Abstract
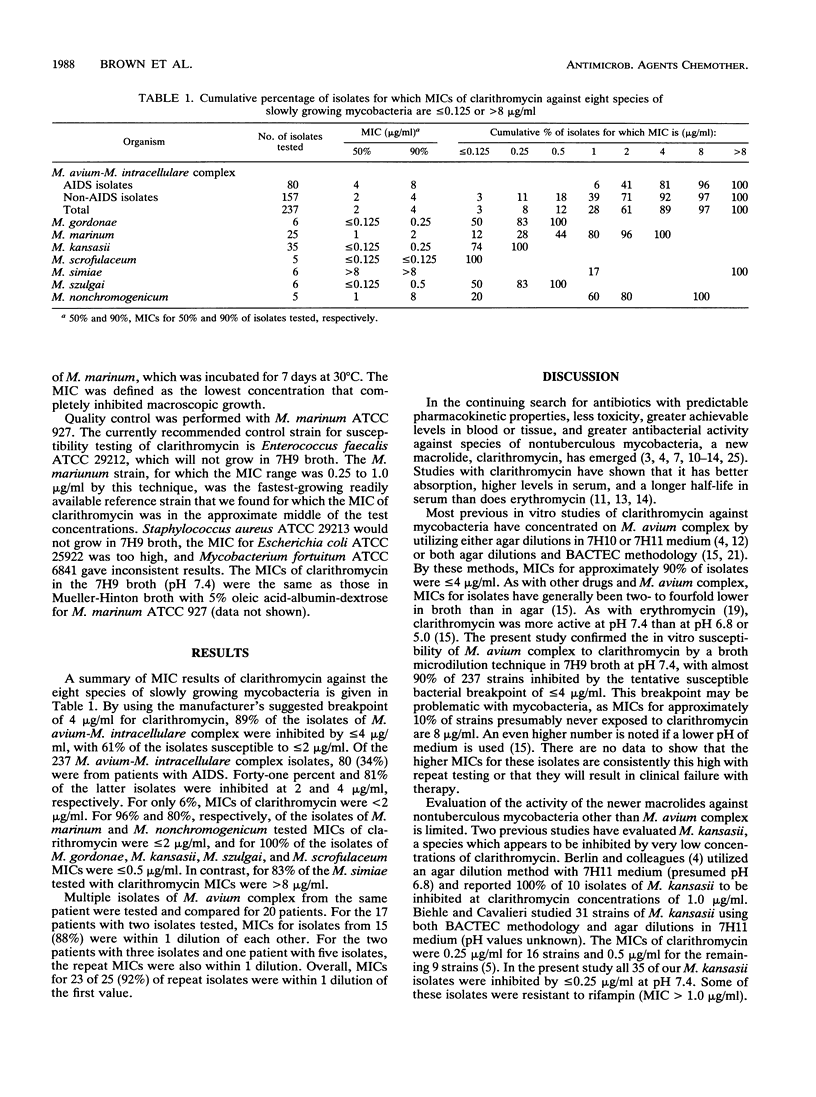
MICs of clarithromycin against 324 clinical isolates belonging to eight species of slowly growing nontuberculous mycobacteria were determined by using a broth microdilution system. Isolates were inoculated into twofold drug dilutions in Middlebrook 7H9 broth (pH corrected to 7.4) and then incubated at 30 degrees C for 7 days for Mycobacterium marinum and for 14 days for all other species. The MIC for 90% of the strains (MIC90) was less than or equal to 0.5 micrograms/ml for isolates of Mycobacterium gordonae (6 strains), Mycobacterium scrofulaceum (5 strains), Mycobacterium szulgai (6 strains), and Mycobacterium kansasii (35 strains). MICs for M. marinum (25 strains) and Mycobacterium avium complex (237 strains) were higher, but 100% and 89% of the strains, respectively, were susceptible to less than or equal to 4 micrograms/ml. In contrast, MICs for five of six M. simiae strains were greater than 8 micrograms/ml, and the range of MICs for Mycobacterium nonchromogenicum varied from less than or equal to 0.125 to 8 micrograms/ml. For the 237 isolates of M. avium complex, the MIC50 was 2 micrograms/ml and the MIC90 was 8 micrograms/ml. MICs for most isolates (77%) were in the 1- to 4-micrograms/ml range. For the 80 isolates in this group known to be from AIDS patients, the MIC50 was 4 micrograms/ml and the MIC90 was 8 micrograms/ml. These MIC studies combined with preliminary clinical trials suggest that clarithromycin may be useful for drug therapy of most species of the slowly growing nontuberculous mycobacteria except M. simiae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguado J. M., Gómez-Garcés J. L., Manrique A., Soriano F. Pulmonary infection by Mycobacterium gordonae in an immunocompromised patient. Diagn Microbiol Infect Dis. 1987 Aug;7(4):261–263. doi: 10.1016/0732-8893(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Bailey W. C. Treatment of atypical mycobacterial disease. Chest. 1983 Nov;84(5):625–628. doi: 10.1378/chest.84.5.625. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C. In vitro activities of azithromycin (CP 62,993), clarithromycin (A-56268; TE-031), erythromycin, roxithromycin, and clindamycin. Antimicrob Agents Chemother. 1988 May;32(5):752–754. doi: 10.1128/aac.32.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin O. G., Young L. S., Floyd-Reising S. A., Bruckner D. A. Comparative in vitro activity of the new macrolide A-56268 against mycobacteria. Eur J Clin Microbiol. 1987 Aug;6(4):486–487. doi: 10.1007/BF02013117. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Wallace R. J., Jr, Onyi G. O., De Rosas V., Wallace R. J., 3rd Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob Agents Chemother. 1992 Jan;36(1):180–184. doi: 10.1128/aac.36.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., McKitrick J. C., Klein R. S. Mycobacterium gordonae in the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Sep;101(3):400–400. doi: 10.7326/0003-4819-101-3-400_1. [DOI] [PubMed] [Google Scholar]
- Chiu J., Nussbaum J., Bozzette S., Tilles J. G., Young L. S., Leedom J., Heseltine P. N., McCutchan J. A. Treatment of disseminated Mycobacterium avium complex infection in AIDS with amikacin, ethambutol, rifampin, and ciprofloxacin. California Collaborative Treatment Group. Ann Intern Med. 1990 Sep 1;113(5):358–361. doi: 10.7326/0003-4819-113-5-358. [DOI] [PubMed] [Google Scholar]
- Dautzenberg B., Truffot C., Legris S., Meyohas M. C., Berlie H. C., Mercat A., Chevret S., Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. A controlled clinical trial. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am Rev Respir Dis. 1990 Oct;142(4):940–953. doi: 10.1164/ajrccm/142.4.940. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Bailer R., Swanson R., Hanson C. W., McDonald E., Ramer N., Hardy D., Shipkowitz N., Bower R. R., Gade E. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob Agents Chemother. 1986 Dec;30(6):865–873. doi: 10.1128/aac.30.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D. J., McDaniel D., Hanson C. W., Swanson R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother. 1989 Sep;33(9):1531–1534. doi: 10.1128/aac.33.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini F., Scaglione F., Pintucci G., Maccarinelli G., Dugnani S., Demartini G. The diffusion of clarithromycin and roxithromycin into nasal mucosa, tonsil and lung in humans. J Antimicrob Chemother. 1991 Feb;27 (Suppl A):61–65. doi: 10.1093/jac/27.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J., Comstock R. D. Clarithromycin minimal inhibitory and bactericidal concentrations against Mycobacterium avium. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):856–858. doi: 10.1164/ajrccm/145.4_Pt_1.856. [DOI] [PubMed] [Google Scholar]
- Hoover W. W., Barrett M. S., Jones R. N. Clarithromycin in vitro activity enhanced by its major metabolite, 14-hydroxyclarithromycin. Diagn Microbiol Infect Dis. 1992 Mar-Apr;15(3):259–266. doi: 10.1016/0732-8893(92)90122-a. [DOI] [PubMed] [Google Scholar]
- Lillo M., Orengo S., Cernoch P., Harris R. L. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis. 1990 Sep-Oct;12(5):760–767. doi: 10.1093/clinids/12.5.760. [DOI] [PubMed] [Google Scholar]
- Lorian V., Sabath L. D. Effect of pH on the activity of erythromycin against 500 isolates of gram-negative bacilli. Appl Microbiol. 1970 Nov;20(5):754–756. doi: 10.1128/am.20.5.754-756.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney J. M., Gregg C. R., Stephens D. S., Manian F. A., Rimland D. Infections caused by Mycobacterium szulgai in humans. Rev Infect Dis. 1987 Nov-Dec;9(6):1120–1126. doi: 10.1093/clinids/9.6.1120. [DOI] [PubMed] [Google Scholar]
- Naik S., Ruck R. In vitro activities of several new macrolide antibiotics against Mycobacterium avium complex. Antimicrob Agents Chemother. 1989 Sep;33(9):1614–1616. doi: 10.1128/aac.33.9.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderhof J. C., Wallace R. J., Jr, Kilburn J. O., Butler W. R., Warren N. G., Tsukamura M., Steele L. C., Wong E. S. Chronic tenosynovitis of the hand due to Mycobacterium nonchromogenicum: use of high-performance liquid chromatography for identification of isolates. Rev Infect Dis. 1991 Sep-Oct;13(5):857–864. doi: 10.1093/clinids/13.5.857. [DOI] [PubMed] [Google Scholar]
- Silcox V. A., David H. L. Differential identification of Mycobacterium kansasii and Mycobacterium marinum. Appl Microbiol. 1971 Feb;21(2):327–334. doi: 10.1128/am.21.2.327-334.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Brown B. A., Onyi G. O. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis. 1992 Aug;166(2):405–412. doi: 10.1093/infdis/166.2.405. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Nash D. R., Steele L. C., Steingrube V. Susceptibility testing of slowly growing mycobacteria by a microdilution MIC method with 7H9 broth. J Clin Microbiol. 1986 Dec;24(6):976–981. doi: 10.1128/jcm.24.6.976-981.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Sramek H. A. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992 Jan;5(1):1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. S., Riordan D. C. Mycobacterium marinum (atypical acid-fast bacillus) infections of the hand. J Bone Joint Surg Am. 1973 Jul;55(5):1042–1050. [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- Yeager H., Jr, Raleigh J. W. Pulmonary disease due to Mycobacterium intracellulare. Am Rev Respir Dis. 1973 Sep;108(3):547–552. doi: 10.1164/arrd.1973.108.3.547. [DOI] [PubMed] [Google Scholar]


