Abstract
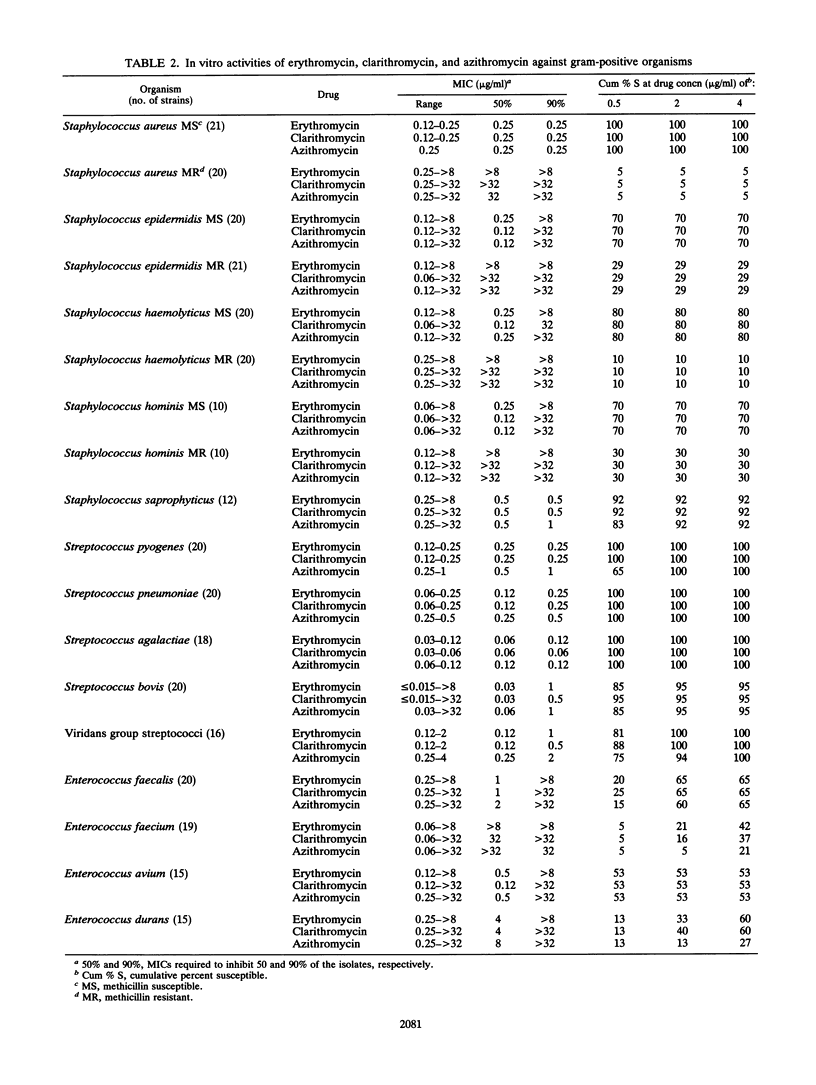
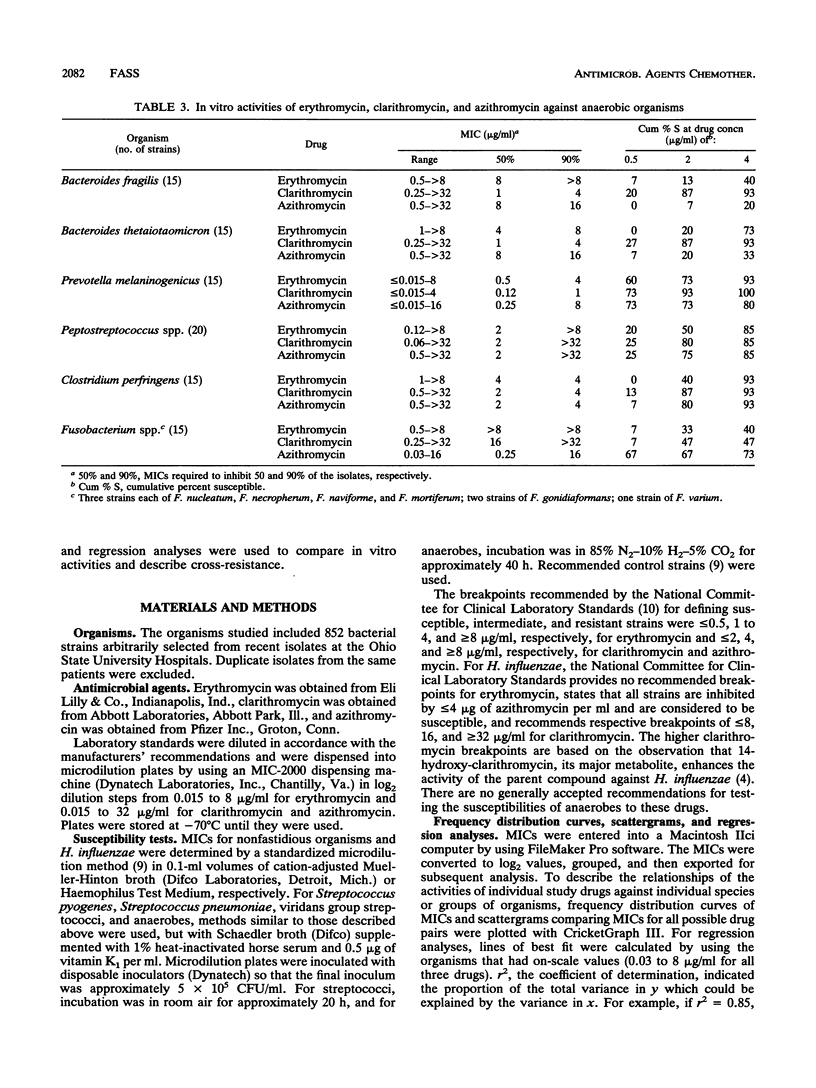
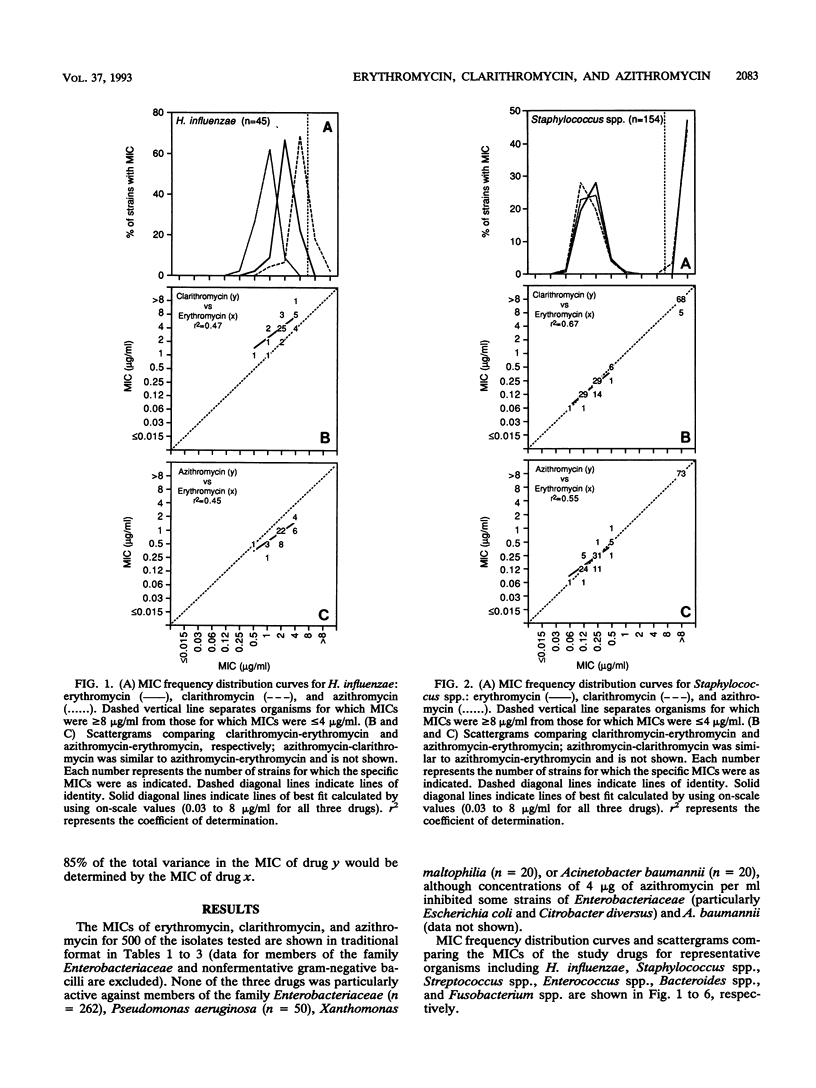
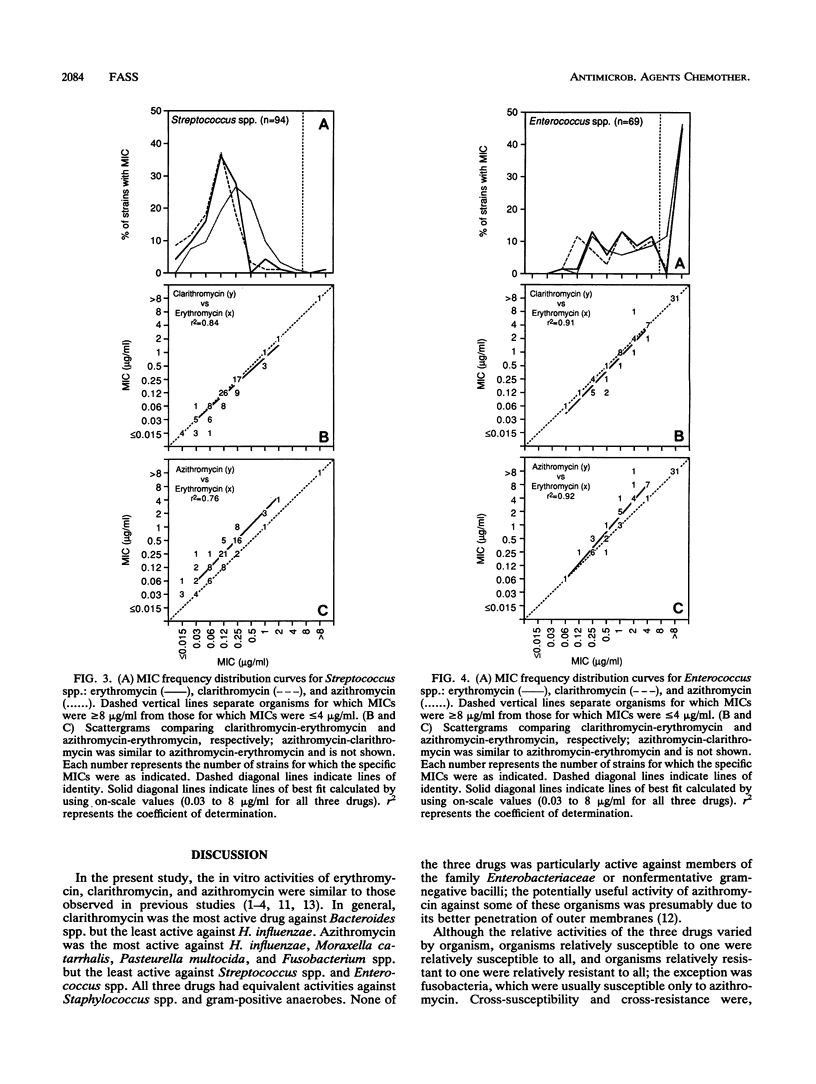
MICs of erythromycin, clarithromycin, and azithromycin for 852 recent clinical isolates were determined by broth microdilution methods. Frequency distribution curves, scattergrams, and regression analyses were used to compare in vitro activities and describe cross-resistance. Clarithromycin was the most active drug against Bacteroides spp. but the least active against Haemophilus influenzae. Azithromycin was most active against H. influenzae, Moraxella catarrhalis, Pasteurella multocida, and Fusobacterium spp. but the least active against Streptococcus spp. and Enterococcus spp. All three drugs had equivalent activities against Staphylococcus spp. and gram-positive anaerobes. None of the three drugs was particularly active against members of the family Enterobacteriaceae or nonfermentative gram-negative bacilli, although concentrations of 4 micrograms of azithromycin per ml inhibited some strains of the family Enterobacteriaceae (particularly Escherichia coli and Citrobacter diversus) and Acinetobacter baumannii. Although relative drug activities varied by organism, organisms relatively susceptible to one were relatively susceptible to all and organisms relatively resistant to one were relatively resistant to all; an exception was fusobacteria, which were usually susceptible only to azithromycin. Cross-susceptibility and cross-resistance were, therefore, the rule (except for Fusobacterium spp.), although the percentage of susceptible organisms could be varied considerably on the basis of the selection of breakpoints.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N., Thornsberry C. In vitro activities of azithromycin (CP 62,993), clarithromycin (A-56268; TE-031), erythromycin, roxithromycin, and clindamycin. Antimicrob Agents Chemother. 1988 May;32(5):752–754. doi: 10.1128/aac.32.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Hensey D. M., Beyer J. M., Vojtko C., McDonald E. J., Fernandes P. B. Comparative in vitro activities of new 14-, 15-, and 16-membered macrolides. Antimicrob Agents Chemother. 1988 Nov;32(11):1710–1719. doi: 10.1128/aac.32.11.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Swanson R. N., Rode R. A., Marsh K., Shipkowitz N. L., Clement J. J. Enhancement of the in vitro and in vivo activities of clarithromycin against Haemophilus influenzae by 14-hydroxy-clarithromycin, its major metabolite in humans. Antimicrob Agents Chemother. 1990 Jul;34(7):1407–1413. doi: 10.1128/aac.34.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst H. A. New macrolides: expanded horizons for an old class of antibiotics. J Antimicrob Chemother. 1991 Dec;28(6):787–790. doi: 10.1093/jac/28.6.787. [DOI] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: structural modifications and in vitro activity. Antimicrob Agents Chemother. 1989 Sep;33(9):1413–1418. doi: 10.1128/aac.33.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991 Jul;35(7):1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991 Jul;35(7):1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Outer membrane permeability barrier to azithromycin, clarithromycin, and roxithromycin in gram-negative enteric bacteria. Antimicrob Agents Chemother. 1993 Feb;37(2):354–356. doi: 10.1128/aac.37.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Maskell J. P., Shain H., Chrysos G., Sefton A. M., Fraser H. Y., Hardie J. M. Comparative in-vitro activity of azithromycin, macrolides (erythromycin, clarithromycin and spiramycin) and streptogramin RP 59500 against oral organisms. J Antimicrob Chemother. 1992 Jul;30(1):27–37. doi: 10.1093/jac/30.1.27. [DOI] [PubMed] [Google Scholar]


