Abstract
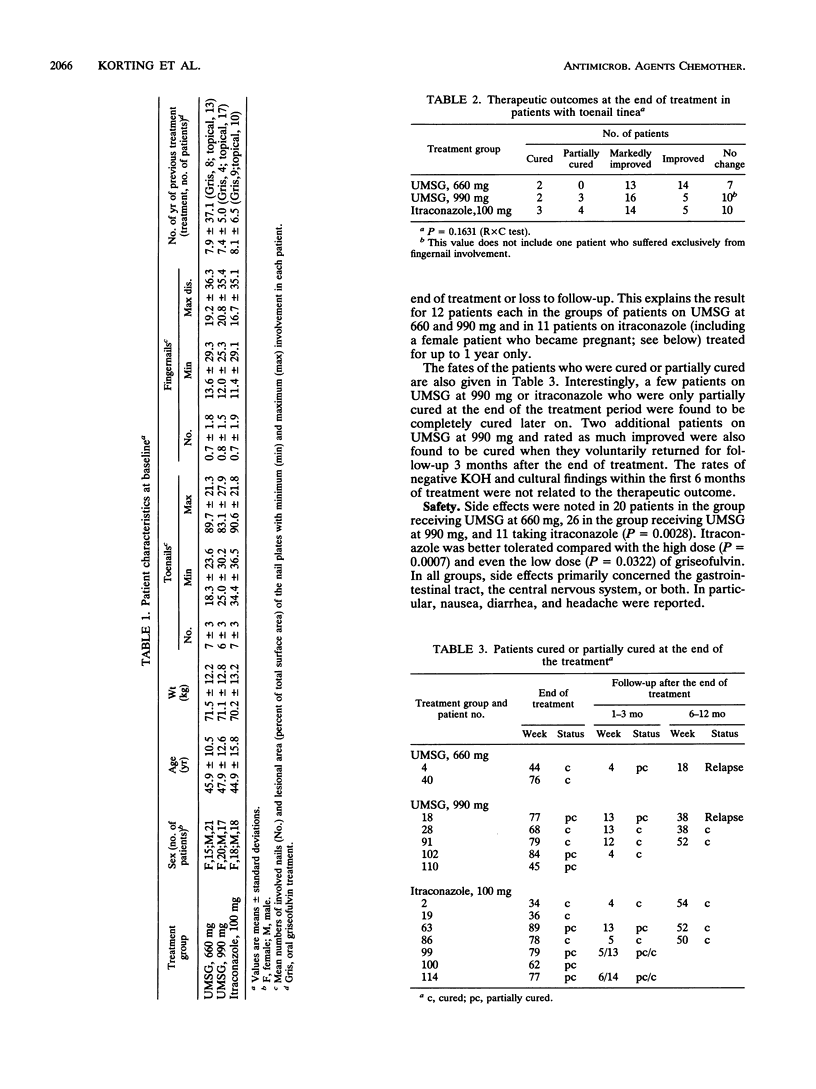
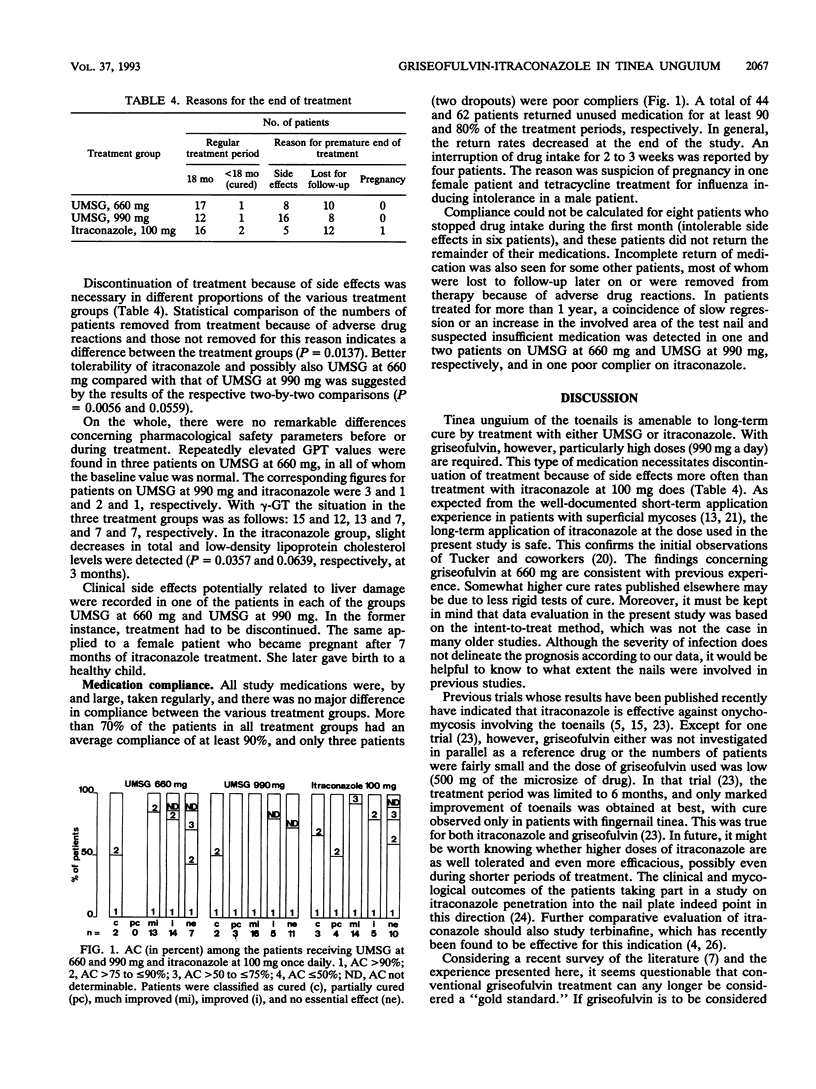
Toenail tinea is a very recalcitrant dermatosis. Griseofulvin at > or = 500 mg/day is the current medication of choice, but it is minimally successful. In a controlled open trial ultramicrosize griseofulvin (UMSG) at doses of 660 and 990 mg/day was compared with itraconazole at 100 mg/day in 109 patients. At 4-week intervals, the patients were evaluated for their clinical and mycological statuses and adverse reactions. Treatment was given for up to 18 months. Compliance was checked by tablet counting. Response (cure, partial cure, marked improvement) was analyzed by the intent-to-treat method. Cured and partially cured patients were followed up. Except for one early dropout, the toenails (mean, 6 to 7) were involved. Cure or partial cure was found in 6% (UMSG at 660 mg), 14% (UMSG at 990 mg), and 19% (itraconazole at 100 mg) of patients (P = 0.2097); marked improvement was found in 36, 44, and 39% of patients in the three treatment groups, respectively. Most patients had to be treated for 18 months. Failure was related to short medication periods (adverse drug reactions, dropout). While stable cure was not obtained with UMSG at 660 mg, the higher dose of UMSG and itraconazole gave stable cures in the other patients. Side effects of nausea, diarrhea, and headache were found in 20, 26, and 11 patients, respectively (P = 0.0028), and the numbers in whom medication had to be discontinued differed, too (P = 0.0137). While there was no major difference with glutamic-pyruvic transaminase and gamma-GT, total and low-density lipoprotein cholesterol levels declined slightly in the itraconazole group (P = 0.0357 and P = 0.0639, respectively, at 3 months). More than 70% of the patients had an average compliance of > or = 90%; four patients (two dropouts) were poor compliers. In conclusion, it appears questionable whether griseofulvin can continue to be considered the "gold standard" in the treatment of toenail tinea. At present, itraconazole at 100 mg shows better efficacy and is better tolerated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cauwenbergh G., Degreef H., Heykants J., Woestenborghs R., Van Rooy P., Haeverans K. Pharmacokinetic profile of orally administered itraconazole in human skin. J Am Acad Dermatol. 1988 Feb;18(2 Pt 1):263–268. doi: 10.1016/s0190-9622(88)70037-7. [DOI] [PubMed] [Google Scholar]
- Galimberti R., Negroni R., Iglesia de Elias Costa M. R., Casalá A. M. The activity of ketoconazole in the treatment of onychomycosis. Rev Infect Dis. 1980 Jul-Aug;2(4):596–598. doi: 10.1093/clinids/2.4.596. [DOI] [PubMed] [Google Scholar]
- Hay R. J., Clayton Y. M., Moore M. K., Midgely G. An evaluation of itraconazole in the management of onychomycosis. Br J Dermatol. 1988 Sep;119(3):359–366. doi: 10.1111/j.1365-2133.1988.tb03229.x. [DOI] [PubMed] [Google Scholar]
- Korting H. C., Schäfer-Korting M. Is tinea unguium still widely incurable? A review three decades after the introduction of griseofulvin. Arch Dermatol. 1992 Feb;128(2):243–248. [PubMed] [Google Scholar]
- Lake-Bakaar G., Scheuer P. J., Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed) 1987 Feb 14;294(6569):419–422. doi: 10.1136/bmj.294.6569.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Lim J., DiGiore C., Gural R., Symchowicz S. Comparative bioavailability of a microsize and ultramicrosize griseofulvin formulation in man. J Int Med Res. 1982;10(4):274–277. doi: 10.1177/030006058201000415. [DOI] [PubMed] [Google Scholar]
- Meinhof W., Girardi R. M., Stracke A. Patient noncompliance in dermatomycosis. Results of a survey among dermatologists and general practitioners and patients. Dermatologica. 1984;169 (Suppl 1):57–66. [PubMed] [Google Scholar]
- Mäenpä H., Manninen V., Heinonen O. P. Comparison of the digoxin marker with capsule counting and compliance questionnaire methods for measuring compliance to medication in a clinical trial. Eur Heart J. 1987 Oct;8 (Suppl 1):39–43. doi: 10.1093/eurheartj/8.suppl_i.39. [DOI] [PubMed] [Google Scholar]
- Mäenpä H., Manninen V., Heinonen O. P. Compliance with medication in the Helsinki Heart Study. Eur J Clin Pharmacol. 1992;42(1):15–19. doi: 10.1007/BF00314913. [DOI] [PubMed] [Google Scholar]
- Robertson M. H., Rich P., Parker F., Hanifin J. M. Ketoconazole in griseofulvin-resistant dermatophytosis. J Am Acad Dermatol. 1982 Feb;6(2):224–229. doi: 10.1016/s0190-9622(82)70015-5. [DOI] [PubMed] [Google Scholar]
- Schäfer-Korting M., Korting H. C., Lukacs A., Heykants J., Behrendt H. Levels of itraconazole in skin blister fluid after a single oral dose and during repetitive administration. J Am Acad Dermatol. 1990 Feb;22(2 Pt 1):211–215. doi: 10.1016/0190-9622(90)70026-e. [DOI] [PubMed] [Google Scholar]
- Schäfer-Korting M., Korting H. C., Mutschler E. Human plasma and skin blister fluid levels of griseofulvin after its repeated administration. Eur J Clin Pharmacol. 1985;29(3):351–354. doi: 10.1007/BF00544093. [DOI] [PubMed] [Google Scholar]
- Schäfer-Korting M., Korting H. C., Mutschler E. Human plasma and skin blister fluid levels of griseofulvin following a single oral dose. Eur J Clin Pharmacol. 1985;29(1):109–113. doi: 10.1007/BF00547378. [DOI] [PubMed] [Google Scholar]
- Tucker R. M., Haq Y., Denning D. W., Stevens D. A. Adverse events associated with itraconazole in 189 patients on chronic therapy. J Antimicrob Chemother. 1990 Oct;26(4):561–566. doi: 10.1093/jac/26.4.561. [DOI] [PubMed] [Google Scholar]
- Van Cauteren H., Heykants J., De Coster R., Cauwenbergh G. Itraconazole: pharmacologic studies in animals and humans. Rev Infect Dis. 1987 Jan-Feb;9 (Suppl 1):S43–S46. doi: 10.1093/clinids/9.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- Van Cutsem J., Van Gerven F., Janssen P. A. Activity of orally, topically, and parenterally administered itraconazole in the treatment of superficial and deep mycoses: animal models. Rev Infect Dis. 1987 Jan-Feb;9 (Suppl 1):S15–S32. doi: 10.1093/clinids/9.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- Walsøe I., Stangerup M., Svejgaard E. Itraconazole in onychomycosis. Open and double-blind studies. Acta Derm Venereol. 1990;70(2):137–140. [PubMed] [Google Scholar]
- Willemsen M., De Doncker P., Willems J., Woestenborghs R., Van de Velde V., Heykants J., Van Cutsem J., Cauwenbergh G., Roseeuw D. Posttreatment itraconazole levels in the nail. New implications for treatment in onychomycosis. J Am Acad Dermatol. 1992 May;26(5 Pt 1):731–735. doi: 10.1016/0190-9622(92)70102-l. [DOI] [PubMed] [Google Scholar]
- Zaias N., Drachman D. A method for the determination of drug effectiveness in onychomycosis. Trials with ketoconazole and griseofulvin ultramicrosize. J Am Acad Dermatol. 1983 Dec;9(6):912–919. doi: 10.1016/s0190-9622(83)70208-2. [DOI] [PubMed] [Google Scholar]
- Zaias N., Serrano L. The successful treatment of finger Trichophyton rubrum onychomycosis with oral terbinafine. Clin Exp Dermatol. 1989 Mar;14(2):120–123. doi: 10.1111/j.1365-2230.1989.tb00907.x. [DOI] [PubMed] [Google Scholar]


