Abstract
Many neurons in the mammalian retina are coupled by means of gap junctions. Here, we show that, in rabbit retina, an antibody to connexin 36 heavily labels processes of AII amacrine cells, a critical interneuron in the rod pathway. Image analysis indicates that Cx36 is primarily located at dendritic crossings between overlapping AII amacrine cells. This finding suggests that Cx36 participates in homotypic gap junctions between pairs of AII amacrine cells. Cx36 was also found at AII/cone bipolar contacts, previously shown to be gap junction sites. This finding suggests that Cx36 participates at gap junctions that may be heterotypic. These results place an identified neuronal connexin in the context of a well-defined retinal circuit. The absence of Cx36 in many other neurons known to be coupled suggests the presence of additional unidentified connexins in mammalian neurons. Conversely, Cx36 labeling in other regions of the retina is not associated with AII amacrine cells, indicating some other cell types use Cx36.
Keywords: AII amacrine cell, gap junction, Neurobiotin, calretinin, bipolar cell
Evidence that gap junctions participate in many neural activities is rapidly growing (Galarreta and Hestrin 1999; Gibson et al., 1999; Venance et al., 2000). However, identification of neural connexins (Cx) has been slow. The first connexins definitively localized to neurons are connexin35 from skate and perch retina (O’Brien et al., 1996, 1998; White et al., 1999; Al-Ubaidi et al., 2000) and its close homolog from murine brain, connexin36, which are broadly distributed in brain and retina (Condorelli et al., 1998; Söhl et al., 1998).
The retina has been an especially fruitful site for identification of gap junctional circuits (Vaney, 1999), with positive evidence for gap junctions found between members of all five major classes of retinal neuron. Evidence for more neuronal connexin types has come from recent studies finding three new connexins in zebrafish retina (Dermietzel et al., 2000) and differential tracer selectivities across coupled cell types (Mills and Massey, 1995, 2000). The presence of chemical rectification in some circuits (Robinson et al., 1993; Vaney, 1994, 1997; Mills, 1999; Zahs and Newman, 1997) suggests the presence of heterotypic connexons and hence multiple connexin types.
AII amacrine cells are well-known to make gap junctions, both homologous gap junctions with neighboring AII amacrine cells and heterologous gap junctions with ON cone bipolar cells (Famiglietti and Kolb, 1975; Smith et al., 1986; Strettoi et al., 1992; Massey and Mills, 1999). The AII amacrine cell is a critical interneuron in rod pathway vision in mammals. Signals from cones are immediately divided into “ON” and “OFF” channels at cone bipolar cells that respond to light with opposite polarity (see Fig. 1). However, there is only a single type of rod bipolar cell, which produces “ON” responses. Rod bipolar cells do not synapse directly onto ganglion cells. Instead, the rod signal passes into both ON and OFF channels by means of the AII amacrine cell. The AII accomplishes this by making sign-conserving gap junctions with ON cone bipolar cells and sign-inverting inhibitory synapses onto OFF cone bipolar cells. Coupling between the AII amacrine cells probably functions to improve signal/noise ratio at low light levels (Vaney, 1994; Vardi and Smith, 1996), whereas modulation of AII/cone bipolar coupling may aid in the transition from rod to cone vision (Vaney, 1994; Mills and Massey, 1995).
Fig. 1.
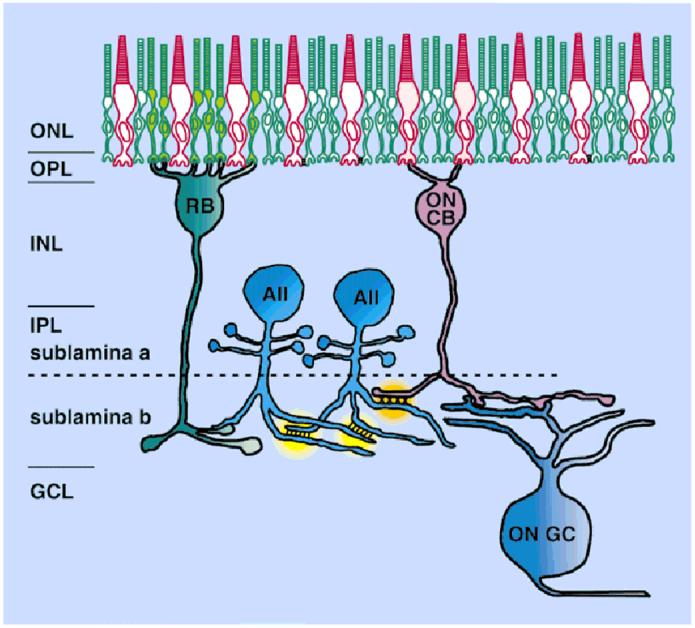
A schematic diagram of the role of the AII amacrine cell in the rod pathway. Each AII amacrine cell makes gap junctions not only with neighboring AII cells, but also with ON cone bipolar cells. These sign-preserving gap junctions with the bipolar cells distribute the signals from rod bipolar cells, which depolarize to light, into ON cone bipolar cells, which also depolarize to light. Sign-inverting inhibitory synapses from AII amacrine cells onto OFF cone bipolar cells occur in sublamina a (not shown), which serve to distribute the rod signal with the appropriate hyperpolarization to light into the OFF channel. The need for separate rod and cone ganglion cells is, thereby, avoided. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; RB, rod bipolar cell; CB, cone bipolar cell; GC, ganglion cell.
In this study, we labeled mammalian retina with an antibody to Cx36 and show that it is intimately associated with the fine dendritic processes of AII amacrine cells in the area where they are known to make gap junctions. By careful, quantitative inspection of this apparent association, we have positively localized an identified neural connexin in a specific retinal type and also placed it in the context of the neural circuit in which it participates. Another study finding similar results in localizing Cx36 to AII amacrine cells in rat retina has recently appeared (Feigenspan et al., 2001).
MATERIALS AND METHODS
Retinas were isolated from adult rabbits as previously described (Mills and Massey, 1991). Animals were deeply anesthetized with 1.5 g/kg urethane i.p. and killed with an overdose of an intracardial injection of 5 ml of urethane, in accordance with all guidelines of the institution and the NIH. Lucifer Yellow or Neurobiotin was injected into individual cells in unfixed pieces of retina perfused with oxygenated Ames media at 35°C. Cells were targeted by prior incubation with 4,6-diamino-2-phenylindole (DAPI) or acridine orange. Neurobiotin was visualized by incubation in 1:200 streptavidin-Cy3 (Jackson Immunoresearch, West Grove, PA).
Immunocytochemistry and imaging
Antibody concentrations were rabbit anti-connexin36 (Zymed Laboratories, San Francisco, CA), 1:2000; goat anti-calretinin (Chemicon; Temecula, CA), 1:5,000 inwhole-mount, 1:50,000 in sections; mouse anti-calbindin-28K Da (Sigma; St. Louis, MO), 1:500 in whole-mount, 1:1,000 in sections; mouse protein kinase α (PKC; Transduction Labs), 1:250; goat anti-serotonin (Incstar, Stillwater, MN),1:500. We used a Zeiss LSM-410 confocal microscope to image tracer and antibodies labeled with Cy3, Cy5, or Alexa488 conjugated to streptavidin or secondary antibodies raised in donkey (Jackson Immunoresearch). Digital images acquired from the confocal microscope were processed in Adobe Photoshop (Adobe Systems, Inc.; San Jose, CA) to enhance brightness and color contrast of the multichannel signals. No filtering techniques were applied.
Quantitative assessment of colocalization in confocal images
Images were analyzed with custom software that allowed the level of association between two labeled structures to be distinguished from chance. Repeating structures of interest, such as dendritic terminals or contacts between identified cells, were clipped from the image by centering a 32 × 32 pixel box on the structure to be analyzed. Alignment and averaging of these boxes produces an image that reveals the spatial distribution of the immunofluorescence in relation to a specific type of structure. A strong level of association between a label and the selected structure is revealed by a large peak in the center of the average intensity plot. A 3 × 3 median filter was sometimes applied for smoothing. Each averaged image was divided into annuli of equal radii; Duncan’s test was used to establish the level of significance for each annulus. Control images were produced by rotating one image channel out of phase, and were always flat.
Reverse transcription-polymerase chain reaction amplification of rabbit connexin36
A cDNA fragment of connexin 36 was cloned from rabbit retina by using reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted from rabbit retina by using an RNeasy Mini kit (Qiagen, Valencia, CA). First-strand cDNA was made by reverse transcription with an oligo(dT) primer and Superscript II reverse transcriptase (Gibco-BRL, Rockville, MD).
A primer pair was derived from Mouse Cx36, spanning the first extracellular loop to the carboxyl terminus coding sequence (Forward: 5′-ATGTTTGTGTGCAACACCCTG-CAGCCC-GGCTG-3′; Reverse: 5′-CGGAGTCACTGGAC-TGAGTCCTGCCGAATTGG-3′). One hundred fifty nanograms of first-strand cDNA was amplified with 35 cycles of PCR by using 100 pmol of each primer. PCR products were gel purified and cloned into pGEM-T vectors (Promega, Madison, WI). The cDNA clones were sequenced on both strands, and the sequence was analyzed by using GeneRunner (Hastings Software, Inc) and BLAST software (National Center for Biotechnology Information).
Western blot analysis of rabbit connexin36
A portion of the rabbit Cx36 cDNA coding for the intracellular loop (corresponding to amino acids 94-198 of the mouse Cx36 sequence) was cloned into pGex2KG. The resulting construct coded for a 39-kDa fusion protein containing glutathione-S-transferase and the rabbit Cx36 intracellular loop (Fig. 2). The fusion protein was expressed in Escherichia coli and used for Western blot screening of anti-Cx36 antisera.
Fig. 2.

Western blot analysis shows that Zymed anti-Cx36 antiserum recognizes rabbit Cx36. A portion of the rabbit Cx36 cDNA coding for the intracellular loop (amino acids 94-198) was subcloned into a GST expression vector (pGex2KG) and expressed in Escherichia coli. Here, a crude bacterial lysate was blotted, probed with the Cx36 antibody (1:5000), and detected by chemiluminescence.
Lysates of bacteria expressing the Cx36 intracellular loop fusion protein and homogenates of rabbit retina and liver were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis, blotted to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA) and probed with the anti-Cx36 antisera at 1:5000 dilution. Labeled bands were detected by chemiluminescence.
RESULTS
Rabbit retina contains connexin36 cDNA
RT-PCR of rabbit retina cDNA by using Cx36 primers amplified an 801-bp fragment. BLAST search revealed the 801-bp fragment was most similar to connexin36. The sequence was 93% and 89% identical to human and mouse Cx36, respectively, at the nucleotide level, and 96% and 95% similar to human and mouse Cx36, respectively, at the amino acid levels (Belluardo et al., 1999, 2000). Rabbit Cx36 contained the glycine-rich region in the intracellular loop similar to mouse and human Cx36 but unlike the related perch and skate Cx35. This finding confirms that Cx36 is expressed in rabbit retina.
Connexin36 Immunoreactivity
A polyclonal antibody to Cx36 at a dilution of 1:5,000 labeled a ∼33-kDa band in Western blots of rabbit retinal homogenate. The antiserum also labeled a 39-kDa GST fusion protein containing the rabbit Cx36 intracellular loop domain (data not shown). This antibody produced distinct and repeatable staining in the rabbit retina at a dilution of 1:2000 (Fig. 3a). The outer plexiform layer (OPL) contained very faint punctate labeling in sections. More prominent punctate labeling appeared throughout the inner plexiform layer (IPL), densely in sublamina b and sparsely in sublamina a. No Cx36 staining was visible in the ganglion cell layer.
Fig. 3.
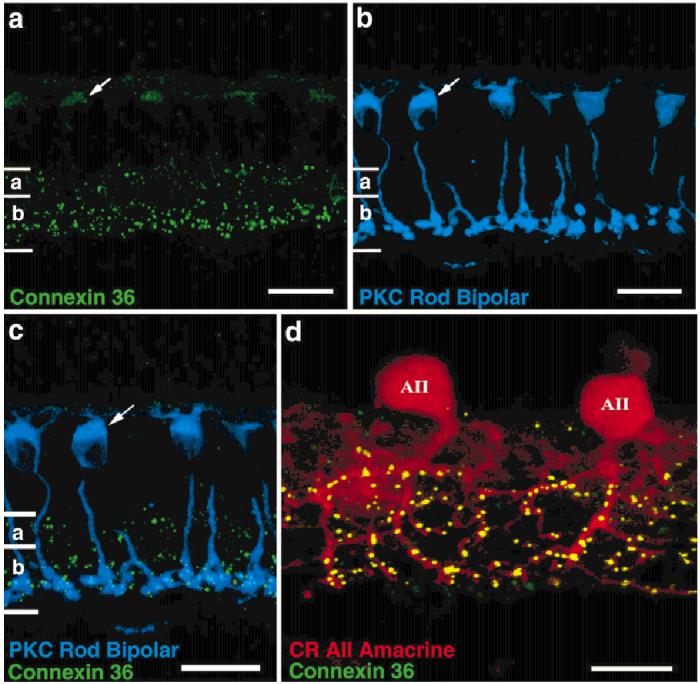
Cx36 immunoreactivity in oblique sections. a: Cx36 immunoreactivity appears as discrete puncta, densely in the ON region and more sparsely in the OFF region of the inner plexiform layer. Light labeling is also sometimes seen in the outer plexiform layer. A more diffuse cytoplasmic stain sometimes appears in rod bipolar cells (arrow). b,c: Staining of rod bipolar cells (blue) by an antibody to protein kinase C (PKC) demonstrates colocalization with the diffuse Cx36 immunostaining (green). d: Strong colocalization of Cx36 immunoreactivity (green) is seen on the processes in sublamina b of AII amacrine cells stained with anti-calretinin (CR, red). Light Cx36 immunoreactivity in sublamina a is not associated with the AII lobular appendages. Scale bars = 20 μm in a-c, 10 μm in d.
We measured Cx36- and PKC-positive pixel densities across the breadth of the retina. The distribution of Cx36 revealed three distinct peaks, a large peak in sublamina b, a small peak in sublamina a, and a peak corresponding to rod bipolar somas in the outer nuclear layer. A smaller, less-reliable peak occurred at the OPL. The greatest concentration of Cx36 is in the lower IPL where the rod bipolar terminals and AII dendrites are located.
In some tissue sections, rod bipolar cells were diffusely stained along their axons and somas, but not on their terminals (Fig. 3a-c, arrow). We suggest this staining is nonspecific because (1) rod bipolar staining appeared to be cytoplasmic, rather than in punctate membrane deposits, as it appeared in all other locations; and (2) rod bipolar staining weakened with dilution of the Cx36 antibody, whereas the punctate staining persisted. Cx36 antibody labeling of rod bipolar cells was confirmed by double immunolabeling with an antibody to PKC, which specifically labels rod bipolar cells in the rabbit retina (Fig. 3b,c).
Because Cx36 faintly labeled rod bipolar cell somas and axons, but not their terminals, we investigated whether Cx36 gap junction plaques were localized on or near the rod bipolar cell terminals. We examined rabbit retinal whole-mounts labeled with PKC, calretinin, and Cx36 to determine the relative locations of rod bipolar terminals, calretinin-stained AII dendrites, and Cx36. The majority of Cx36 plaques near rod bipolar terminals were located a short distance from, rather than on, rod bipolar cell terminals. Colocalization software revealed a rim or caldera, rather than a peak, indicating Cx36 is adjacent to rod bipolar terminals. Gap junctions frequently occur between postsynaptic AII amacrine cell processes in this vicinity (Kolb, 1979; Dacheux and Raviola, 1986; Smith et al., 1986; Strettoi et al., 1992; Massey and Mills, 1999). Although a few Cx36 plaques appeared to be located on rod bipolar cell terminals (cyan puncta in Fig. 4a), calretinin labeling of the same tissue revealed that overlying AII amacrine cell dendrites intersected at these points (Fig. 4a,b). Based on these findings, we conclude that the cytoplasmic Cx36 labeling of rod bipolar cell somas and axons is nonspecific. Finally, there is no available evidence that rod bipolar cells make gap junctions. In fact, detailed electron microscopic observations reveal an absence of gap junctions on rod bipolar terminals (Kolb, 1979; McGuire et al., 1984; Freed et al., 1987; Sterling et al., 1988).
Fig. 4.
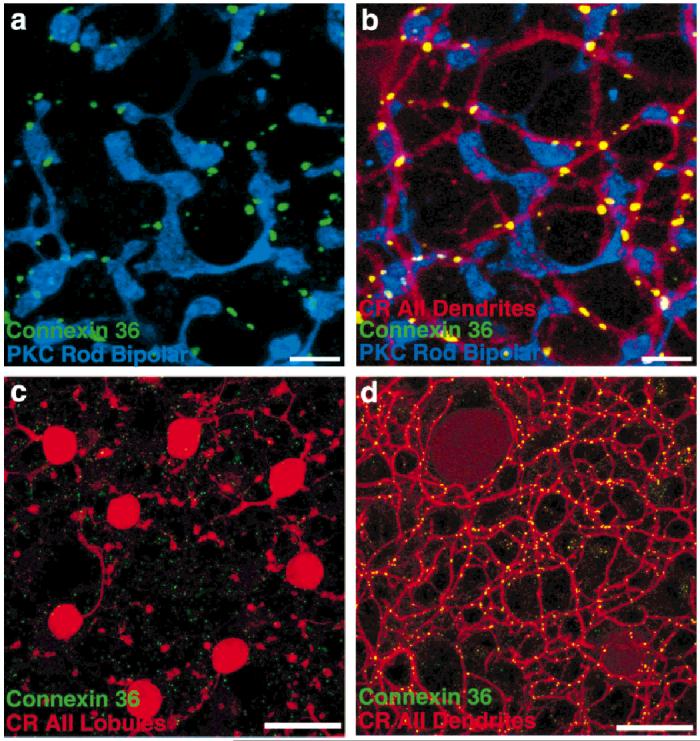
Cx36 immunoreactivity in retinal whole-mounts. a: Rod bipolar cells, although sometimes stained diffusely by the Cx36 antibody in the somatic and axonal regions, are not stained at all at their terminals, which are stained by an antibody to protein kinase C (PKC, blue). Some Cx36-immunoreactive puncta (green) do appear nearby, but rarely appear on the terminals. b: The dendrites of AII amacrine cells, stained by anti-calretinin (CR, red), are intimately associated with the Cx36 puncta. The few colocalizations of rod bipolar cell terminals with Cx36 (e.g., lower right) can invariably be accounted for by passing AII amacrine cell processes. c: Cx36 immunoreactivity is sparse and faint in sublamina a. The puncta that are found do not colocalize with the lobules of AII amacrine cells, which do not make gap junctions in this area. d: In the same field as c, focus on sublamina b demonstrates the extent to which Cx36-immunoreactive puncta are located on AII amacrine cell dendrites. Scale bars = 10 μm in a,b, 20 μm in c,d.
Cx36 immunoreactivity is primarily on puncta on AII dendrites
AII amacrine cells send a thick primary dendrite into the IPL before branching into many tapering dendrites in sublamina b. Because AII amacrine cells are well coupled to other AII amacrine cells and also to ON cone bipolar cells, the Cx36 punctate labeling in sublamina b was investigated to determine Cx36’s role in the gap junctions formed by AII amacrine cells. Calretinin antibody at a high dilution (1:50,000 in sections) specifically labels the AII amacrine cells (Massey and Mills, 1996). Oblique Vibratome sections, double labeled for Cx36 and calretinin, revealed punctate Cx36 staining all along the AII amacrine cell dendrites (Fig. 3d). Light Cx36 labeling appeared at the very top of the IPL but was not localized to AII amacrine cell somas. Again, no Cx36 staining was visible in the ganglion cell layer.
Cx36 immunoreactivity occurs at junctions between AII amacrine cells
Serial image scans were taken every 0.5 μm that encompassed the full span of the AII amacrine cell. Cx36 staining was anticorrelated with AII somas and lobules in sublamina a (Fig. 4c). This finding suggests that the sparse Cx36-immunoreactive puncta in sublamina a are located on an unidentified amacrine or bipolar cell type. Figure 4d demonstrates that Cx36-containing plaques are colocalized with the AII dendritic matrix in sublamina b. A pixel scattergram confirmed colocalization in sublamina b and its lack in sublamina a by the presence or absence, respectively, of pixels containing both markers (data not shown). Figure 5a demonstrates at higher power the location of Cx36-immunoreactive puncta at the junctions between AII amacrine cells. There are very few isolated green pixels, where Cx36 immunoreactivity appears unassociated with AII amacrine cell processes.
Fig. 5.

Cx36 puncta occur where AII amacrine cell dendrites cross. a: A high-power photomicrograph shows that almost all Cx36 immunoreactivity (green) in sublamina b is located on AII amacrine cells stained by anti-calretinin (CR, red) and that virtually every crossing of AII amacrine cell processes contains a Cx36 puncta. b-d: A focal series demonstrates that the Cx36 puncta occur only where adjacent AII process actually abut, in three-dimensional space. (b) The vertical dendrite is in focus. (c) Cx36 puncta are brightest when both processes are in focus. (d) Cx36 puncta have begun to fade when the horizontal dendrite is in focus. Scale bars = 5 μmina,2 μm in d (applies to b-d).
We estimated the percentage of Cx36 plaques that contact AII amacrine cells. Counting the Cx36 plaques in sublamina b in whole-mount revealed that 98% of the plaques occurred on AII amacrine dendrites. Of these, 84% of the Cx36 plaques on AII dendrites occurred where AII dendrites intersected and indicate homologous AII gap junction coupling. The remaining 16% of Cx36 plaques that are on AII dendrites do not intersect other AII dendrites. This finding suggests heterologous coupling of AII dendrites to ON-cone bipolar cells also occurs and places a lower limit on its relative frequency.
To examine the homologous junctions more closely, the apparent junction of two AII dendrites marked with Cx36 plaques was examined with serial confocal scans of 0.2 μm at high magnification. Figure 5b-d shows three narrow confocal images of two AII amacrine cell dendrites oriented at right angles to one another when viewed en face but at slightly different planes of focus. When the vertical AII dendrite is in its focal plane, weak Cx36 punctate labeling begins. The Cx36 signal occurs strongly only in the middle panel, where the vertical and horizontal dendrites abut. In the third panel, when the horizontal dendrite is in focus, the dendrites have diverged and no Cx36 immunoreactivity is found. This illustrates the general finding that Cx36 puncta appear only on contact points between dendrites, within the limits of confocal resolution.
An individual AII amacrine cell makes numerous Cx36-containing gap junctions with neighboring AII amacrine cells
We investigated the extent to which a single AII amacrine cell is coupled to other AII amacrine cells by measuring the number of contacts between a Neurobiotin-injected AII amacrine cell and the surrounding calretininstained AII amacrine cells. Figure 6a shows the lobular appendages in sublamina a of the injected cell (blue) and the surrounding AII lobules (red). The coverage factor of the lobules is near 1 (Mills and Massey, 1991). This lack of overlap allows little opportunity for gap junctional coupling.
Fig. 6.
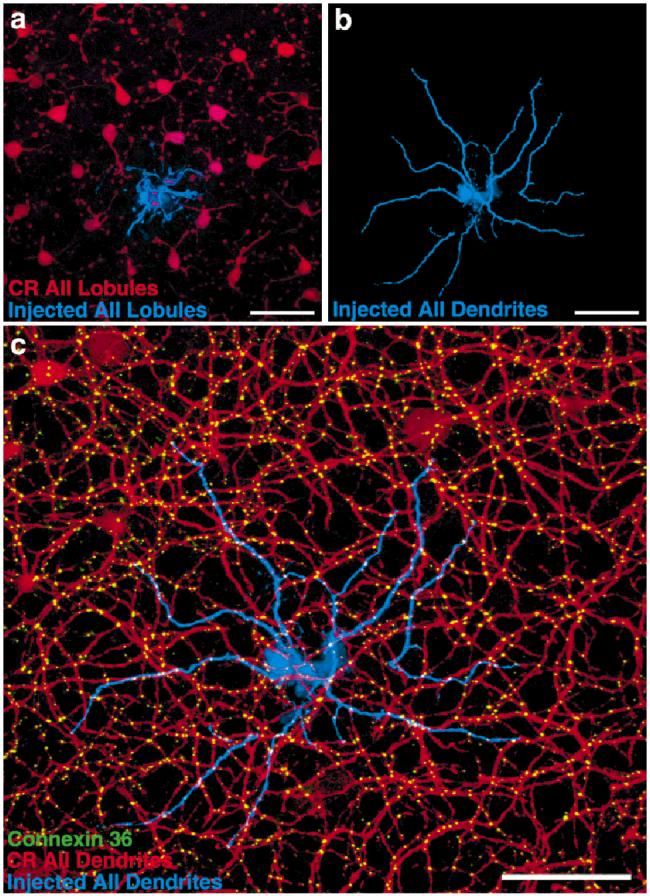
An individually injected AII amacrine cell is extensively coupled with its anti-calretinin stained neighbors. a: There is little spatial overlap in sublamina a, hence little opportunity for gap junctions, between the lobules of Neurobiotin-stained AII amacrine cells (blue) and those of neighboring AII amacrine cells stained with anticalretinin (CR, red). b: Inspection of the fine dendrites in sublamina b confirms the Neurobiotin fill as an AII amacrine cell. c: Cx36 immunoreactivity (green) occurs at virtually all instances where the dye-injected AII amacrine cell (blue) contacts neighboring AII amacrine cells stained with anti-calretinin (red). Scale bars = 40 μm in a-c.
The fine dendrites in sublamina b of the injected cell are seen in isolation in Figure 6b, and with the calretinin-(red) and Cx36-(green) immunoreactivity in Figure 6c. The dendritic overlap is such that any point is within the dendritic field of three to eight different AII amacrine cells (Mills and Massey, 1991). The dendrites of a single cell do not intersect one another. As before, the great majority of Cx36 puncta appear on AII amacrine cell processes that contact other AII processes. The extent of coupling between the injected cells and its neighbors is great, with over 100 puncta on the injected cell, 66% contacting calretinin-stained processes.
Figure 7 demonstrates that Cx36 immunoreactivity on the Neurobiotin-injected cell occurs where it intersects other AII processes stained with calretinin. Several 24 × 24 pixel squares were centered on intersections between the injected amacrine cell and calretinin-stained processes (Fig. 7a). When these squares were averaged, a large peak of associated Cx36 immunoreactivity appears (Fig. 7b). (In Duncan’s test, the central 1.8 μm, mean Cx36 intensity = 34.1 was greater [P < 0.05] than the peripheral portion of the clipped area, mean = 14.3.) If the image of the injected AII amacrine cell is rotated, and squares are centered on the new intersections of processes, no peaks appear at above chance levels (Fig. 7c). (In Duncan’s test, no portions of the clipped area were significantly different, means = 15.5-22.4.)
Fig. 7.
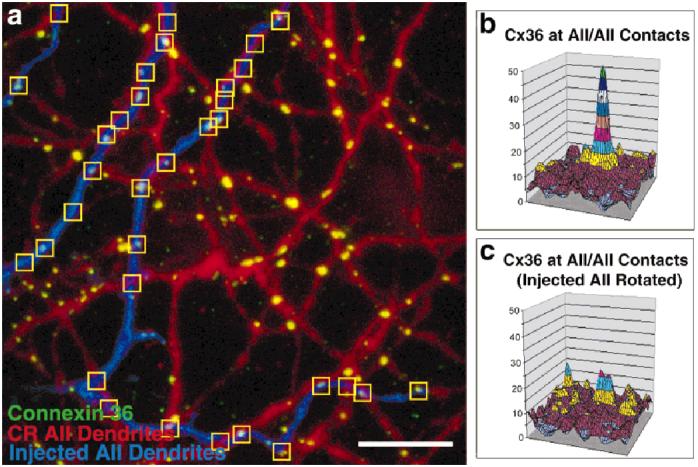
Association of Cx36 with AII amacrine cell processes is not a chance occurrence. a: Squares (36 × 36 pixels) are centered on crossings of AII amacrine cell processes. b: When all such squares are averaged, a distinct peak of Cx36 immunoreactivity appears. c: When the AII image is rotated by 180 degrees, the proximity of Cx36 immunoreactivity peak to AII amacrine cell dendrites is at chance level. CR, anti-calretinin. Scale bar = 10 μm in a.
Cx36 is contained in junctions between AII amacrine cells and ON cone bipolar cells
AII amacrine cells also form heterologous gap junctions with ON cone bipolar cells, as demonstrated by electron microscopy (Famiglietti and Kolb, 1975; McGuire et al., 1984; Dacheux and Raviola, 1986; Freed et al., 1987; Sterling et al., 1988; Cohen and Sterling, 1990; Strettoi et al., 1992; Massey and Mills, 1999), tracer coupling (Vaney, 1991; Mills and Massey, 1995; Bloomfield et al., 1997) and physiological recording (Xin and Bloomfield, 1999). To determine whether Cx36 forms gap junctions between AII and ON cone bipolar cells, retinal whole-mount tissue was labeled with antibodies to Cx36, calretinin, and calbindin. Calbindin antibodies label a single type of ON cone bipolar cell in the rabbit retina (Massey and Mills, 1996). Figure 8a shows that some Cx36-immunoreactive puncta appear on calbindin-stained processes in sublamina b (arrows). When the calretinin image is observed, there are also AII amacrine cell processes at these sites (Fig. 8b,c; arrows). Hence, Cx36 also participates in coupling between AII amacrine cells and an identified type of ON cone bipolar cell. Several types of ON cone bipolar cell are known to make gap junctions with the AII amacrine cell. We have also found Cx36 immunoreactivity on ON cone bipolar cells stained by Neurobiotin injection into AII amacrine cells, and which are not immunoreactive for calbindin (not shown). This finding indicates that gap junctions from AII amacrine cells to other types of ON cone bipolar cell can also contain Cx36.
Fig. 8.
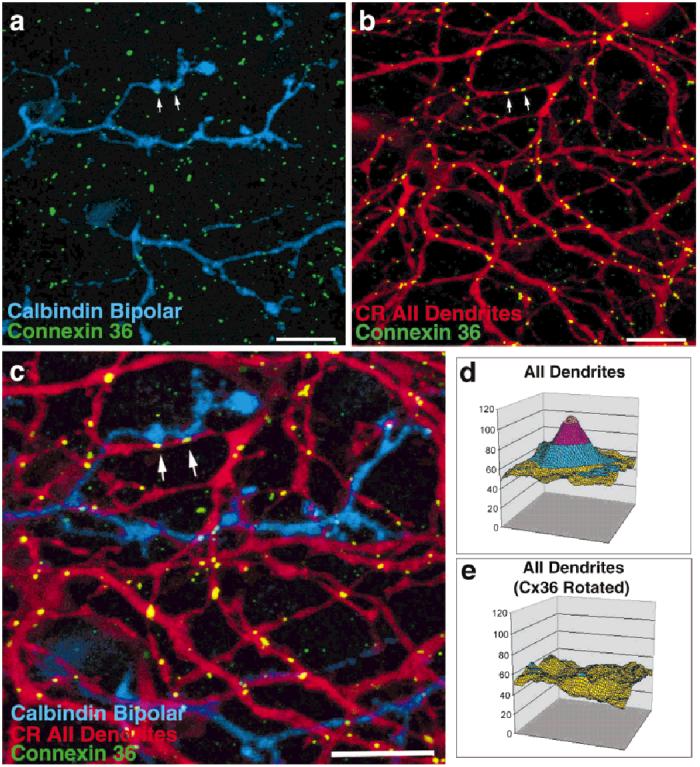
Cx36 immunoreactivity is also found at the intersections of AII amacrine cell processes with calbindin-positive ON cone bipolar cells. a: The arrows point to Cx36-immunoreactive puncta (green) that appear in conjunction with bipolar cell processes stained with an antibody to calbindin (blue). b,c: Calretinin-stained AII amacrine cell processes (CR, red) are invariably found to abut these bipolar cell-Cx36-immunoreactive puncta. d: Averaging of locations where Cx36 immunoreactivity puncta occur on calbindin-positive bipolar cell dendrites reveals that calretinin-positive AII amacrine cell processes also occur. e: Sites selected for combined calbindin and Cx36 immunoreactivity after rotation of the Cx36-immunoreactive image by 180 degrees are not associated with AII amacrine cell processes. Scale bars = 10 μm in a-c.
The number of contacts of Cx36 puncta with calretininstained AII dendrites and calbindin-positive ON cone bipolar cells were counted. The majority (66%) of Cx36 puncta on calbindin-positive bipolar cells were located where two AII dendrites crossed. However, 34% of Cx36 puncta located on calbindin-positive bipolar cells contacted single, uncrossed AII dendrites. The lack of a second AII dendrite in the vicinity of the Cx36-containing calbindin bipolar cell process strongly suggests that AII-ON cone bipolar gap junctions also utilize Cx36.
Our signal colocalization software was used to measure the level of association of Cx36 plaques with AII-ON cone bipolar cell junctions. Cx36 plaques contacting a calbindin bipolar cell were selected, while the calretinin image was turned off to eliminate observer bias. The markers for Cx36 and calbindin bipolar cells were of course colocalized by the selection procedure. However, a large peak indicating the presence of calretinin-stained AII processes also appeared (Fig. 8d). (In Duncan’s test, the central 1.8 μm, mean Cx36 intensity = 47.3 was greater [P < 0.05] than the peripheral portion of the clipped area, mean = 6.1.) This finding indicates that Cx36 immunoreactivity on calbindin-stained ON cone bipolar cells occurs where an AII amacrine cell contacts the bipolar cell. To verify this conclusion, all occurrences of Cx36 puncta contacting ON cone bipolar cells were reanalyzed after the Cx36 image was rotated 180 degrees. (In Duncan’s test, no portions of the clipped area were significantly different, means = 6.4 -9.9.) In this condition, AII dendrites were no longer colocalized with Cx36/calbindin (Fig. 8e). A complementary analysis of junctions between calretinin- and calbindin-stained processes revealed a peak of Cx36 immunoreactivity. Both analyses support the idea that Cx36 forms gap junctional channels between AII amacrine cells and ON cone bipolar cells.
Many retinal gap junctions do not contain Cx36
A great many retinal neurons are coupled by gap junctions (Vaney, 1994). Horizontal cells are perhaps the best-known example. Because very faint Cx36 labeling was detected in the OPL of Vibratome sections, the expression of Cx36 in the horizontal cell layer was examined. The antibody to calbindin also stains A-type horizontal cells (Röhrenbeck et al., 1987; Massey and Mills, 1996). However, Cx36 staining at the level of horizontal cell somas revealed only a faint diffuse staining of rod bipolar cell somas. Very faint punctate labeling was also observed in the OPL, compared with the intense Cx36 staining of inner plexiform layer in the same tissue region. Contrast the near-exclusive association of Cx36-immunoreactive puncta (green) with AII amacrine cell (red) and calbindin bipolar cells (blue) in Figure 9a, with the lack of association of the faint punctate labeling in the OPL with A-type horizontal cells (Fig. 9b).
Fig. 9.

Many retinal gap junctions are not stained with Cx36 antibody. (a,b) Labeling with antibodies to calbindin (blue), Cx36 (green), and calretinin (CR, red) show that Cx36 is intimately associated with AII amacrine cells and calbindin-stained bipolar cells in sublamina b (a), whereas in the outer plexiform layer (OPL) at the same location (b), the light Cx36 staining does not colocalize with calbindin staining of horizontal cells or an ON cone bipolar cell subtype (blue). c: The coupled matrix of S1/S2 cells is not localized with Cx36 immunoreactivity. d: Squares containing Cx36 immunoreactivity do not contain processes stained with anti-serotonin at levels above chance. e: The average density of Cx36 in the selected squares occurs in “holes” (blue patch in d) in the center of the S1/S2 signal. Scale bars = 20 μm in a,b, 12 μm in c.
Two closely related amacrine cells (S1/S2) that accumulate indolamines form a dense meshwork of highly overlapping dendrites. Like AII amacrine cells, they receive synaptic input from and make reciprocal synapses back onto rod bipolar terminals (Sandell et al., 1989). They are also extensively tracer coupled. To determine whether Cx36 formed gap junctions in the S1/S2 network, tissue was labeled with antibodies to serotonin and Cx36. As S1/S2 and AII amacrine cell processes converge at the rod bipolar cell synapse, some association must occur. Nevertheless, when images (Fig. 9c) were analyzed with the signal colocalization software, Cx36 immunoreactivity (Fig. 9e) occurred at a hole in the S1/S2 signal (Fig. 9d). (Duncan’s test found a significant dip, mean Cx36 intensity = 69.5 in the central 3.6 μm, compared with the peripheral regions, mean = 80.8, P < 0.05.) This finding indicates that Cx36 does not form gap junctions between S1/S2 cells. These holes were also found to contain calretinin-labeled AII amacrine cell processes where the Cx36 immunoreactivity appears.
DISCUSSION
A functional analysis of the role of gap junctions within the tissue where they occur requires knowledge both of the physiological properties of the connexins that exist there and their anatomic localization. Connexin35(36) is the first connexin positively identified as neural. This study localizes it to a specific well-known neural type, the AII amacrine cell. Other retinal cell types known to contain gap junctions were found to lack Cx36 immunoreactivity. This finding is an important step in the process of contrasting gap junctional properties in various neural circuits and drawing inferences regarding their possible functions.
Where is Cx36 in the retina?
The one cell type that definitively contains Cx36 is the AII amacrine cell. The majority of Cx36 plaques in the ON portion (sublamina b) of the IPL are colocalized with AII amacrine cells. This finding is apparent both from visual inspection and from quantitative examination of the spatial relationship that occurs between Cx36-positive puncta and AII amacrine cell processes in sublamina b of the IPL. Furthermore, the majority of these sites occur where two processes from AII amacrine cells abut, indicating the formation of homologous and homotypic gap junctions. The remaining Cx36 puncta occur where an AII amacrine cell apparently contacts ON cone bipolar cells, presumably the site of heterologous gap junctions. These appear on bipolar cells immunoreactive for calbindin but also on cone bipolar cells types stained by Neurobiotin diffusion through the AII/ON cone bipolar cell gap junctions.
Some Cx36 immunoreactivity also occurs in sublamina a, where OFF cone bipolar cells ramify, and in the OPL, where the dendrites of horizontal cells and bipolar cells contact photoreceptors. The anticorrelation of Cx36 immunoreactivity with the lobules of AII amacrine cells in sublamina a suggests the presence of gap junctions between a different type of amacrine cell or between bipolar cells. The lack of association of Cx36 puncta with horizontal cells and S1/S2 amacrine cells suggests the presence of yet-unidentified connexin types in these cells.
As noted, there is always some Cx36 puncta not associated with AII amacrine cells, although very little occurs in sublamina b. These are most likely to be other cell types that we have not been able to stain selectively. That most of these other puncta occur outside of sublamina b may indicate that spatial segregation is required to prevent inappropriate linkage of cell types that share a common connexin type, but should not pool their signals by means of gap junctions.
Do ON cone bipolar cells contain Cx36?
The AII/AII gap junctions have distinguishably different permeabilities than those between AII amacrine cells and ON cone bipolar cells. This difference could occur if AII amacrine cells produced only one type of connexin and ON cone bipolar cells produced another type. AII amacrine cells would then be interconnected by homotypic gap junctions, while they might be connected to ON cone bipolar cells by heterotypic gap junctions. An alternative is that the AII amacrine cell might produce two different connexin types, and make homotypic channels with each type of target cell. The presence of Cx36 immunoreactivity at both sites suggests that this is not the case. If the AII amacrine cell produces two connexin types, another possibility is that the channels with ON cone bipolar cells could be a mixture of homotypic and heterotypic channels, or even heteromeric.
It is likely that the AII amacrine cell to ON cone bipolar cell gap junction contains at least some heterotypic junctions. The two channels are differentially permeable to different tracers (Mills and Massey, 1995, 2000) and are differentially regulated by cyclic nucleotides (Mills and Massey, 1995) and carbenoxelone (Vaney et al., 1998). Differences also appear in the ultrastructure of these gap junctions, with a structure described as dense or fluffy appearing on the AII cell side of the bipolar cell/AII amacrine cell gap junction (Kolb, 1979; Strettoi et al., 1992) but missing in AII/AII gap junctions. This study has shown that Cx36 immunoreactivity appears both at AII/ AII and AII/cone bipolar cell gap junctions. If the channels cannot be identical, from the previous work, then the bipolar cell hemichannel must contain a connexin with different gating and permeability characteristics than Cx36, as expressed in AII amacrine cells. However, it is possible that the bipolar cell channel might be coded for by Cx36 mRNA and derive its different characteristics from posttranslational modifications. Whether this putative modified channel would be immunoreactive for our Cx36 antibody is unknown, and the resolution of this study does not permit conclusions on whether AII amacrine cell to ON cone bipolar cell gap junctions were stained on both sides of the channel or only one.
Gap junctional plaques composed of multiple connexin types arranged in different homotypic mixtures could possibly account for some of these findings, but would still require at least two functionally different channel types. Heteromeric combinations of multiple connexins would have effects impossible to predict, although there is evidence that such variations might confer differential permeability to mid-size molecules (Bevans et al., 1998).
Comparison with other studies
A similar study that recently appeared (Feigenspan et al., 2001) also found Cx36 immunoreactivity colocalized to the processes of AII amacrine cells in mouse and rat retina, as labeled by intracellular injection or parvalbumin immunoreactivity. The findings of the studies are in agreement regarding the major retinal locations of Cx36 immunoreactivity. This study strengthens these findings by (1) extension to another species, (2) quantitative examination of level of association, (3) examination of Cx36-association between tracer-coupled AII amacrine cells and calretinin-stained AIIs, (4) demonstration of Cx36 immunoreactivity between AII amacrine cells and a well-characterized ON cone bipolar cell (stained with anti-calbindin), and (5) demonstration of several negative cases of Cx36 immunoreactivity on neurons known to contain gap junctions, presumably of other connexin types.
How many connexin types are likely in neurons?
Although Cx36 is the first identified neural mammalian connexin, it seems likely that there are many more types in retina alone. Four connexins, three previously unknown, have been found recently in zebrafish retina, none of which were closely related to Cx36 (Dermietzel et al., 2000). Table 1 summarizes several observed properties of gap junctions in rabbit retinal neurons. Their permeabilities can be differentiated based on their ability to pass Lucifer Yellow, selectivity for cation size, and closure in response to carbenoxelone or putative phosphorylating agents.
TABLE 1.
Functional Differences Between Identified Retinal Gap Junctions1
| Tracer permeability to | ||||||
|---|---|---|---|---|---|---|
| Cell type | Cx36-IR | Lucifer Yellow2 | Large cations | Effect of phosphorylation | Sensitivity to carbenoxelone | Class |
| A-type horizontal cells | - | +a,b | Highc | Closef | Highh | 1 |
| B-type horizontal cells | - | - | Moderatec | ? | High | 2 |
| S1 amacrine cells | - | - | Moderatec | ? | Lowh | 3 |
| AII amacrine cells | ||||||
| To other AII | + | - | Moderatec,d | Closed,e | Highh | 4 |
| To bipolar cells | + | - | Lowc,d | Closec | Moderateh | 5 |
| Rod-cone | - | - | ? | Openg | ? | 6 |
Five lines of evidence imply the existence of several functionally discriminable gap junctional channel classes in retinal neurons. “Class” refers to classification of the junctions into arbitrary groups with demonstrably different properties and may or may not correspond to distinct connexin classes. The phosphorylation results are inferences based on presumptive stimulation of kinases. Some functional differences might be attributable to differential regulatory processes in the individual cells or varying combinations of fewer connexin types. This table does not include evidence for other connexin types in retinal glia. IR, immunoreactivity; 1, found; 2, not found; ?, data not available. Superscript letters indicate the following references: a, Dacheux and Raviola, 1982; b-d, Mills and Massey, 1994, 1995, 2000; e, f, Hampson et al., 1992, 1994; g, Mangel and Wang, 2000; h, Vaney et al., 1998.
Two exceptions have been reported to the general rule that Lucifer Yellow will pass the gap junctions of only A-type horizontal cell in mammalian retina. Lucifer Yellow-coupling was reported between B-type horizontal cells in mouse retina (He et al., 2000) and between the cat homolog of the S1 amacrine cell in cat retina (Vaney, 1996). In each case, the rabbit counterpart will not pass Lucifer Yellow.
The first column of the table indicates which cells in the rabbit retina contain gap junctional connexins that are recognized by the antibody we used. As noted, some other Cx36 puncta were present, but we were unable to identify the specific cell types. However, those cell types listed as negative in the table were not associated with Cx36 puncta in this study. It is always possible that a different Cx36 antibody might produce more staining, even of some of these cell types.
The second two columns of Table 1 describe the permeability of the gap junctions in these cell types to negatively charged (Lucifer Yellow) and positively charged tracers. Lucifer Yellow will pass only through A-type horizontal cells in rabbit retina. This finding indicates the presence of a structural barrier to anions in all other gap junctional channels, including Cx36, which does not exist in A-type horizontal cell channels. Conversely, all known gap junctional channels in the retina will pass Neurobiotin and other biotinylated tracers (Vaney, 1991). Nevertheless, their permeability declines to differing degrees as a function of increasing tracer size, even when measures are taken to equate for total gap junctional area and open probability (Mills and Massey, 2000). These results suggested the presence of at least three distinctly different permeabilities.
The next two columns show differences in retinal gap junctions to agents that presumably alter the open probability of gap junctions, either by phosphorylation of structural gates or by a likely “nonspecific” method, carbenoxelone. Most known retinal gap junctions appear to decrease their open probability in response to phosphorylation, but indirect evidence suggests that the rod-cone gap junction increases its open probability as protein kinase A is increased (Mangel and Wang, 2000) and, therefore, is distinctly different from the other retinal types. Carbenoxelone has been reported to have differential effects at different rabbit retinal gap junctions and seems to distinguish between S1- and B-type horizontal cell gap junctions (Vaney et al., 1998), which had otherwise similar ion selectivities (columns 2 and 3).
These observations suggest that a minimum of six different classes of gap junctional behavior exist, based on Cx36 immunoreactivity and the other criteria cited. As discussed, some of these properties might arise from complex arrangements of fewer connexins, posttranslational modifications, or regulation by different internal environments (Vaney and Weiler, 2000).
How might the distinct properties of Cx36 affect signal processing?
The single channel conductance reported for Cx35(36) was only 15 pS (Srinivas et al., 1999), the smallest value of any presently measured connexin type. This finding compares with approximately 50 -70 pS for the unknown connexin type(s) of horizontal cells in retina of various fish (McMahon et al., 1989; DeVries and Schwartz, 1992; McMahon and Brown, 1994; Lu and McMahon, 1996). The novel zebrafish connexins cloned by Dermietzel et al. (2000) ranged in main unitary conductance from approximately 55-275 pS. Yet, we previously found that the AII amacrine cell-to-ON cone bipolar cell gap junction contained channels significantly less permeable to large tracers than those between adjoining AII amacrine cells (Mills and Massey, 1995, 2000). This finding suggests that at least the conductance state permeant to Neurobiotin is even smaller for these channels. However, it cannot be assumed that electrical coupling will reflect tracer coupling (Veenstra et al., 1994, 1995; Kwak et al., 1995).
Another notable feature of Cx36 is the weak voltage-gating properties of Cx36 channels in expression systems. This finding might be appropriate for AII amacrine cells coupled to ON cone bipolar cells, as the resting membrane potential of the AII amacrine cell is likely to be >20 mV more negative than that of ON cone bipolar cells (Boos et al., 1993). These and other functional questions concerning the behavior of Cx36 channels in intact tissue can be more easily addressed now that the identity of the cells that express them is known.
ACKNOWLEDGMENTS
We thank Dr. Reto Weiler for helpful commentary and encouragement. S.L.M., S.C.M., and J.O. received support from the NIH. The Department of Ophthalmology and Visual Science received an unrestricted award from Research to Prevent Blindness.
Glossary
The following terms are used in this study to distinguish difference in gap junctional channel structures:
- hemichannel
the half of a channel formed by one of the participating cells
- homomeric (heteromeric)
a hemichannel whose six constituent connexin proteins are (are not) all the same
- homotypic (heterotypic)
a gap junctional channel whose two hemichannels are the same (different)
- homologous (heterologous)
a gap junctional channel between two cells of the same (different) morphologic type
Footnotes
Grant sponsor: NIH; Grant number: EY10121; Grant number: EY06515; Grant number: EY12857; Grant number: EY10608; Grant sponsor: Department of Ophthalmology and Visual Science from Research to Prevent Blindness.
LITERATURE CITED
- Al-Ubaidi MR, White TW, Ripps H, Poras I, Avner P, Gomès D, Bruzzone R. Functional properties, developmental regulation, and chromosomal localization of murine Connexin36, a gap-junctional protein expressed preferentially in retina and brain. J Neurosci Res. 2000;59:813–826. doi: 10.1002/(SICI)1097-4547(20000315)59:6<813::AID-JNR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Trovato-Salinaro A, Mudo G, Hurd YL, Condorelli DF. Structure, chromosomal localization, and brain expression of human Cx36 gene. J Neurosci Res. 1999;57:740–752. [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. Expression of Connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. doi: 10.1016/s0006-8993(00)02300-3. [DOI] [PubMed] [Google Scholar]
- Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci. 1997;14:565–576. doi: 10.1017/s0952523800012220. [DOI] [PubMed] [Google Scholar]
- Boos R, Schneider H, Wässle H. Voltage- and transmitter-gated currents of AII-amacrine cells in a slice preparation of the rat retina. J Neurosci. 1993;13:2874–2888. doi: 10.1523/JNEUROSCI.13-07-02874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Demonstration of cell types among cone bipolar neurons of cat retina. Philos Trans R Soc Lond B. 1990;330:305–321. doi: 10.1098/rstb.1990.0201. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Parenti R, Spinella F, Trovato Salinaro A, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. Horizontal cells in the retina of the rabbit. J Neurosci. 1982;2:1486–1493. doi: 10.1523/JNEUROSCI.02-10-01486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Kremer M, Paputsoglu G, Stang A, Skerrett IM, Gomes D, Srinivas M, Janssen-Bienhold U, Weiler R, Nicholson BJ, Bruzzone R, Spray DC. Molecular and functional diversity of neural connexins in the retina. J Neurosci. 2000;20:8331–8343. doi: 10.1523/JNEUROSCI.20-22-08331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries S, Schwartz E. Hemi-gap junction channels in solitary horizontal cells of the catfish retina. J Physiol (Lond) 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Teubner B, Willecke K, Weiler R. Expression of neuronal connexin36 in AII amacrine cells of the mammalian retina. J Neurosci. 2001;21:230–239. doi: 10.1523/JNEUROSCI.21-01-00230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed MA, Smith RG, Sterling P. Rod bipolar array in the cat retina: pattern of input from rods and GABA-accumulating amacrine cells. J Comp Neurol. 1987;266:445–455. doi: 10.1002/cne.902660310. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Hampson ECGM, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson ECGM, Weiler R, Vaney DI. pH-gated dopaminergic modulation of horizontal cell gap junctions in mammalian retina. Proc R Soc Lond B. 1994;255:67–72. doi: 10.1098/rspb.1994.0010. [DOI] [PubMed] [Google Scholar]
- He S, Weiler R, Vaney DI. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J Comp Neurol. 2000;418:33–40. doi: 10.1002/(sici)1096-9861(20000228)418:1<33::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Kwak BR, van Veen TAB, Analber LJS, Jongsma HJ. TPA increases conductance but decreases permeability in neonatal rat cardiomyocyte gap junction channels. Exp Cell Res. 1995;200:456–463. doi: 10.1006/excr.1995.1337. [DOI] [PubMed] [Google Scholar]
- Lu C, McMahon DG. Gap junction channel gating at bass retinal electrical synapses. Vis Neurosci. 1996;13:1049–1057. doi: 10.1017/s0952523800007707. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel S, Wang Y. Two dopamine systems in the fish retina. Invest Ophthalmol Vis Sci (Suppl) 2000;41:S112. [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive bipolar cell type in the rabbit retina. J Comp Neurol. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. Gap junctions between AII amacrine cells and calbindin-positive bipolar cells in the rabbit retina. Vis Neurosci. 1999;16:1181–1189. doi: 10.1017/s0952523899166173. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Brown DR. Modulation of gap-junction channel gating at zebrafish retinal electrical synapses. J Neurophysiol. 1994;72:2257–2268. doi: 10.1152/jn.1994.72.5.2257. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Knapp AG, Dowling JE. Horizontal cell gap junctions: single channel conductance and modulation by dopamine. Proc Natl Acad Sci USA. 1989;86:7639–7643. doi: 10.1073/pnas.86.19.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL. Unusual coupling properties of a cone bipolar cell in mammalian retina. Vis Neurosci. 1999;16:1029–1035. doi: 10.1017/s0952523899166057. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Labeling and distribution of AII amacrine cells in the rabbit retina. J Comp Neurol. 1991;304:491–501. doi: 10.1002/cne.903040312. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Distribution and coverage of A- and B-type horizontal cells stained with Neurobiotin in the rabbit retina. Vis Neurosci. 1994;11:549–560. doi: 10.1017/s0952523800002455. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. A series of biotinylated tracers distinguishes three types of gap junction in retina. J Neurosci. 2000;20:8629–8636. doi: 10.1523/JNEUROSCI.20-22-08629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, al-Ubaidi MR, Ripps H. Connexin35: a gap-junctional protein expressed preferentially in the skate retina. Mol Biol Cell. 1996;7:233–243. doi: 10.1091/mbc.7.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Bruzzone R, White TW, al-Ubaidi MR, Ripps H. Cloning and expression of two related connexins from the perch retina define a distinct subgroup of the connexin family. J Neurosci. 1998;18:7625–7637. doi: 10.1523/JNEUROSCI.18-19-07625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR, Hampson ECG, Munro MN, Vaney DI. Unidirectional coupling of gap junctions between neuroglia. Science. 1993;262:1072–1074. doi: 10.1126/science.8093125. [DOI] [PubMed] [Google Scholar]
- Röhrenbeck J, Wässle H, Heizmann CW. Immunocytochemical labelling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci Lett. 1987;77:255–260. doi: 10.1016/0304-3940(87)90508-8. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Masland RH, Raviola E, Dacheux RF. Connections of indoleamine-accumulating cells in the rabbit retina. J Comp Neurol. 1989;283:303–313. doi: 10.1002/cne.902830210. [DOI] [PubMed] [Google Scholar]
- Smith RG, Freed MA, Sterling P. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. J Neurosci. 1986;6:3605–3617. doi: 10.1523/JNEUROSCI.06-12-03505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söhl G, Degen J, Teubner B, Willecke K. The murine gap junction gene connexin36 is highly expressed in mouse retina and regulated during brain development. FEBS Lett. 1998;428:27–31. doi: 10.1016/s0014-5793(98)00479-7. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the on-beta ganglion cell. J Neurosci. 1988;8:623–642. doi: 10.1523/JNEUROSCI.08-02-00623.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Patterns of neuronal coupling in the retina. Prog Retinal Res. 1994;13:301–355. [Google Scholar]
- Vaney DI. The A17 (AI) amacrine cell of the cat retina. Invest Ophthalmol Vis Sci. 1996;37:3099. [Google Scholar]
- Vaney DI. Do amacrine cells show tracer coupling to retinal ganglion cells? Invest Ophthalmol Vis Sci. 1997;38:S233. [PubMed] [Google Scholar]
- Vaney DI. Neuronal coupling in the central nervous system: lessons from the retina. Novartis Found Symp. 1999;219:113–125. doi: 10.1002/9780470515587.ch8. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Weiler R. Gap junctions in the eye: evidence for heteromeric, heterotypic and mixed-homotypic junctions. Brain Res Rev. 2000;32:115–120. doi: 10.1016/s0165-0173(99)00070-3. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. J Neurosci. 1998;18:10594–10602. doi: 10.1523/JNEUROSCI.18-24-10594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Smith RG. The AII amacrine network: coupling can increase correlated activity. Vision Res. 1996;36:3743–3757. doi: 10.1016/0042-6989(96)00098-3. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang H-Z, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994;75:483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang H-Z, Beblo DA, Chilton MG, Harris AL, Beyer EC, Brink PR. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ Res. 1995;77:1156–1165. doi: 10.1161/01.res.77.6.1156. [DOI] [PubMed] [Google Scholar]
- Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Nat Acad Sci USA. 2000;97:10260–10265. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Deans MR, O’Brien J, Al-Ubaidi M, Goodenough DA, Ripps H, Bruzzone R. Functional characteristics of skate connexin35, a member of the γ subfamily of connexins expressed in the vertebrate retina. Eur J Neurosci. 1999;11:1883–1890. doi: 10.1046/j.1460-9568.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci. 1999;16:653–665. doi: 10.1017/s0952523899164058. [DOI] [PubMed] [Google Scholar]
- Zahs KR, Newman EA. Asymmetric gap junctional coupling between glial cells in the rat retina. Glia. 1997;10:10–22. [PubMed] [Google Scholar]


