Abstract
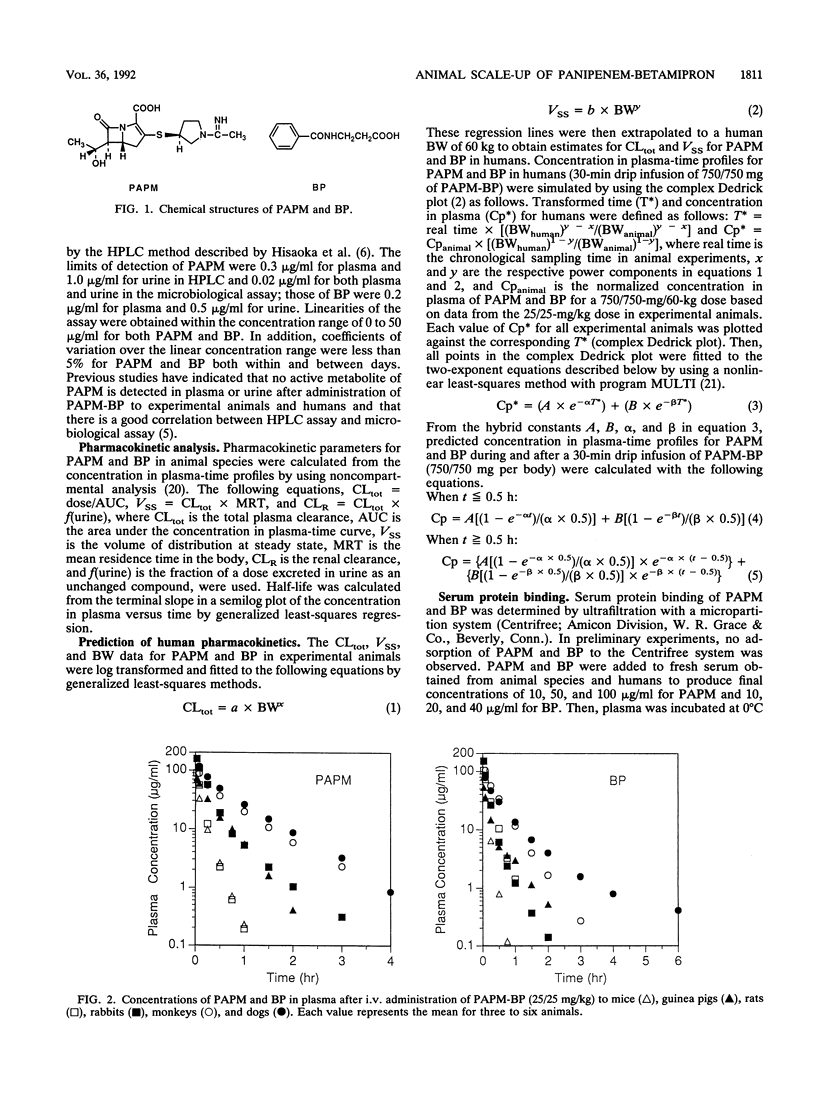
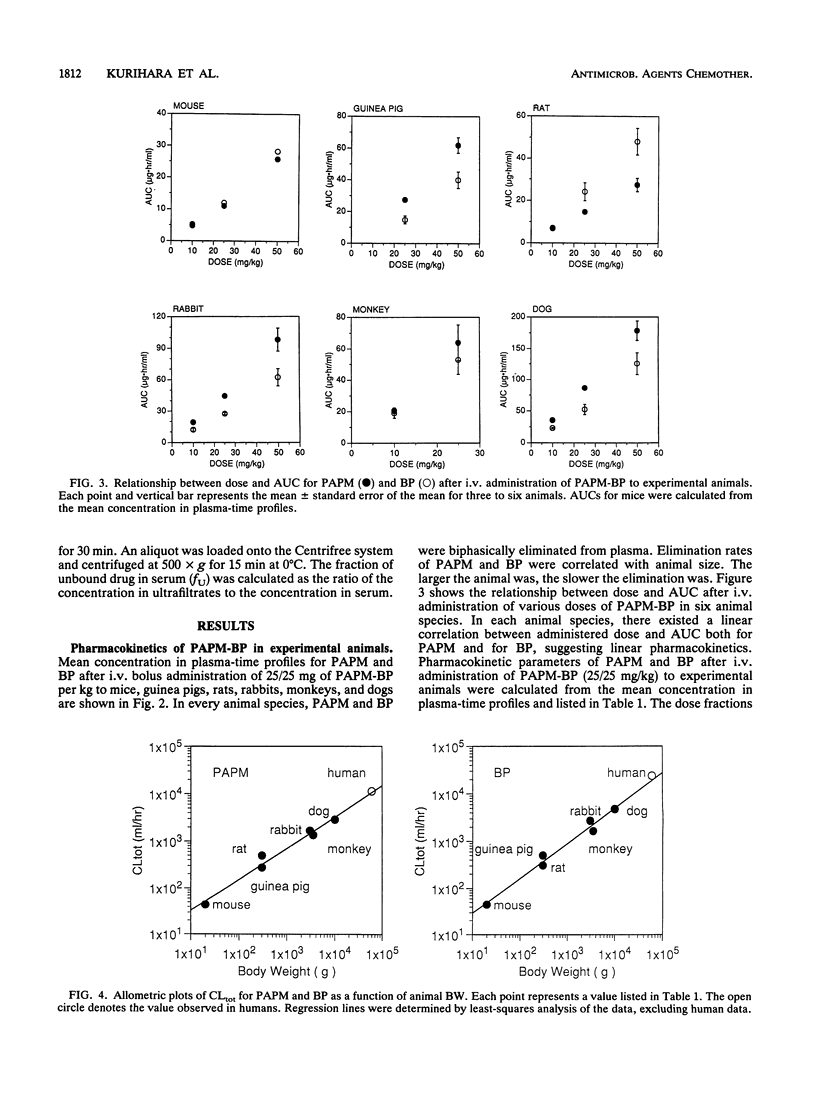
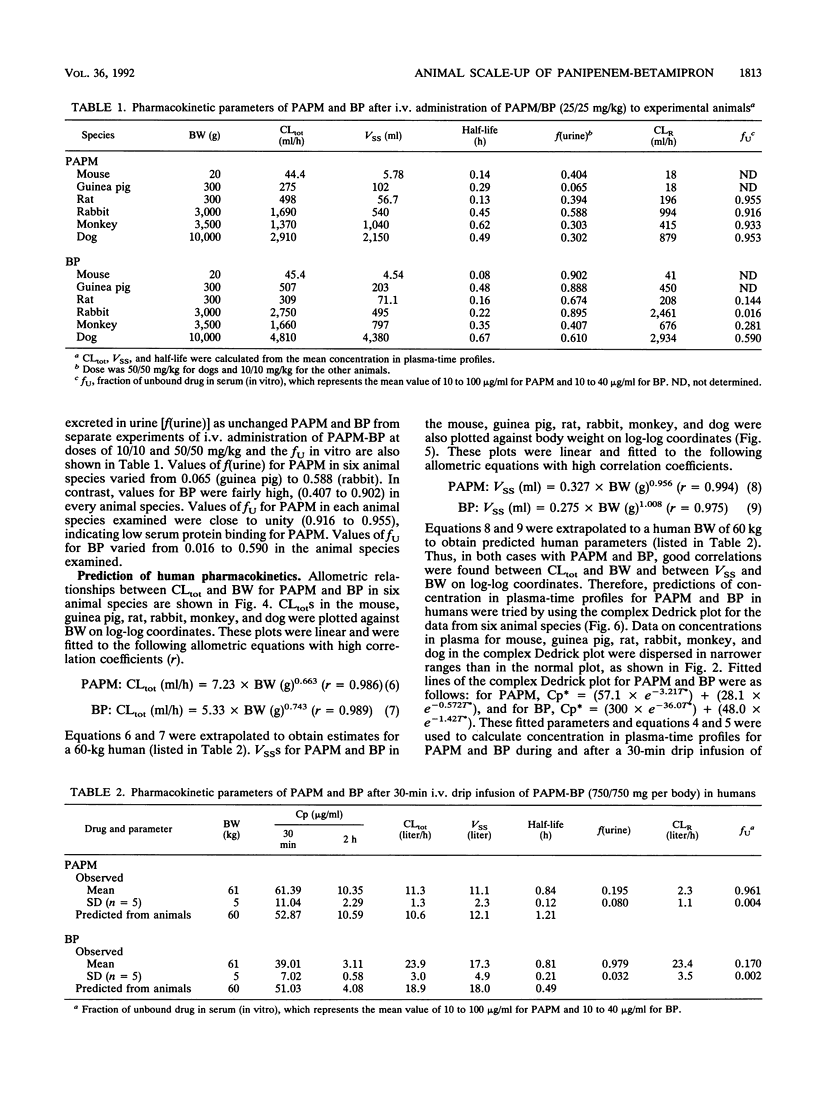
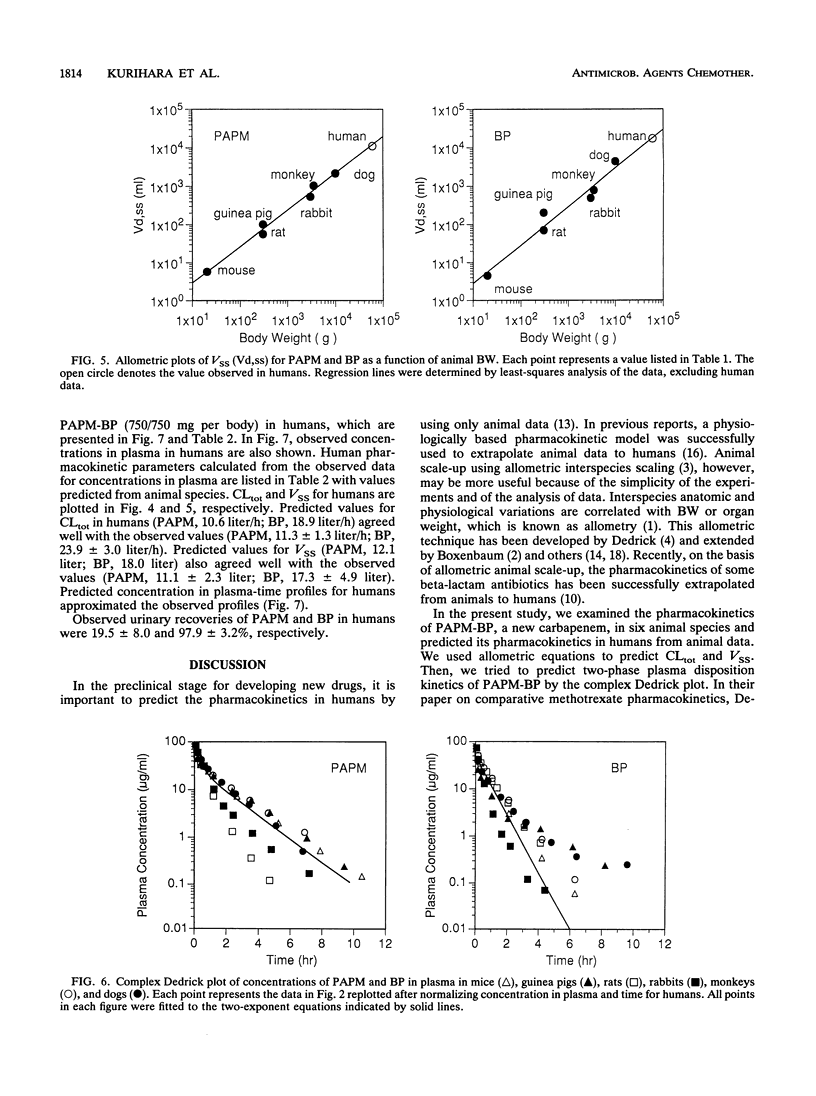
The pharmacokinetic behavior of panipenem (PAPM)-betamipron (BP), a new carbapenem, in humans was successfully predicted from data collected from six animal species. PAPM and BP were biphasically eliminated from plasma after intravenous (i.v.) administration of PAPM-BP to mice, guinea pigs, rats, rabbits, monkeys, and dogs. Elimination rates of PAPM and BP were correlated with animal size: the larger the animal was, the slower the elimination was. As for PAPM and BP, log-log plots of total plasma clearance (CLtot) versus body weight and log-log plots of distribution volume at steady state (VSS) versus body weight for six animal species were linear, with high correlation coefficients. These allometric equations were extrapolated to predict CLtot and VSS for PAPM and BP in humans. In addition, concentration in plasma-time profiles for humans were predicted by using two-exponent equations fitted to the complex Dedrick plot of animal data. Predicted values for CLtot and VSS for PAPM and BP in humans agreed well with observed values in humans given 750/750 mg of PAPM-BP as an i.v. drip infusion for 30 min. Predicted concentration in plasma-time profiles for humans approximated observed profiles. Thus, the pharmacokinetics of PAPM-BP extrapolated well from animal species to humans when allometric equations and the complex Dedrick plot were used.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boxenbaum H. Interspecies pharmacokinetic scaling and the evolutionary-comparative paradigm. Drug Metab Rev. 1984;15(5-6):1071–1121. doi: 10.3109/03602538409033558. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J Pharmacokinet Biopharm. 1982 Apr;10(2):201–227. doi: 10.1007/BF01062336. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H., Ronfeld R. Interspecies pharmacokinetic scaling and the Dedrick plots. Am J Physiol. 1983 Dec;245(6):R768–R775. doi: 10.1152/ajpregu.1983.245.6.R768. [DOI] [PubMed] [Google Scholar]
- Dedrick R. L. Animal scale-up. J Pharmacokinet Biopharm. 1973 Oct;1(5):435–461. doi: 10.1007/BF01059667. [DOI] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Hajdu R., Kahan F. M. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase. Antimicrob Agents Chemother. 1982 Jul;22(1):62–70. doi: 10.1128/aac.22.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H., Suzuki H., Sugiyama Y., Sawada Y., Iga T., Hanano M., Kawaguchi Y. Prediction of the pharmacokinetics of cefodizime and cefotetan in humans from pharmacokinetic parameters in animals. J Pharmacobiodyn. 1990 Oct;13(10):602–611. doi: 10.1248/bpb1978.13.602. [DOI] [PubMed] [Google Scholar]
- McGovren J. P., Williams M. G., Stewart J. C. Interspecies comparison of acivicin pharmacokinetics. Drug Metab Dispos. 1988 Jan-Feb;16(1):18–22. [PubMed] [Google Scholar]
- Mitsuhashi Y., Sugiyama Y., Ozawa S., Nitanai T., Sasahara K., Nakamura K., Tanaka M., Nishimura T., Inaba M., Kobayashi T. Prediction of ACNU plasma concentration-time profiles in humans by animal scale-up. Cancer Chemother Pharmacol. 1990;27(1):20–26. doi: 10.1007/BF00689271. [DOI] [PubMed] [Google Scholar]
- Mordenti J. Forecasting cephalosporin and monobactam antibiotic half-lives in humans from data collected in laboratory animals. Antimicrob Agents Chemother. 1985 Jun;27(6):887–891. doi: 10.1128/aac.27.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordenti J. Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci. 1986 Nov;75(11):1028–1040. doi: 10.1002/jps.2600751104. [DOI] [PubMed] [Google Scholar]
- Nakashima E., Yokogawa K., Ichimura F., Kurata K., Kido H., Yamaguchi N., Yamana T. A physiologically based pharmacokinetic model for biperiden in animals and its extrapolation to humans. Chem Pharm Bull (Tokyo) 1987 Feb;35(2):718–725. doi: 10.1248/cpb.35.718. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Björnegård B., Burman L. A., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Kahan J. S., Kropp H. Urinary recovery of N-formimidoyl thienamycin (MK0787) as affected by coadministration of N-formimidoyl thienamycin dehydropeptidase inhibitors. Antimicrob Agents Chemother. 1983 Feb;23(2):300–307. doi: 10.1128/aac.23.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M. Physiologic pharmacokinetic models and interanimal species scaling. Pharmacol Ther. 1985;29(1):49–68. doi: 10.1016/0163-7258(85)90016-6. [DOI] [PubMed] [Google Scholar]
- Swabb E. A., Bonner D. P. Prediction of aztreonam pharmacokinetics in humans based on data from animals. J Pharmacokinet Biopharm. 1983 Jun;11(3):215–223. doi: 10.1007/BF01061865. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978 Dec;6(6):547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]



