Abstract
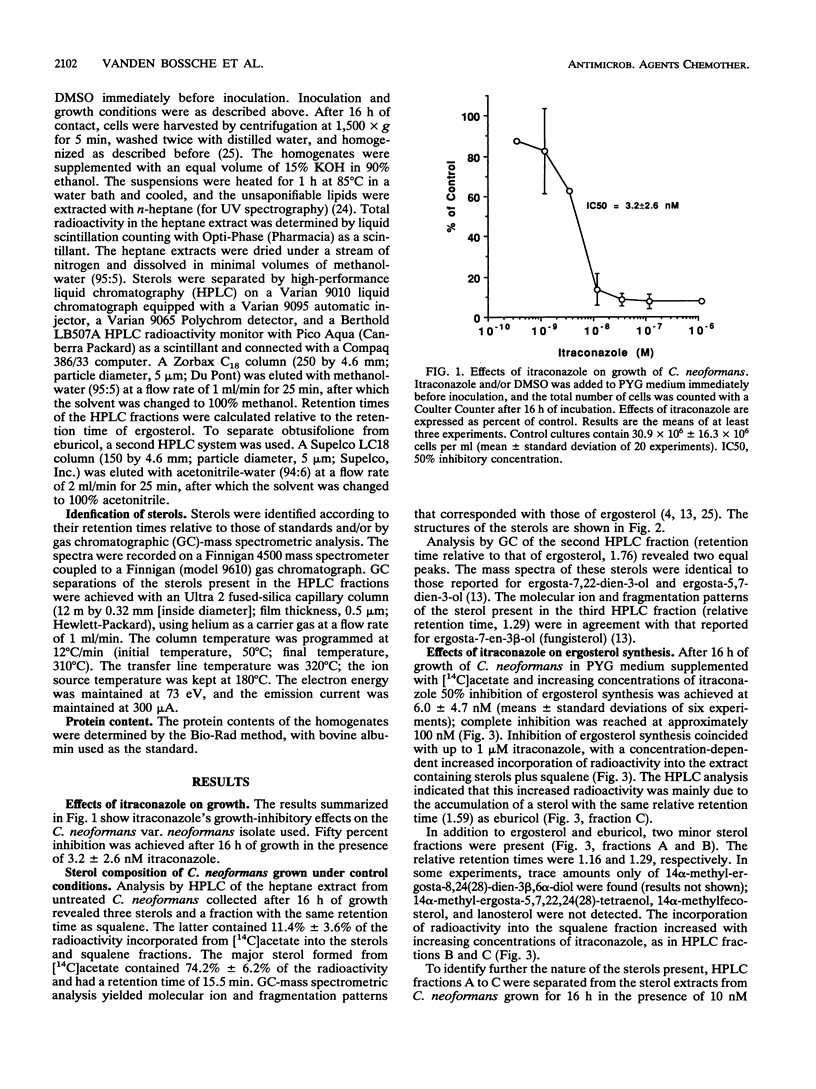
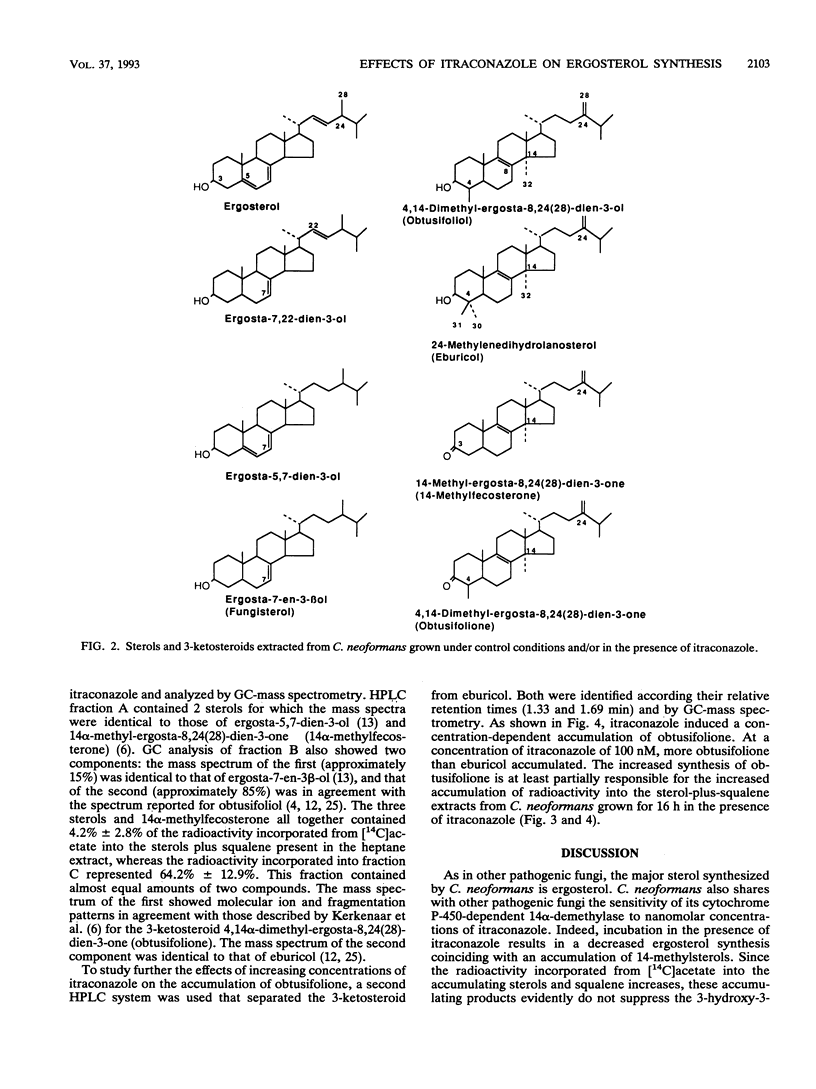
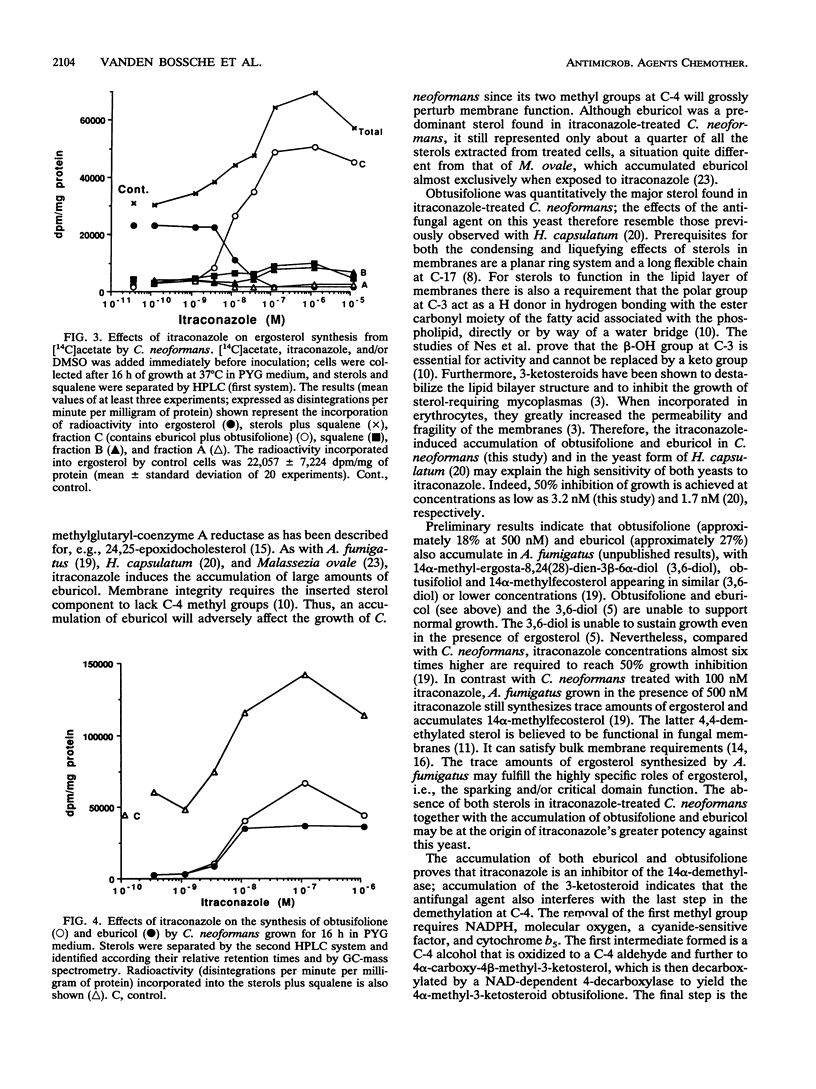
As in other pathogenic fungi, the major sterol synthesized by Cryptococcus neoformans var. neoformans is ergosterol. This yeast also shares with most pathogenic fungi a susceptibility of its cytochrome P-450-dependent ergosterol synthesis to nanomolar concentrations of itraconazole. Fifty percent inhibition of ergosterol synthesis was reached after 16 h of growth in the presence of 6.0 +/- 4.7 nM itraconazole, and complete inhibition was reached at approximately 100 nM itraconazole. This inhibition coincided with the accumulation of mainly eburicol and the 3-ketosteroid obtusifolione. The radioactivity incorporated from [14C]acetate in both compounds represents 64.2% +/- 12.9% of the radioactivity incorporated into the sterols plus squalene extracted from cells incubated in the presence of 10 nM itraconazole. The accumulation of obtusifolione as well as eburicol indicates that itraconazole inhibits not only the 14 alpha-demethylase but also (directly or indirectly) the NADPH-dependent 3-ketosteroid reductase, i.e., the enzyme catalyzing the last step in the demethylation at C-4. This latter inhibition obviates the synthesis of 4,4-demethylated 14 alpha-methylsterols that may function at least partly as surrogates of ergosterol. Eburicol and obtusifolione are unable to support cell growth, and the 3-ketosteroid has been shown to disturb membranes. The complete inhibition of ergosterol synthesis and the accumulation of the 4,4,14-trimethylsterol and of the 3-ketosteroid together with the absence of sterols, such as 14 alpha-methylfecosterol and lanosterol, which can partly fulfill some functions of ergosterol, are at the origin of the high activity of itraconazole against C. neoformans. Fifty percent inhibition of growth achieved after 16 h of incubation in the presence of 3.2 +/- 2.6 nM itraconazole.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gallay J., De Kruijff B. Correlation between molecular shape and hexagonal HII phase promoting ability of sterols. FEBS Lett. 1982 Jun 21;143(1):133–136. doi: 10.1016/0014-5793(82)80289-5. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Lauwers W. J., Willemsens G., Vanden Bossche H., Opperdoes F. R. Perturbation of sterol biosynthesis by itraconazole and ketoconazole in Leishmania mexicana mexicana infected macrophages. Mol Biochem Parasitol. 1989 Mar 1;33(2):123–134. doi: 10.1016/0166-6851(89)90026-1. [DOI] [PubMed] [Google Scholar]
- Kelly S. L., Rowe J., Watson P. F. Molecular genetic studies on the mode of action of azole antifungal agents. Biochem Soc Trans. 1991 Aug;19(3):796–798. doi: 10.1042/bst0190796. [DOI] [PubMed] [Google Scholar]
- Nes W. D., Janssen G. G., Crumley F. G., Kalinowska M., Akihisa T. The structural requirements of sterols for membrane function in Saccharomyces cerevisiae. Arch Biochem Biophys. 1993 Feb 1;300(2):724–733. doi: 10.1006/abbi.1993.1100. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Low C., Bottema C. D., Parks L. W. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1985 Dec 4;837(3):336–343. doi: 10.1016/0005-2760(85)90057-8. [DOI] [PubMed] [Google Scholar]
- Taylor F. R., Kandutsch A. A., Gayen A. K., Nelson J. A., Nelson S. S., Phirwa S., Spencer T. A. 24,25-Epoxysterol metabolism in cultured mammalian cells and repression of 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem. 1986 Nov 15;261(32):15039–15044. [PubMed] [Google Scholar]
- Taylor F. R., Rodriguez R. J., Parks L. W. Requirement for a second sterol biosynthetic mutation for viability of a sterol C-14 demethylation defect in Saccharomyces cerevisiae. J Bacteriol. 1983 Jul;155(1):64–68. doi: 10.1128/jb.155.1.64-68.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Bossche H., Marichal P., Gorrens J., Coene M. C., Willemsens G., Bellens D., Roels I., Moereels H., Janssen P. A. Biochemical approaches to selective antifungal activity. Focus on azole antifungals. Mycoses. 1989;32 (Suppl 1):35–52. doi: 10.1111/j.1439-0507.1989.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H., Marichal P., Gorrens J., Geerts H., Janssen P. A. Mode of action studies. Basis for the search of new antifungal drugs. Ann N Y Acad Sci. 1988;544:191–207. doi: 10.1111/j.1749-6632.1988.tb40404.x. [DOI] [PubMed] [Google Scholar]
- van den Bossche H., Willemsens G., Cools W., Lauwers W. F., Le Jeune L. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chem Biol Interact. 1978 Apr;21(1):59–78. doi: 10.1016/0009-2797(78)90068-6. [DOI] [PubMed] [Google Scholar]
- vanden Bossche H., Marichal P., Odds F. C., Le Jeune L., Coene M. C. Characterization of an azole-resistant Candida glabrata isolate. Antimicrob Agents Chemother. 1992 Dec;36(12):2602–2610. doi: 10.1128/aac.36.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]



