Abstract
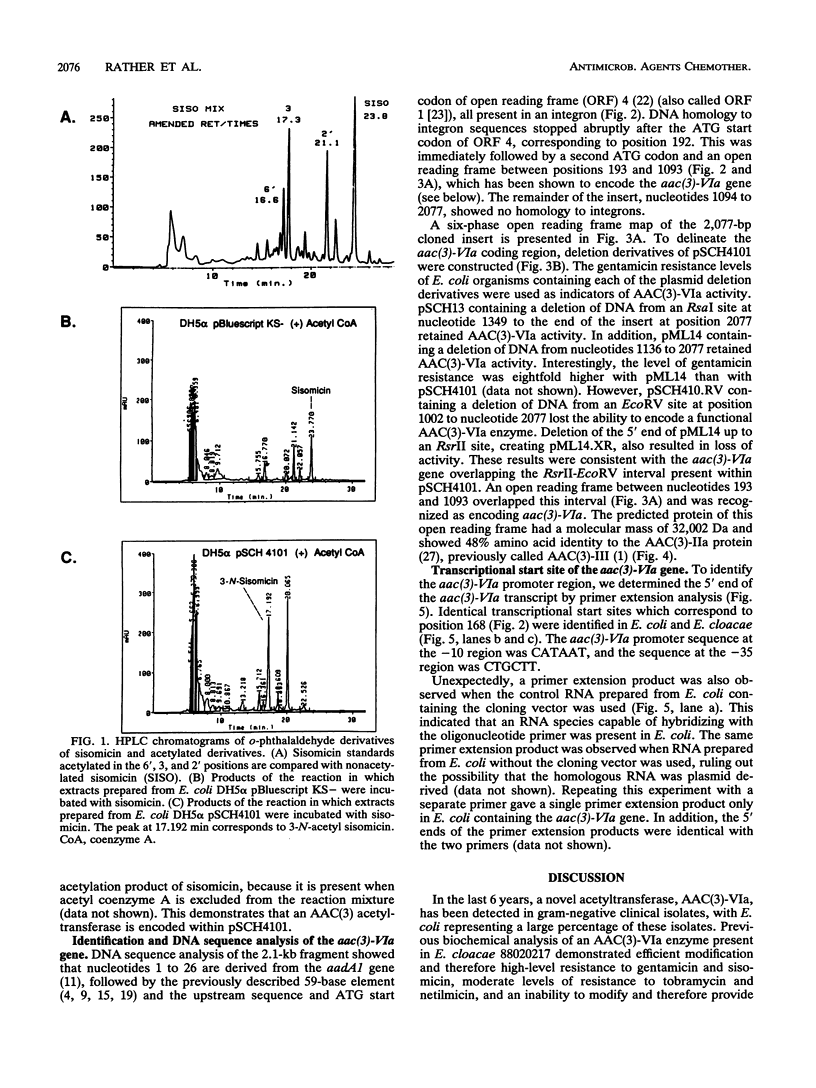
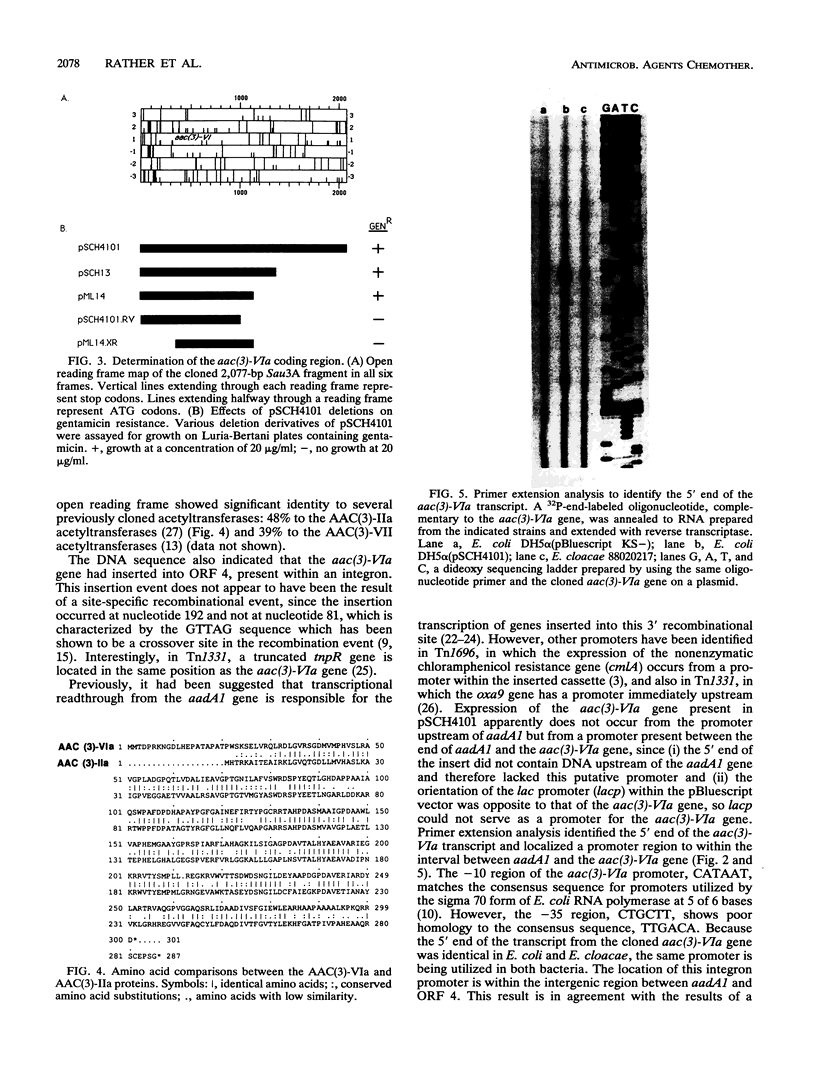
Biochemical analysis (G. A. Papanicolaou, R. S. Hare, R. Mierzwa, and G. H. Miller, abstr. 152, Program Abstr. 29th Intersci. Conf. Antimicrob. Agents Chemother., 1989) demonstrated the presence of a novel 3-N-acetyltransferase in Enterobacter cloacae 88020217. This organism was resistant to gentamicin, and the MIC of 2'-N-ethylnetilmicin for it was fourfold lower than that of 6'-N-ethylnetilmicin, a resistance pattern which suggested 2'-acetylating activity. However, high-pressure liquid chromatography analysis demonstrated that the enzyme acetylated sisomicin in the 3 position. We have cloned the structural gene for this enzyme from a large (> 70-kb) conjugative plasmid present in E. cloacae. Subcloning experiments have localized the aac(3)-VIa gene to a 2.1-kb Sau3A fragment. The deduced AAC(3)-VIa protein showed 48% amino acid identity to the AAC(3)-IIa protein and 39% identity to the AAC(3)-VII protein. Examination of the 5'-flanking sequences demonstrated that the aac(3)-VIa gene was located 167 bp downstream of the aadA1 gene and was present in an integron. In addition, the aac(3)-VIa gene is also downstream of a 59-base element often seen in an integron environment. Primer extension analysis has identified a promoter for the aac(3)-VIa gene downstream of both the aadA1 gene and a 59-base element.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allmansberger R., Bräu B., Piepersberg W. Genes for gentamicin-(3)-N-acetyl-transferases III and IV. II. Nucleotide sequences of three AAC(3)-III genes and evolutionary aspects. Mol Gen Genet. 1985;198(3):514–520. doi: 10.1007/BF00332949. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette L., Champetier S., Buisson J. P., Roy P. H. Characterization of the nonenzymatic chloramphenicol resistance (cmlA) gene of the In4 integron of Tn1696: similarity of the product to transmembrane transport proteins. J Bacteriol. 1991 Jul;173(14):4493–4502. doi: 10.1128/jb.173.14.4493-4502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F. H., Groot Obbink D. J., Ackerman V. P., Hall R. M. Nucleotide sequence of the AAD(2'') aminoglycoside adenylyltransferase determinant aadB. Evolutionary relationship of this region with those surrounding aadA in R538-1 and dhfrII in R388. Nucleic Acids Res. 1986 Nov 11;14(21):8625–8635. doi: 10.1093/nar/14.21.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Resistance towards aminoglycoside-aminocyclitol antibiotics in bacteria. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):57–69. doi: 10.1093/jac/8.suppl_a.57. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Guerineau F., Brooks L., Mullineaux P. Expression of the sulfonamide resistance gene from plasmid R46. Plasmid. 1990 Jan;23(1):35–41. doi: 10.1016/0147-619x(90)90042-b. [DOI] [PubMed] [Google Scholar]
- Hall R. M., Brookes D. E., Stokes H. W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991 Aug;5(8):1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S., Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid. 1985 Jan;13(1):17–30. doi: 10.1016/0147-619x(85)90052-6. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- López-Cabrera M., Pérez-González J. A., Heinzel P., Piepersberg W., Jiménez A. Isolation and nucleotide sequencing of an aminocyclitol acetyltransferase gene from Streptomyces rimosus forma paromomycinus. J Bacteriol. 1989 Jan;171(1):321–328. doi: 10.1128/jb.171.1.321-328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., de la Cruz F. Genetic elements involved in Tn21 site-specific integration, a novel mechanism for the dissemination of antibiotic resistance genes. EMBO J. 1990 Apr;9(4):1275–1281. doi: 10.1002/j.1460-2075.1990.tb08236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., de la Cruz F. Transposon Tn21 encodes a RecA-independent site-specific integration system. Mol Gen Genet. 1988 Feb;211(2):320–325. doi: 10.1007/BF00330610. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Bissonnette L., Roy P. H. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 beta-lactamase gene. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7378–7382. doi: 10.1073/pnas.84.21.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather P. N., Moran C. P., Jr Compartment-specific transcription in Bacillus subtilis: identification of the promoter for gdh. J Bacteriol. 1988 Nov;170(11):5086–5092. doi: 10.1128/jb.170.11.5086-5092.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F. R., Nücken E. J., Henschke R. B. Structure and function of hot spots providing signals for site-directed specific recombination and gene expression in Tn21 transposons. Mol Microbiol. 1989 Nov;3(11):1545–1555. doi: 10.1111/j.1365-2958.1989.tb00140.x. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Cramer C. A., Rizzo M., Mierzwa R., Gewain K., Miller G. H., Hare R. S. Isolation, characterization, and DNA sequence analysis of an AAC(6')-II gene from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Dec;33(12):2052–2062. doi: 10.1128/aac.33.12.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Rather P. N., Hare R. S., Miller G. H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993 Mar;57(1):138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes H. W., Hall R. M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989 Dec;3(12):1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Sundström L., Rådström P., Swedberg G., Sköld O. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol Gen Genet. 1988 Aug;213(2-3):191–201. doi: 10.1007/BF00339581. [DOI] [PubMed] [Google Scholar]
- Swedberg G. Organization of two sulfonamide resistance genes on plasmids of gram-negative bacteria. Antimicrob Agents Chemother. 1987 Feb;31(2):306–311. doi: 10.1128/aac.31.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky M. E., Crosa J. H. Genetic organization of antibiotic resistance genes (aac(6')-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid. 1993 Jan;29(1):31–40. doi: 10.1006/plas.1993.1004. [DOI] [PubMed] [Google Scholar]
- Tolmasky M. E. Sequencing and expression of aadA, bla, and tnpR from the multiresistance transposon Tn1331. Plasmid. 1990 Nov;24(3):218–226. doi: 10.1016/0147-619x(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Vliegenthart J. S., Ketelaar-van Gaalen P. A., van de Klundert J. A. Nucleotide sequence of the aacC2 gene, a gentamicin resistance determinant involved in a hospital epidemic of multiply resistant members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1989 Aug;33(8):1153–1159. doi: 10.1128/aac.33.8.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlleben W., Arnold W., Bissonnette L., Pelletier A., Tanguay A., Roy P. H., Gamboa G. C., Barry G. F., Aubert E., Davies J. On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I(AAC(3)-I), another member of the Tn21-based expression cassette. Mol Gen Genet. 1989 Jun;217(2-3):202–208. doi: 10.1007/BF02464882. [DOI] [PubMed] [Google Scholar]
- de la Cruz F., Grinsted J. Genetic and molecular characterization of Tn21, a multiple resistance transposon from R100.1. J Bacteriol. 1982 Jul;151(1):222–228. doi: 10.1128/jb.151.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]