Abstract
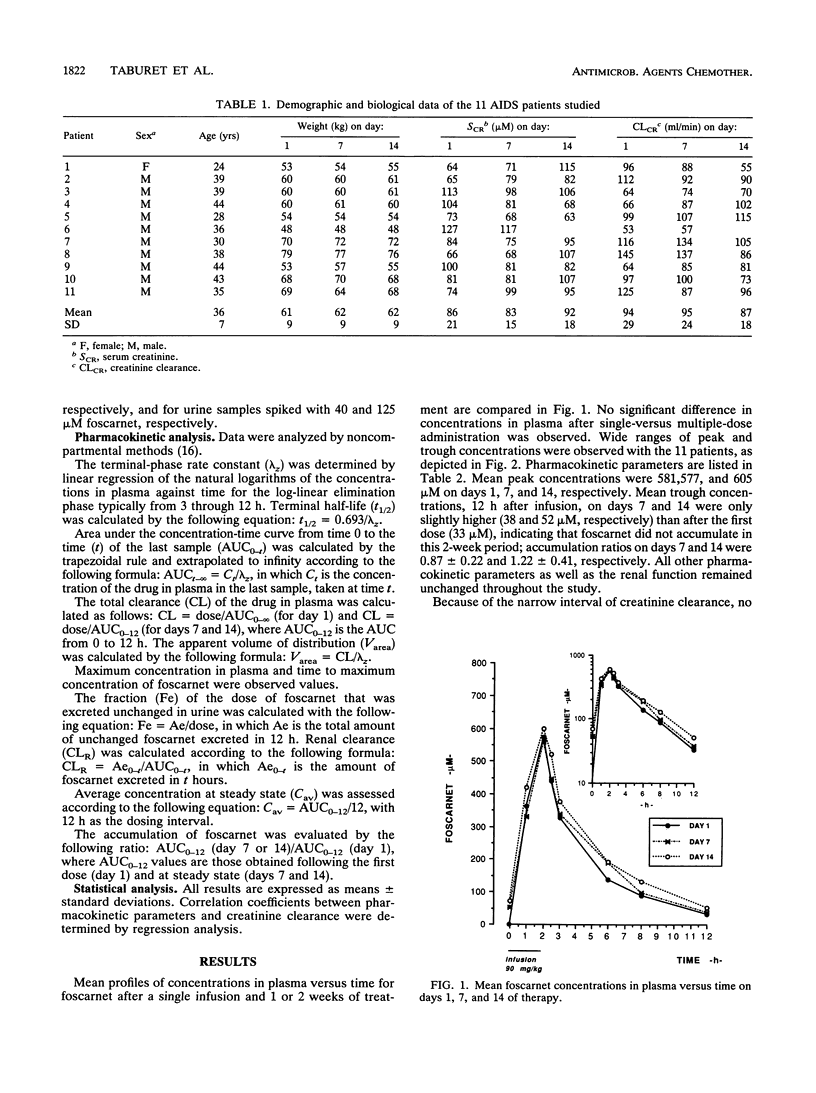
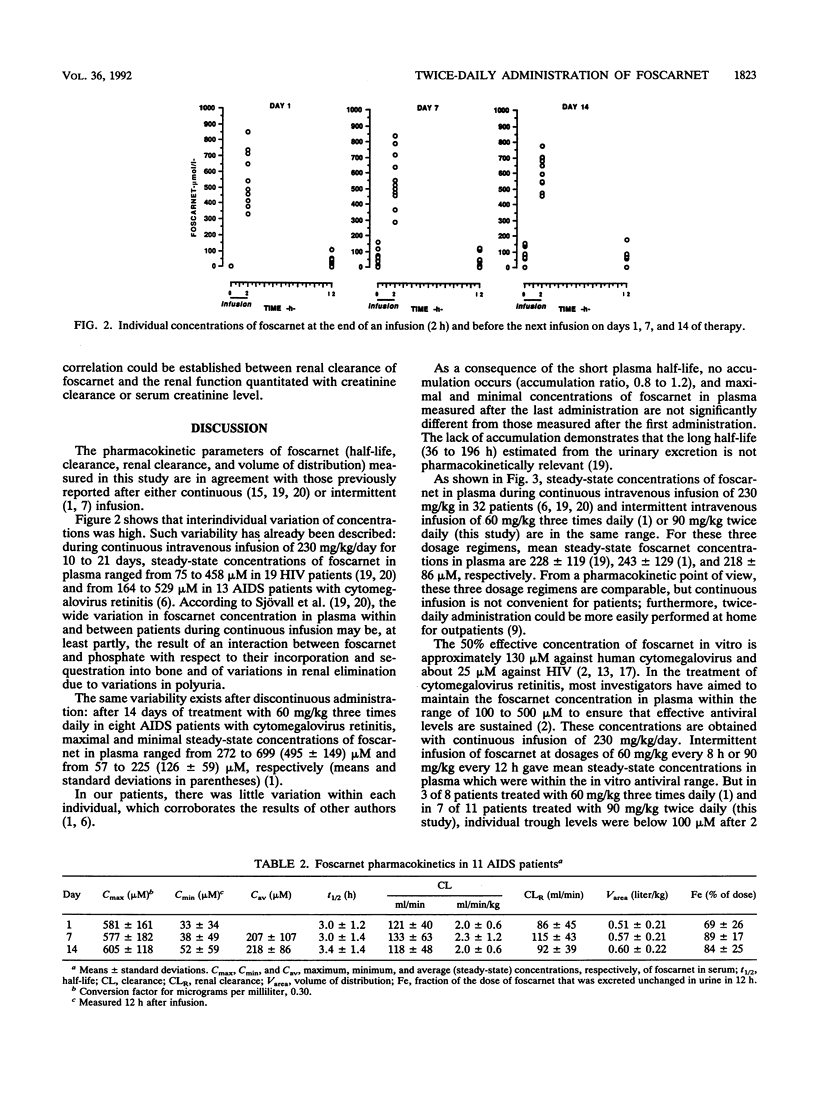
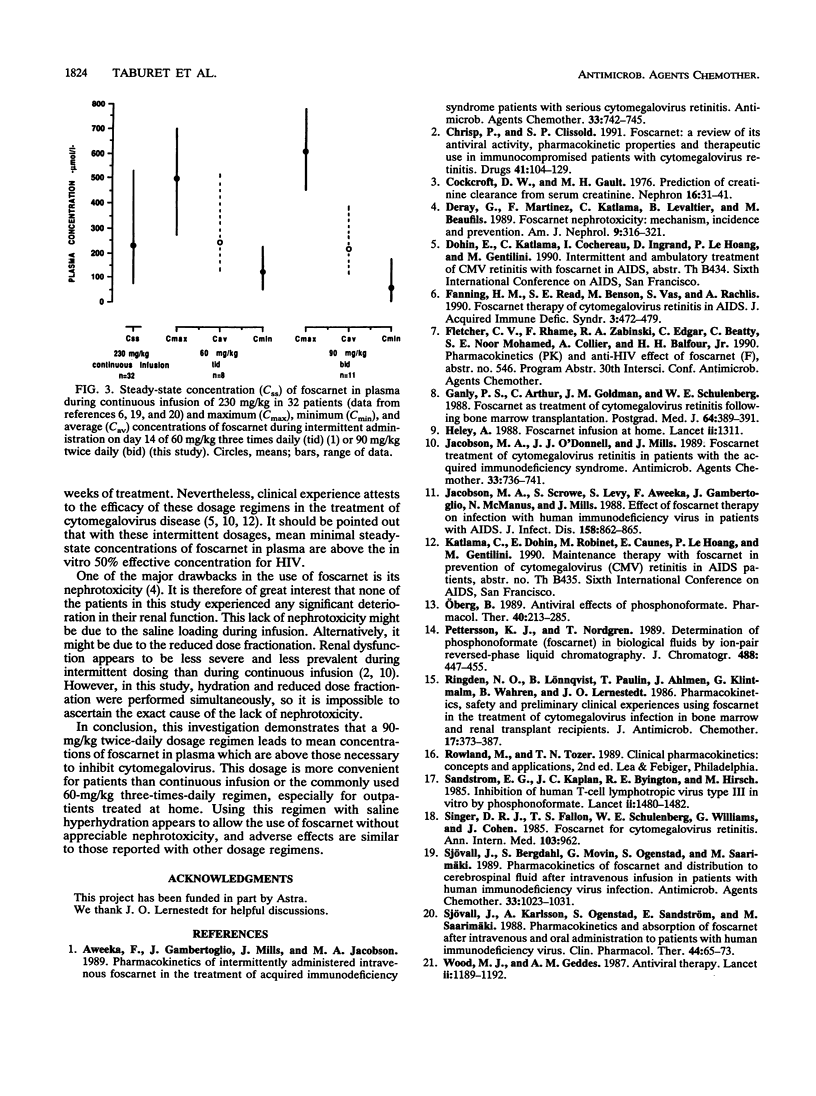
The pharmacokinetics of foscarnet were evaluated in 11 AIDS patients with cytomegalovirus disease after twice-daily infusion of 90 mg/kg of body weight for 2 weeks. All patients were hydrated during foscarnet infusion. Blood and urine samples were collected on days 1, 7, and 14 of therapy. Foscarnet concentrations were measured by high-pressure liquid chromatography. Despite large interindividual variations, no significant differences were seen between day 1, day 7, and day 14 concentrations in plasma. Mean peak and trough concentrations on day 14 of therapy were 605 +/- 118 and 52 +/- 59 microM, respectively. In all patients, peak concentrations were well above those necessary to inhibit cytomegalovirus. Pharmacokinetic parameters remained stable throughout the study. On day 14, the mean half-life was 3.4 h, total and renal clearances were 118 and 92 ml/min, respectively, and the volume of distribution was 0.6 liter/kg. These data and previous clinical trials demonstrate that this more convenient dosage regimen can be safely used for patients with cytomegalovirus disease. The side effects were comparable to those reported with other dosage regimens, although no renal impairment was seen in this study, probably because of the hydration.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aweeka F., Gambertoglio J., Mills J., Jacobson M. A. Pharmacokinetics of intermittently administered intravenous foscarnet in the treatment of acquired immunodeficiency syndrome patients with serious cytomegalovirus retinitis. Antimicrob Agents Chemother. 1989 May;33(5):742–745. doi: 10.1128/aac.33.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisp P., Clissold S. P. Foscarnet. A review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs. 1991 Jan;41(1):104–129. doi: 10.2165/00003495-199141010-00009. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Deray G., Martinez F., Katlama C., Levaltier B., Beaufils H., Danis M., Rozenheim M., Baumelou A., Dohin E., Gentilini M. Foscarnet nephrotoxicity: mechanism, incidence and prevention. Am J Nephrol. 1989;9(4):316–321. doi: 10.1159/000167987. [DOI] [PubMed] [Google Scholar]
- Fanning M. M., Read S. E., Benson M., Vas S., Rachlis A., Kozousek V., Mortimer C., Harvey P., Schwartz C., Chew E. Foscarnet therapy of cytomegalovirus retinitis in AIDS. J Acquir Immune Defic Syndr. 1990;3(5):472–479. [PubMed] [Google Scholar]
- Ganly P. S., Arthur C., Goldman J. M., Schulenburg W. E. Foscarnet as treatment for cytomegalovirus retinitis following bone marrow transplantation. Postgrad Med J. 1988 May;64(751):389–391. doi: 10.1136/pgmj.64.751.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heley A. Foscarnet infusion at home. Lancet. 1988 Dec 3;2(8623):1311–1311. doi: 10.1016/s0140-6736(88)92920-0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., Crowe S., Levy J., Aweeka F., Gambertoglio J., McManus N., Mills J. Effect of Foscarnet therapy on infection with human immunodeficiency virus in patients with AIDS. J Infect Dis. 1988 Oct;158(4):862–865. [PubMed] [Google Scholar]
- Jacobson M. A., O'Donnell J. J., Mills J. Foscarnet treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Antimicrob Agents Chemother. 1989 May;33(5):736–741. doi: 10.1128/aac.33.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B. Antiviral effects of phosphonoformate (PFA, foscarnet sodium). Pharmacol Ther. 1989;40(2):213–285. doi: 10.1016/0163-7258(89)90097-1. [DOI] [PubMed] [Google Scholar]
- Pettersson K. J., Nordgren T., Westerlund D. Determination of phosphonoformate (foscarnet) in biological fluids by ion-pair reversed-phase liquid chromatography. J Chromatogr. 1989 Mar 24;488(2):447–455. doi: 10.1016/s0378-4347(00)82968-0. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Lönnqvist B., Paulin T., Ahlmén J., Klintmalm G., Wahren B., Lernestedt J. O. Pharmacokinetics, safety and preliminary clinical experiences using foscarnet in the treatment of cytomegalovirus infections in bone marrow and renal transplant recipients. J Antimicrob Chemother. 1986 Mar;17(3):373–387. doi: 10.1093/jac/17.3.373. [DOI] [PubMed] [Google Scholar]
- Sandstrom E. G., Kaplan J. C., Byington R. E., Hirsch M. S. Inhibition of human T-cell lymphotropic virus type III in vitro by phosphonoformate. Lancet. 1985 Jun 29;1(8444):1480–1482. doi: 10.1016/s0140-6736(85)92255-x. [DOI] [PubMed] [Google Scholar]
- Singer D. R., Fallon T. J., Schulenburg W. E., Williams G., Cohen J. Foscarnet for cytomegalovirus retinitis. Ann Intern Med. 1985 Dec;103(6 ):962–962. doi: 10.7326/0003-4819-103-6-962_2. [DOI] [PubMed] [Google Scholar]
- Sjövall J., Bergdahl S., Movin G., Ogenstad S., Saarimäki M. Pharmacokinetics of foscarnet and distribution to cerebrospinal fluid after intravenous infusion in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1989 Jul;33(7):1023–1031. doi: 10.1128/aac.33.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjövall J., Karlsson A., Ogenstad S., Sandström E., Saarimäki M. Pharmacokinetics and absorption of foscarnet after intravenous and oral administration to patients with human immunodeficiency virus. Clin Pharmacol Ther. 1988 Jul;44(1):65–73. doi: 10.1038/clpt.1988.114. [DOI] [PubMed] [Google Scholar]
- Wood M. J., Geddes A. M. Antiviral therapy. Lancet. 1987 Nov 21;2(8569):1189–1193. doi: 10.1016/s0140-6736(87)91329-8. [DOI] [PubMed] [Google Scholar]



