled to horseradish peroxidase to screen a barley aleurone cDNA expression library for CaM binding proteins. The deduced amino acid sequence of one cDNA obtained by this screen was shown to be a unique protein of 702 amino acids with CaM and cyclic nucleotide binding domains at the carboxyl terminus and high similarity to olfactory and K+ channels. This cDNA was designated HvCBT1 (Hordeum vulgare CaM binding transporter). Hydropathy plots of HvCBT1 showed the presence of six putative transmembrane domains, but sequence alignment indicated a pore domain that was unlike the consensus domains in K+ and olfactory channels. Expression of a subclone of amino acids 482–702 in Escherichia coli generated a peptide that bound CaM. When a fusion protein of HvCBT1 and green fluorescent protein was expressed in barley aleurone protoplasts, fluorescence accumulated in the plasma membrane. Expression of HvCBT1 in the K+ transport deficient Saccharomyces cerevisiae mutant CY162 showed no rescue of the mutant phenotype. However, growth of CY162 expressing HvCBT1 with its pore mutated to GYGD, the consensus sequence of K+ channels, was compromised. We interpret these data as indicating that HvCBT1 acts to interfere with ion transport.
Calcium is an ubiquitous signaling molecule in eukaryotic cells, and the maintenance of cytosolic Ca2+ concentration at around 100 nM is crucial for the role of Ca2+ in signaling (1, 2). Cellular proteins that bind Ca2+ play key roles in cytosolic Ca2+ homeostasis and signal transduction (2, 3). Proteins such as calsequestrin and calreticulin that have a high binding capacity but a low affinity for Ca2+ (4, 5) function as Ca2+ buffers, whereas proteins such as calmodulin (CaM) that change conformation after Ca2+ binding (6) act as Ca2+ triggers. Ca2+ trigger proteins function in signal transduction by interacting with target proteins. A large number of CaM binding proteins have been identified in animal cells, but far fewer have been identified in plant cells (7, 8). The CaM binding proteins from plants that have been studied in most detail are enzymes such as glutamate decarboxylase (9), NAD kinase (8), and protein kinases (10, 11). Several cDNAs encoding CaM binding proteins have also been cloned from plants, including cDNAs for glutamate decarboxylase from petunia (12), protein kinase homologs from maize and lily (13, 14), and a protein from Arabidopsis with a kinesin heavy-chain motor domain (15).
We have used the barley aleurone cell as a model to study the Ca–CaM signaling pathway in plants. The cereal aleurone is a secretory tissue whose activity is controlled by the plant hormones gibberellic acid (GA) and abscisic acid (ABA) (16). Cytosolic Ca2+, cGMP, and CaM are key components of the GA signaling pathway (1, 17–19). Three potential targets of the Ca–CaM signaling pathway have been identified in the aleurone cell. The activities of a Ca2+ ATPase on the endoplasmic reticulum (20), a slow vacuolar cation channel on the protein storage vacuole (21), and a Ca2+ transporter on the tonoplast (22) are stimulated by CaM. Recent work with a slow vacuolar channel indicates that Ca/CaM regulates the activity of this channel via protein phosphorylation (23).
We have applied a novel approach using covalently modified CaM to isolate cDNAs encoding CaM binding proteins from the barley aleurone cell. Recombinant Arabidopsis CaM2 (24) was covalently coupled to horseradish peroxidase (HRP), and this conjugate was used to screen barley aleurone cDNA expression libraries. We report on the isolation and characterization of a full-length cDNA clone encoding a CaM binding transporter from barley designated HvCBT1 (Hordeum vulgare CaM binding transporter). HvCBT1 is a member of a family of plant transporters that is likely to be regulated by both cyclic nucleotide monophosphates (cNMPs) and CaM.
MATERIALS AND METHODS
Plant Material.
Barley (Hordeum vulgare L. cv Himalaya, 1985 and 1991 harvests; Agronomy Department, Washington State University, Pullman, WA) grains were used for all experiments. Aleurone layers were isolated as described (19). For isolation of scutella, grains were germinated for 24 h.
CaM-HRP Conjugate.
Arabidopsis CaM2 (ACaM2) cDNA inserted in the pET-5a vector (Novagen) was provided by R. Zielinski (University of Illinois) and expressed in Escherichia coli BL21(DE3). ACaM2 protein was isolated and purified as described (19). The single C of ACaM2 (24) was coupled to HRP with a maleimide linker (Sulfo-succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate as described by the manufacturer (Pierce). The ACaM2-HRP conjugate was purified on a Superdex-75 column (Pharmacia) by using a Pharmacia FPLC system.
Screening of cDNA Libraries with CaM-HRP.
cDNA libraries of barley aleurone mRNA were obtained from Stratagene (H2O-imbibed aleurone layers) and F. Gubler (Commonwealth Scientific and Industrial Research Organization, Canberra, Australia; GA-treated aleurone layers). Plaques were plated at 25,000 plaque-forming units per 15-cm Petri dish and incubated for 3 h at 42°C. Plaques were overlaid with a Hybond-N membrane (Amersham) saturated in 1 mM isopropyl β-d-thiogalactoside and incubated for 5 h at 37°C. Membranes were extensively washed in CaM binding buffer (150 mM NaCl/1 mM CaCl2/50 mM Tris⋅HCl, pH 7.5) and blocked with BSA (1 mg/ml) in CaM binding buffer for 30 min at room temperature before incubation with the CaM-HRP conjugate (0.6 μg/ml) in CaM binding buffer with BSA (0.1 mg/ml, 3 h to overnight at room temperature). After incubation, membranes were washed for four 20-min periods in CaM binding buffer. HRP activity was localized on membranes by using the 4CN-plus substrate (DuPont). A total of 106 plaque-forming units were screened.
DNA Sequencing.
Sequence reactions with Taq polymerase and fluorescent terminators were carried out according to the manufacturer’s instructions (Applied Biosystems) and analyzed on an Applied Biosystems PRISM model 377 sequencer. Protein comparisons were performed by using the bestfit, gap, and fasta algorithms (University of Wisconsin Computer Group). Related sequences were searched in available protein and nucleotide databases.
Rapid Amplification of cDNA Ends (RACE).
Rapid amplification of 5′ ends of cDNA (5′ RACE, GIBCO/BRL) was used to obtain full-length cDNA of HvCBT1. Poly(A)+ RNA from scutella of germinated barley grains was reverse-transcribed by using a HvCBT1-specific primer RACE1 (5′-TATGTTGACAGATACCGCTTTGC-3′, Fig. 1A). PCR amplification was performed with the HvCBT1-specific primer RACE2 (5′-AGCGCAGGACACTTGCAGTG-3′, Fig. 1A) upstream of RACE1 and the anchor primer was supplied by the manufacturer. A single DNA molecule of 0.7 kbp was obtained. An NcoI site was introduced before the second ATG at position 246 (Fig. 1A) and used to generate constructs expressing the full-length HvCBT1. All PCR products were sequenced.
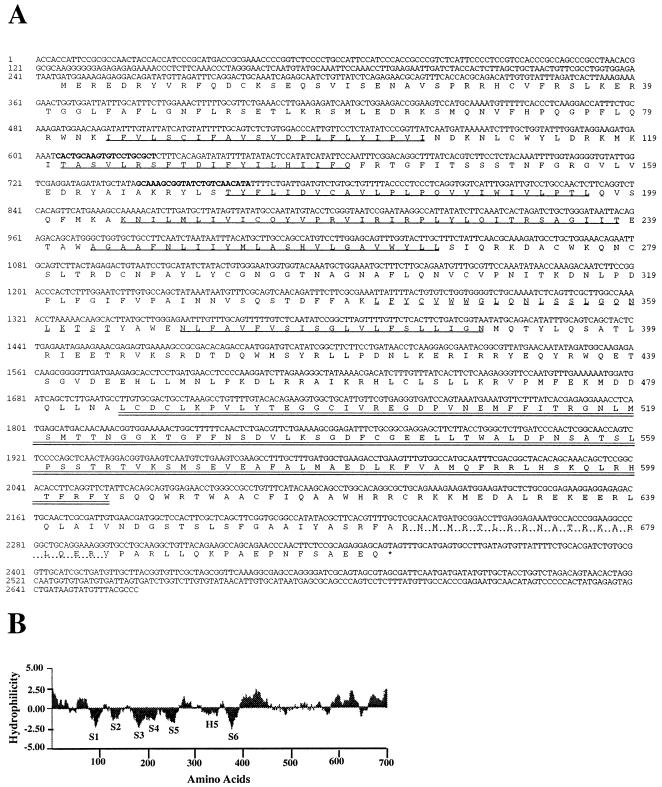
Figure 1.
(A) Nucleotide sequence of the full-length HvCBT1 along with the amino acid sequence of the longest ORF. The RACE primers are shown in boldface type, the six putative transmembrane domains are underlined, the H5 domain is dashed underlined, the putative cyclic nucleotide binding domain is double underlined, and the CaM binding domain is dotted underlined. (B) Hydropathy plot of the deduced amino acid sequence of HvCBT1. The H5 region as well as the six transmembrane domains (S1–S6) are indicated. Negative values indicate hydrophobic regions.
HvCBT1-Green Fluorescent Protein (GFP) Fusion Construct.
The pDMC207 vector (25) containing the GUS reporter driven by a rice actin promoter and terminated by the rbcs terminator was digested with NcoI and KpnI to remove GUS and the rbcs terminator. HvCBT1 was cut with EspI at position 2,195, blunted with Pfu polymerase, and digested with NcoI to obtain HvCBT1 lacking the carboxyl-terminal 50 amino acids. Bluescript SK-SGFP-TYG [synthetic GFP with Ser at position 65 changed to Thr (26), henceforth referred to as GFP] was digested with HindIII, blunted with Pfu polymerase, and digested with KpnI to excise full-length GFP terminated by a nos terminator. HvCBT1 was ligated with GFP into pDMC207 so that the HvCBT1-GFP fusion was driven by the rice actin promoter and terminated by the nos terminator. Five new amino acids between HvCBT1 and GFP were derived from multiple cloning sites. For control experiments, an NcoI–KpnI fragment of Bluescript SK-GFP, containing the full-length GFP and the nos terminator was directly cloned into NcoI/KpnI-digested pDMC207.
RNA Isolation and RNA Gel Blotting.
Total RNA was isolated and RNA gel blots prepared as described (19). Poly(A)+ RNA was selected by chromatography on poly(U)-Sepharose according to the protocol of the manufacturer (Pharmacia). Hybridizations were carried out as described (19).
HvCBT1 Protein Purification, Antibody Generation, and Far Western Blotting.
To localize the CaM binding domain of HvCBT1, amino acids 482–702 were inserted into the PvuII site of pRSETC (Novagen; ref. 27). The HvCBT1 protein, fused to 10 amino acids of the T7 gene 10 major capsid protein with six His at the amino terminus, was expressed in E. coli BL21(DE3) pLysE. The HvCBT1 fusion protein was detected in E. coli lysates by protein blotting after SDS/PAGE using two methods. Protein blots were incubated with ACaM2 and CaM binding was determined by using a mAb to CaM (19) or incubated with biotinylated bovine brain CaM whose binding was determined with avidin-coupled alkaline phosphatase.
The HvCBT1 fusion protein was purified under denaturing conditions on a Ni column as described by the manufacturer (Qiagen, Chatsworth, CA). Purified protein was injected into rabbits by using AlOH3 as a carrier and adjuvant (Pierce). Rabbit serum was first depleted of antibodies reacting with E. coli proteins by affinity purification on an E. coli lysate column (Pierce), and antibodies to the HvCBT1 fusion protein were purified on a Ni column (28).
Yeast Constructs and Transformation.
The pJR1133 (J. Rine, University of California, Berkeley) and pYES2 (Novagen) E. coli-yeast shuttle vectors were used for all manipulations. The full-length HvCBT1 fragment was cloned into pJR1133 between the GPD promoter and the PGK terminator. To mutate the GQNL sequence in HvCBT1 into GYGD primers for the sequence 5′-LGYGDKTSTY-3′ (GYGD-forward) and 5′-KDGYGLSSLNQ-3′ (GYGD-reverse) were designed. A full-length HvCBT1-GYGD was generated by PCR and cloned into pYES2 between the GAL promoter and the CYC1 terminator. Yeast was transformed using the LiAc method (29). For all PCRs, Pfu polymerase (Stratagene) was used and all PCR products were sequenced.
Protoplast Transfection and Microscopy.
Aleurone protoplasts were isolated and transfected as described (30). Transfected protoplasts were incubated in the dark at room temperature for 2–4 days. Protoplasts were viewed by bright-field and fluorescence microscopy as described (31). In 10 replicated experiments, the efficiency of transfection with HvCBT1-GFP averaged 5% of living cells.
RESULTS
Molecular Cloning of HvCBT1.
To identify cDNAs from barley aleurone encoding CaM binding proteins, we developed an enzyme-linked protein–protein interaction screening strategy. We purified recombinant Arabidopsis ACaM2 to homogeneity from E. coli and determined that recombinant ACaM2 activated CaM-dependent phosphodiesterase (data not shown). We covalently linked ACaM2 to HRP via Cys-26 and showed that calcineurin, a CaM-dependent protein phosphatase, bound the CaM-HRP conjugate in a Ca2+-dependent fashion (data not shown). A cDNA library prepared from barley aleurone mRNA was expressed in λ-ZAPII, and the expressed proteins were screened with the CaM-HRP conjugate. Expressed proteins that bound CaM-HRP in the presence of Ca2+ were identified by using a colorimetric stain for HRP. As a positive control a CaM-dependent protein kinase (ICM-1, ref. 32) was expressed in λgt11, and plaques expressing ICM-1 bound CaM-HRP in a Ca2+-dependent manner (data not shown). The CaM-binding plaque-forming units from the barley aleurone cDNA expression library were sequenced, and one of them, HvCBT1, showed high similarity to ion channels.
RNA gel blots of barley aleurone mRNA probed with HvCBT1 cDNA showed only one mRNA band that was approximately 500 bases longer than the cDNA (data not shown). 5′ RACE of HvCBT1 using poly(A)+ RNA from barley scutellum in which HvCBT1 was also expressed (data not shown) was used to obtain a full-length HvCBT1 cDNA. The 5′ RACE product had 126 nucleotides that overlapped the 5′ end of the original cDNA.
The Primary Structure of HvCBT1 Shows Similarity to Ion Channels.
Because HvCBT1 cDNA was obtained from a λ-ZAPII cDNA library, we deduced that the sequence of the full-length cDNA contained an ORF of 2,106 nucleotides encoding a 702 amino acid polypeptide (Fig. 1A). Two ATG at positions 243 and 246 were identified (Fig. 1A), but only the second ATG had adjacent sequences having consensus with plant translation initiation sites (33).
Analysis of all available data bases showed similarities among HvCBT1 and olfactory (34) and K+ channels (35) and with cAMP- and cGMP-dependent protein kinases (36). The identity with K+ and olfactory channels ranged from 20 to 24%, and similarity to these channels was higher (about 47%). Similarity with protein kinases was at the carboxyl terminus (amino acids 485–604; Fig. 1A), where a putative cyclic nucleotide binding site is located.
Comparative studies of eukaryotic ion channel sequences show that six transmembrane domains (S1–S6) and a pore region (the H5 or P domain) are ubiquitous features (37–39). Hydropathy analysis of HvCBT1 showed strongly hydrophobic domains (Fig. 1B), suggesting six membrane-spanning domains. The S4 domain of voltage-gated K+ channels consists of a hydrophobic stretch of amino acids interrupted by Arg or Lys at every third residue and is thought to serve as a voltage sensor (37). HvCBT1 has four positive residues (all Arg) in the S4 domain (Fig. 2A).
Figure 2.
Alignment of amino acid sequences of HvCBT1 and ion channels. (A) Alignment of the S4 regions of HvCBT1, AKT1, KAT1, Shaker, olfactory channel, and Eag. Positively charged amino acids are highlighted. (B) Alignment of the consensus amino acid sequence for the H5 region of K+ channels, the amino acid sequences for the H5 regions of HvCBT1, AKT1, KAT1, and Shaker. The conserved GYGD sequence is highlighted, identical amino acids are represented by dashes, and asterisks indicate amino acids that are not conserved.
Fig. 2B shows an alignment of sequences in the H5 region of HvCBT1, K+ and olfactory channels. GYGD is a consensus sequence in the H5 region of K+ channels but is absent in HvCBT1, which has GQNL at the aligned position (Fig. 2B). The presence of the much smaller Gln in GQNL of HvCBT1 is more characteristic of nonspecific ion channels (40). Two Trp residues are found in the pore region of almost all K+ channels and are present in the aligned position in HvCBT1 (Fig. 2B). Two Ser residues are conservative substitutions in HvCBT1 for the Thr residues that are found in many other K+ channels (Fig. 2B). The region between Ser-5 and His-5 of HvCBT1 is much longer (approximately 90 amino acid residues) than that in other ion channels cloned and sequenced to date, which average about 40 amino acids.
The Carboxyl Terminus of HvCBT1 Binds CaM.
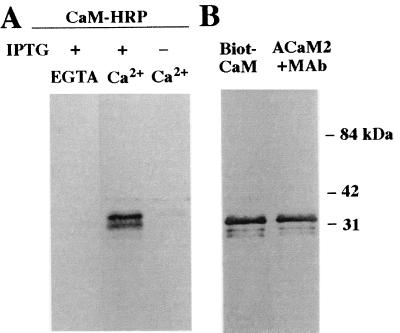
To determine whether CaM binding by HvCBT1 was a result of a specific interaction between hydrophilic regions and CaM, part of the hydrophilic carboxyl terminus of HvCBT1 (amino acids 482–702) was subcloned and expressed in E. coli. Fig. 3A shows that a major band of about 33 kDa and a minor band of 31 kDa were detected when protein blots of recombinant protein were probed with CaM-HRP. Affinity-purified recombinant HvCBT1 also bound biotinylated bovine brain CaM and recombinant ACaM2 on protein blots (Fig. 3B).
Figure 3.
Far Western analysis of the carboxyl terminus of HvCBT1 for CaM binding. (A) Crude protein extracts of E. coli grown with (+) or without (−) 0.4 mM isopropyl β-d-thiogalactoside and harboring the expression vector pRSETC with cDNA sequences for the carboxyl terminus of HvCBT1 inserted were separated by SDS/PAGE and transferred to nitrocellulose. The nitrocellulose filter was incubated with the CaM-HRP conjugate to assess CaM binding in the presence of 1 mM EGTA or 1 mM Ca2+. (B) Highly purified carboxyl terminus of HvCBT1 was separated by SDS/PAGE and transferred to nitrocellulose. CaM binding was assessed with biotinylated bovine brain CaM (Biot-CaM) or with purified ACaM2 plus monoclonal antibody (MAb) to CaM. Molecular mass markers are shown to the right.
HvCBT1 Localizes to the Plasma Membrane of Aleurone Cells.
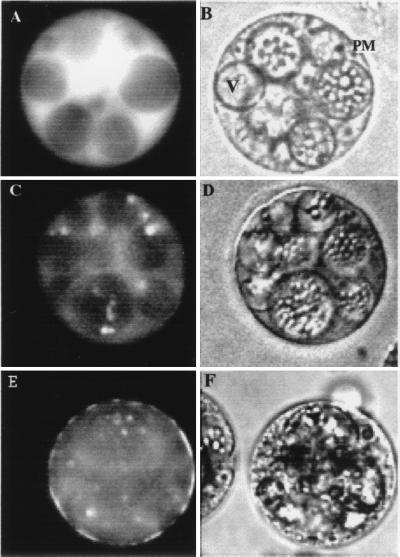
The subcellular location of HvCBT1 in aleurone cells was examined by transient overexpression of a cDNA encoding a near full-length HvCBT1 fused to a full-length cDNA encoding GFP. When GFP alone was expressed in aleurone cells under the control of a rice actin promoter, fluorescence accumulated in the cytosol within 20 h of transfection (Fig. 4A), although fluorescence occasionally accumulated in the nucleus (data not shown). GA-treated cells transfected with an actin-HvCBT1-GFP construct showed accumulation of fluorescence in the cytosol beginning about 24 h after transfection, but after 48 h, fluorescence was lost from the cytosol and accumulated in the plasma membrane (PM, Fig. 4E). Incubation of transfected protoplasts in GA enhanced the appearance of fluorescence in the PM (Fig. 4E). Fluorescence in the PM of GA-treated protoplasts was detected after 24 h of incubation and was prominent after 48 h, in contrast to protoplasts incubated in the presence of ABA when fluorescence was not detected in the PM until 72 h of incubation (Fig. 4C). Fluorescence in the PM of transfected cells was often patchy with areas of bright fluorescence interspersed with areas that lacked measurable fluorescence (Fig. 4E). No fluorescence was detected in nuclei or vacuoles of cells transfected with actin-HvCBT1-GFP (Fig. 4 C and E), and fluorescence was absent from cells transfected with plasmid alone (data not shown).
Figure 4.
Localization of HvCBT1-GFP fusion protein and GFP in barley aleurone protoplasts. Protoplasts were transfected with GFP driven by a rice actin promoter (A and B) or with HvCBT1 fused to GFP driven by the rice actin promoter (C–F). Transfected protoplasts were treated with 5 μM hormone and 20 mM Ca2+. Fluorescent (A and E) and bright-field (B and F) images were taken 48 h after treatment with GA or 72 h after treatment with ABA (C and D). PM, plasma membrane; V, vacuole. (×40.)
Expression of HvCBT1 in Saccharomyces cerevisiae.
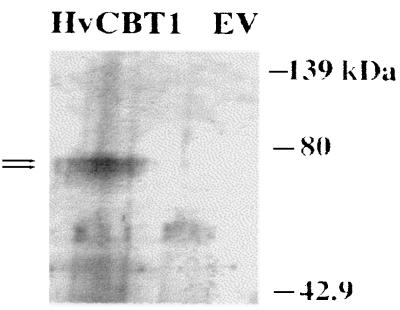
To determine whether HvCBT1 plays a role in K+ acquisition, we transformed the S. cerevisiae mutant CY162 (Δtrk1Δtrk2) with yeast–E. coli shuttle vectors containing various constructs. The CY162 mutant of S. cerevisiae is unable to grow on KCl concentrations below approximately 5 mM, but the mutant phenotype can be rescued by expression of the Arabidopsis thaliana inwardly rectifying K+ channel KAT1 (35). To establish whether HvCBT1 was expressed in CY162, protein blots of extracts of transformed CY162 were probed with HvCBT1 antibodies. Two proteins of 75–77 kDa were recognized in CY162 cells transformed with HvCBT1 but not in cells transformed with empty vector (Fig. 5).
Figure 5.
Protein blot of HvCBT1 protein in CY162. Cell-free protein extracts of CY162 transformed with pJR1133 (lane EV) and pJR1133 harboring HvCBT1 were separated by SDS/PAGE and transferred to nitrocellulose. HvCBT1 protein was detected with polyclonal antibodies against the carboxyl terminus of HvCBT1. Arrows indicate HvCBT1. Molecular mass markers are shown to the right.
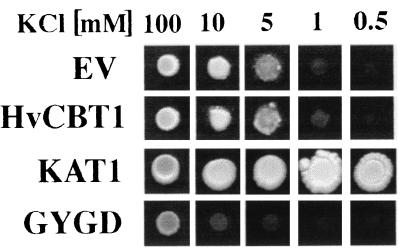
To establish a role for HvCBT1 in K+ homeostasis in yeast, the CY162 mutant was transformed with HvCBT1, and transformed cells were grown on solid medium containing KCl concentrations from 0.5 mM to 100 mM. CY162 transformed with HvCBT1 alone showed no improvement of growth over cells transformed with empty vector in medium where KCl was limiting (Fig. 6). Because HvCBT1 does not possess the consensus sequence GYGD found in all cloned K+ channels, we mutated HvCBT1 to introduce GYGD in the pore region at amino acids 357–360. CY162 transformed with HvCBT1-GYGD grew slowly at KCl concentrations between 0.5 mM and 100 mM, and growth was significantly slower than cells transformed with the vector alone (Fig. 6). As a control, CY162 was transformed with KAT1. As has been shown by others (35), KAT1 complemented CY162 and allowed growth on medium containing micromolar KCl concentrations (Fig. 6).
Figure 6.
Growth of CY162 transformed with various constructs. CY162 was transformed with pYES (EV) or pYES containing HvCBT1 (HvCBT1), KAT1 (KAT1), or HvCBT1-GYGD (GYGD). Single colonies of pure streaks of the transformed CY162 were suspended in water, and equal amounts of cells were dotted on agar plates with the specified concentrations of KCl.
DISCUSSION
By exploiting the ability of CaM to bind target proteins with high affinity, we have isolated a cDNA from barley aleurone that encodes a CaM-binding protein, HvCBT1. HvCBT1 is localized to the PM of the aleurone cell and shows similarity to ion channels previously cloned from animals and K+ channels from plants. HvCBT1 is unable to complement CY162, a S. cerevisiae K+ transport mutant, on medium containing micromolar KCl concentrations. Growth of CY162 on both micro- and millimolar KCl concentrations is compromised when mutations are made in the pore domain of HvCBT1.
Because HvCBT1 cDNA was isolated by interaction of expressed protein with CaM-HRP, we sought to localize the CaM binding domain in the recombinant HvCBT1 molecule. Amphiphilicity profiles of amino acids at the carboxyl terminus of HvCBT1 show that amino acids 664–684 form an amphiphilic helix (data not shown). This region contains several amino acids with high α-helix forming tendency including Met, Ala, Leu, Glu, and a large number of basic amino acids (Lys and Arg) are separated by hydrophobic residues, indicating a basic amphiphilic structure characteristic of other CaM binding domains (41). By subcloning a carboxyl-terminal fragment of HvCBT1, we were able to localize the CaM binding domain to a region encoding amino acids 482–702. The recombinant carboxyl-terminal fragment of HvCBT1 bound the CaM-HRP probe used to isolate HvCBT1 as well as biotinylated bovine brain CaM and Arabidopsis CaM2. In all cases, CaM binding to the carboxyl terminus of HvCBT1 was Ca2+-dependent.
CaM is an important component of the GA signal transduction pathway in barley aleurone. GA increases CaM protein levels in aleurone cells by 2-fold (19), and experiments with CaM antagonists and Ca2+ chelators show that CaM and Ca2+ are essential for GA-stimulated enzyme secretion (17). Because HvCBT1 is a PM-localized CaM-binding ion channel homolog, we infer that CaM plays a crucial role in ion transport in the barley aleurone cell.
The presence of a putative cNMP-binding domain adjacent to the CaM binding domain at the carboxyl terminus also makes the sequence of HvCBT1 unique in plants. The only other ion channel that has been shown to have both CaM and cNMP binding domains is the catfish olfactory channel (42). The CaM binding site in this olfactory channel resides at the amino terminus, upstream of the first transmembrane domain. In the olfactory channel, Ca/CaM reduces the affinity of the channel for either cGMP or cAMP. By altering the sensitivity of the olfactory channel to cNMP, CaM allows this channel to respond to a range of cNMP concentrations in excess of four orders of magnitude (42). The response of the olfactory channel to a broad range of cNMP concentrations suggests a mechanism whereby plant cells, including the aleurone layer, respond to hormones such as GA and ABA.
The amino acid sequence of the putative cNMP binding site in HvCBT1 indicates a higher affinity for cGMP compared with cAMP (36). In this context it is interesting to note that aleurone cells contain cGMP and that the levels of this cNMP are transiently increased by GA (18). Furthermore, inhibition of cGMP accumulation by LY83583 results in a repression of α-amylase synthesis and secretion that can be reversed by the addition of membrane-permeable cGMP analogs (18). These data strongly implicate cGMP in the response of the barley aleurone to GA.
We localized HvCBT1 at the PM of the barley aleurone cell by expressing GFP translationally fused to the carboxyl terminus of HvCBT1. Accumulation of fluorescence in the PM was greatly enhanced by incubation of transfected aleurone protoplasts with GA (Fig. 4). In GA-treated cells fluorescence at the cell surface was detected at 24 h of incubation and was very prominent after 48 h (Fig. 4E). Cells incubated in the absence of hormone or in the presence of ABA showed less-intense fluorescence (Fig. 4C). The effect of GA in enhancing PM fluorescence of HvCBT1-GFP is likely a result of an effect of GA on the secretory machinery of the aleurone cell (16). The endoplasmic reticulum and Golgi apparatus of the barley aleurone cell develop more extensively in GA-treated cells compared with ABA- or non-hormone-treated cells, and this may account for the more efficient insertion of HvCBT1-GFP into the PM.
Fluorescence from HvCBT1-GFP in the PM of the aleurone cell is aggregated into patches in GA-treated cells (Fig. 4E). The aggregation of PM-localized proteins such as ion channels and receptors has been reported by several laboratories (43), including those working on ion channels in fungi (44) and hormone receptors in plants (45). In the oomycete Saprolegnia, stretch-activated channels in hyphal tips are aggregated by the filamentous actin network (46). The auxin binding protein (ABP) is localized to the PM, and in maize coleoptile protoplasts ABP becomes aggregated in response to auxin (45). It has been argued that aggregation of PM proteins is a basic feature of this membrane, allowing for the formation of specific functional domains in the PM (43).
Although the amino acid sequence of HvCBT1 shares many features with those of channels previously cloned from animals and plants, HvCBT1 differs in regions attributed to channel function. The H5 loop of HvCBT1 is rich in Gly but lacks the consensus sequence GYGD found in all inwardly rectifying K+ channels (37, 38) with the exception of the Eag K+ channel family (46). Furthermore, there is no indication for the presence of a second GYGD-like domain as recently described for outwardly rectifying K+ channels with eight membrane-spanning domains (47) and for an inwardly rectifying K+ channel with four transmembrane domains (48). The size of the H5 loop of HvCBT1 is also larger than that has been reported for other K+ channels and contains approximately 90 amino acids compared with the H5 loops of KAT1 and Shaker, which average about 40 amino acids. On the basis of these differences, we conclude that HvCBT1 is a PM-localized transport protein.
We used several approaches to establish the function of HvCBT1. Injection of cRNA encoding HvCBT1 into Xenopus laevis oocytes did not elicit unique ion channel activity, even though parallel experiments with KAT1 cRNA showed inwardly rectifying K+ channel activity typical of KAT1 (data not shown). HvCBT1, unlike KAT1, was unable to complement the K+ transport defective yeast mutant CY162 (Fig. 6) even though the protein was expressed in this mutant (Fig. 5). Because one of the major differences between HvCBT1 and KAT1 is the presence of the GYGD consensus sequence in the H5 domain, we mutated HvCBT1 to introduce GYGD into H5. When CY162 was transformed with HvCBT1-GYGD, CY162 was unable to grow even on medium containing 10 mM KCl, indicating that K+ acquisition by the yeast cell was compromised. The simplest interpretation of this data is that mutation of HvCBT1 in the H5 domain with GYGD causes the mutated protein to alter the mechanism of K+ influx and/or accumulation in this yeast system.
Acknowledgments
Eleanor Crump is thanked for her help in preparation of this manuscript. The yeast strain CY162 was the generous gift of Dr. Richard F. Garber. This work was supported by grants from the National Science Foundation and National Research Initiative Competitive Grants Program/U.S. Department of Agriculture.
ABBREVIATIONS
- ABA
abscisic acid
- CaM
calmodulin
- GA
gibberellic acid
- HRP
horse radish peroxidase, PM, plasma membrane
- GFP
green fluorescent protein
- HvCBT
Hordeum vulgare CaM binding transporter
- cNMP
cyclic nucleotide monophosphate
- ACam2
Arabidopsis CaM2
- RACE
rapid amplification of cDNA ends
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ002610).
References
- 1.Bush D S. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- 2.Clapham D E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 3.Heizman C W, Hunziker W. Trends Biochem Sci. 1991;16:98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- 4.Mery L, Mesaeli N, Michalak M, Opas M, Lew D P, Krause K H. J Biol Chem. 1996;271:9332–9339. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- 5.Sambrook J F. Cell. 1990;61:197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein H, Mehler E L. Annu Rev Physiol. 1994;56:213–236. doi: 10.1146/annurev.ph.56.030194.001241. [DOI] [PubMed] [Google Scholar]
- 7.Poovaiah B W, Reddy A S N. CRC Crit Rev Plant Sci. 1993;21:185–211. doi: 10.1080/07352689309701901. [DOI] [PubMed] [Google Scholar]
- 8.Roberts D M, Harmon A C. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:375–414. [Google Scholar]
- 9.Ling V, Snedden W A, Shelp B J, Assmann S M. Plant Cell. 1994;6:1135–1143. doi: 10.1105/tpc.6.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman A M, Blumenthal D K, Krebs E G. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- 11.Roberts D M, Lukas T J, Watterson D M. CRC Crit Rev Plant Sci. 1986;4:311–339. [Google Scholar]
- 12.Baum G, Chen Y, Arazi T, Takatsuji H, Fromm H. J Biol Chem. 1993;268:19610–19617. [PubMed] [Google Scholar]
- 13.Lu Y T, Hidaka H, Feldman L J. Planta. 1996;199:18–24. doi: 10.1007/BF00196876. [DOI] [PubMed] [Google Scholar]
- 14.Patil S, Takezawa D, Poovaiah B W. Proc Natl Acad Sci USA. 1995;92:4897–4901. doi: 10.1073/pnas.92.11.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy A S N, Safadi F, Narasimhulu S B, Golovkin M, Hu X. J Biol Chem. 1996;271:7052–7060. doi: 10.1074/jbc.271.12.7052. [DOI] [PubMed] [Google Scholar]
- 16.Jones R L, Jacobsen J V. Int Rev Cytol. 1991;126:49–88. doi: 10.1016/s0074-7696(08)60682-8. [DOI] [PubMed] [Google Scholar]
- 17.Gilroy S. Plant Cell. 1996;8:2193–2209. doi: 10.1105/tpc.8.12.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penson S P, Schuurink R C, Fath A, Gubler F, Jacobsen J V, Jones R L. Plant Cell. 1996;8:2325–2333. doi: 10.1105/tpc.8.12.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuurink R C, Chan P V, Jones R L. Plant Physiol. 1996;111:371–380. doi: 10.1104/pp.111.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilroy S, Jones R L. Planta. 1993;190:289–296. [Google Scholar]
- 21.Bethke P C, Jones R L. Plant Cell. 1994;6:277–285. doi: 10.1105/tpc.6.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush D S, Wang T. Planta. 1995;197:19–30. [Google Scholar]
- 23.Bethke P C, Jones R L. Plant J. 1997;11:1227–1235. [Google Scholar]
- 24.Ling V, Perera I, Zielinski R E. Plant Physiol. 1991;96:1196–1202. doi: 10.1104/pp.96.4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElroy D, Chamberlain D A, Moon E, Wilson K J. Mol Breeding. 1995;1:27–37. [Google Scholar]
- 26.Chiu W L, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Curr Biol. 1996;6:325–330. doi: 10.1016/s0960-9822(02)00483-9. [DOI] [PubMed] [Google Scholar]
- 27.Kroll D J, Abdel-Hafiz H A M, Marcell T, Simpson S, Chen C Y, Gutierrez-Hartmann A, Lustbader J W, Hoeffler J P. DNA Cell Biol. 1993;12:441–453. doi: 10.1089/dna.1993.12.441. [DOI] [PubMed] [Google Scholar]
- 28.Gu J, Stephenson C G, Iadarola M J. BioTechniques. 1994;17:257–262. [PubMed] [Google Scholar]
- 29.Ito H, Fukuda Y, Murata K, Kimura A. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin W, Gopalakrishnan B, Muthukrishnan S. Protoplasma. 1996;192:93–108. [Google Scholar]
- 31.Swanson S J, Jones R L. Plant Cell. 1996;8:2211–2221. doi: 10.1105/tpc.8.12.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones D A, Glod J, Wilson-Shaw D, Hahn W E, Sikela J M. FEBS Lett. 1991;289:105–109. doi: 10.1016/0014-5793(91)80919-t. [DOI] [PubMed] [Google Scholar]
- 33.Luehrsen K R, Walbot V. Plant Cell Rep. 1994;13:454–458. doi: 10.1007/BF00231966. [DOI] [PubMed] [Google Scholar]
- 34.Goulding E H, Ngai J, Kramer R H, Colicos S, Axel R, Siegelbaum S A, Chess A. Neuron. 1992;8:45–58. doi: 10.1016/0896-6273(92)90107-o. [DOI] [PubMed] [Google Scholar]
- 35.Anderson J A, Huprikar S S, Kochian L V, Lucas W J. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shabb J B, Corbin J D. J Biol Chem. 1992;267:5723–5726. [PubMed] [Google Scholar]
- 37.Jan L Y, Jan Y N. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura R L, Anderson J A, Gaber R F. J Biol Chem. 1997;272:1011–1018. doi: 10.1074/jbc.272.2.1011. [DOI] [PubMed] [Google Scholar]
- 39.Reuveny E, Jan Y N, Jan L Y. Biophys J. 1996;70:754–761. doi: 10.1016/S0006-3495(96)79615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerr I D, Sansom M S P. Nature (London) 1995;373:112. doi: 10.1038/373112a0. [DOI] [PubMed] [Google Scholar]
- 41.O’Neil K T, Degrado W F. Trends Biochem Sci. 1990;15:59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Chen T Y, Ahamed B, Li J, Yau K W. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- 43.Kusumi A, Sako Y. Curr Opin Cell Biol. 1996;8:566–574. doi: 10.1016/s0955-0674(96)80036-6. [DOI] [PubMed] [Google Scholar]
- 44.Levina N N, Lew R R, Heath I B. J Cell Sci. 1994;107:127–134. doi: 10.1242/jcs.107.1.127. [DOI] [PubMed] [Google Scholar]
- 45.Diekmann W, Venis M A, Robinson D G. Proc Natl Acad Sci USA. 1995;92:3425–3429. doi: 10.1073/pnas.92.8.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brueggemann A, Pardo L A, Stuehmer W, Pongs O. Nature (London) 1993;365:445–448. doi: 10.1038/365445a0. [DOI] [PubMed] [Google Scholar]
- 47.Ketchum K A, Joiner W J, Sellers A J, Kaczmarek L K, Goldstein S A N. Biophys J. 1996;70:A96. [Google Scholar]
- 48.Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. EMBO J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]