Abstract
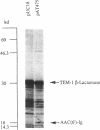
The amikacin resistance gene acc(6')-Ig of Acinetobacter haemolyticus BM2685 encoding an aminoglycoside 6'-N-acetyltransferase was characterized. The gene was identified as a coding sequence of 438 bp corresponding to a protein with a calculated mass of 16,522 Da. Analysis of the deduced amino acid sequence suggested that it was the fourth member of a subfamily of aminoglycoside 6'-N-acetyltransferases. The resistance gene was not transferable either by conjugation to Escherichia coli or to Acinetobacter baumannii or by transformation into Acinetobacter calcoaceticus. Plasmid DNA from strain BM2685 did not hybridize with an intragenic aac(6')-Ig probe. These results suggest a chromosomal location for this gene. The gene was detected by DNA hybridization in all 20 strains of A. haemolyticus tested but not in 179 other Acinetobacter strains, including A. baumannii, A. lwoffii, A. junii, and A. johnsonii and genospecies 3, 6, 11, 13, 14, 15, 16, and 17, of which 162 were amikacin resistant. The probe did not hybridize in dot blot assays with DNAs purified from members of the families Enterobacteriaceae and Pseudomonadaceae that encode 6'-N-acetyltransferases. These data suggest that the aac(6')-Ig gene is species specific and may be used to identify A. haemolyticus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Bouvet P. J., Jeanjean S. Delineation of new proteolytic genomic species in the genus Acinetobacter. Res Microbiol. 1989 May-Jun;140(4-5):291–299. doi: 10.1016/0923-2508(89)90021-1. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplaces N., Gutmann L., Carlet J., Guibert J., Acar J. F. The new quinolones and their combinations with other agents for therapy of severe infections. J Antimicrob Chemother. 1986 Mar;17 (Suppl A):25–39. doi: 10.1093/jac/17.suppl_a.25. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger M., Schmucker R., Kishan V., Hillen W. Analysis and nucleotide sequence of an origin of DNA replication in Acinetobacter calcoaceticus and its use for Escherichia coli shuttle plasmids. Gene. 1990 Mar 1;87(1):45–51. doi: 10.1016/0378-1119(90)90494-c. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Blaser M. J., Santanam P., Hächler H., Kayser F. H., Hare R. S., Miller G. H. Appearance of amikacin and tobramycin resistance due to 4'-aminoglycoside nucleotidyltransferase [ANT(4')-II] in gram-negative pathogens. Antimicrob Agents Chemother. 1990 Dec;34(12):2381–2386. doi: 10.1128/aac.34.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner M., Macicková T., Krcméry V. Occurrence of 4'-O-aminoglycoside-nucleotidyltransferase in clinical strains of Enterobacteriaceae. Infection. 1989 Mar-Apr;17(2):100–101. doi: 10.1007/BF01646887. [DOI] [PubMed] [Google Scholar]
- Kleppe R. K., Kleppe K. Preparations and properties of ribonucleic acid polymerase from Acinetobacter calcoaceticus. J Bacteriol. 1976 Feb;125(2):435–443. doi: 10.1128/jb.125.2.435-443.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Bouvet P., Vieu J. F., Courvalin P. Dissemination of amikacin resistance gene aphA6 in Acinetobacter spp. Antimicrob Agents Chemother. 1990 Jun;34(6):1244–1248. doi: 10.1128/aac.34.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988 Jan;32(1):15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabilat C., Courvalin P. Development of "oligotyping" for characterization and molecular epidemiology of TEM beta-lactamases in members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1990 Nov;34(11):2210–2216. doi: 10.1128/aac.34.11.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen R., Vosman B., Buijsman P., Breek C. K., Hellingwerf K. J. Physiological characterization of natural transformation in Acinetobacter calcoaceticus. J Gen Microbiol. 1993 Feb;139(2):295–305. doi: 10.1099/00221287-139-2-295. [DOI] [PubMed] [Google Scholar]
- Rather P. N., Munayyer H., Mann P. A., Hare R. S., Miller G. H., Shaw K. J. Genetic analysis of bacterial acetyltransferases: identification of amino acids determining the specificities of the aminoglycoside 6'-N-acetyltransferase Ib and IIa proteins. J Bacteriol. 1992 May;174(10):3196–3203. doi: 10.1128/jb.174.10.3196-3203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel A. F., Chabbert Y. A. Taxonomy and epidemiology of gram-negative bacterial plasmids studied by DNA-DNA filter hybridization in formamide. J Gen Microbiol. 1978 Feb;104(2):269–276. doi: 10.1099/00221287-104-2-269. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F. R., Nücken E. J., Henschke R. B. Nucleotide sequence analysis of 2''-aminoglycoside nucleotidyl-transferase ANT(2'') from Tn4000: its relationship with AAD(3'') and impact on Tn21 evolution. Mol Microbiol. 1988 Nov;2(6):709–717. doi: 10.1111/j.1365-2958.1988.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Cramer C. A., Rizzo M., Mierzwa R., Gewain K., Miller G. H., Hare R. S. Isolation, characterization, and DNA sequence analysis of an AAC(6')-II gene from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Dec;33(12):2052–2062. doi: 10.1128/aac.33.12.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Hare R. S., Sabatelli F. J., Rizzo M., Cramer C. A., Naples L., Kocsi S., Munayyer H., Mann P., Miller G. H. Correlation between aminoglycoside resistance profiles and DNA hybridization of clinical isolates. Antimicrob Agents Chemother. 1991 Nov;35(11):2253–2261. doi: 10.1128/aac.35.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Rather P. N., Sabatelli F. J., Mann P., Munayyer H., Mierzwa R., Petrikkos G. L., Hare R. S., Miller G. H., Bennett P. Characterization of the chromosomal aac(6')-Ic gene from Serratia marcescens. Antimicrob Agents Chemother. 1992 Jul;36(7):1447–1455. doi: 10.1128/aac.36.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Kumada T., Hsieh W. C., Chung H. Y., Chong Y., Hare R. S., Miller G. H., Sabatelli F. J., Howard J. Comparison of aminoglycoside resistance patterns in Japan, Formosa, and Korea, Chile, and the United States. Antimicrob Agents Chemother. 1985 Aug;28(2):282–288. doi: 10.1128/aac.28.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Filpula D., Phillips K. L., Plorde J. J. Cloning and sequencing of a gene encoding an aminoglycoside 6'-N-acetyltransferase from an R factor of Citrobacter diversus. J Bacteriol. 1988 Jan;170(1):471–473. doi: 10.1128/jb.170.1.471-473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terán F. J., Suárez J. E., Mendoza M. C. Cloning, sequencing, and use as a molecular probe of a gene encoding an aminoglycoside 6'-N-acetyltransferase of broad substrate profile. Antimicrob Agents Chemother. 1991 Apr;35(4):714–719. doi: 10.1128/aac.35.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran van Nhieu G., Collatz E. Primary structure of an aminoglycoside 6'-N-acetyltransferase AAC(6')-4, fused in vivo with the signal peptide of the Tn3-encoded beta-lactamase. J Bacteriol. 1987 Dec;169(12):5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H., Spohr M. Antimicrobial drug susceptibility of clinical isolates of Acinetobacter species (A. baumannii, A. haemolyticus, genospecies 3, and genospecies 6). Antimicrob Agents Chemother. 1989 Sep;33(9):1617–1619. doi: 10.1128/aac.33.9.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zubay G. The isolation and properties of CAP, the catabolite gene activator. Methods Enzymol. 1980;65(1):856–877. doi: 10.1016/s0076-6879(80)65079-4. [DOI] [PubMed] [Google Scholar]