Abstract
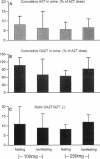
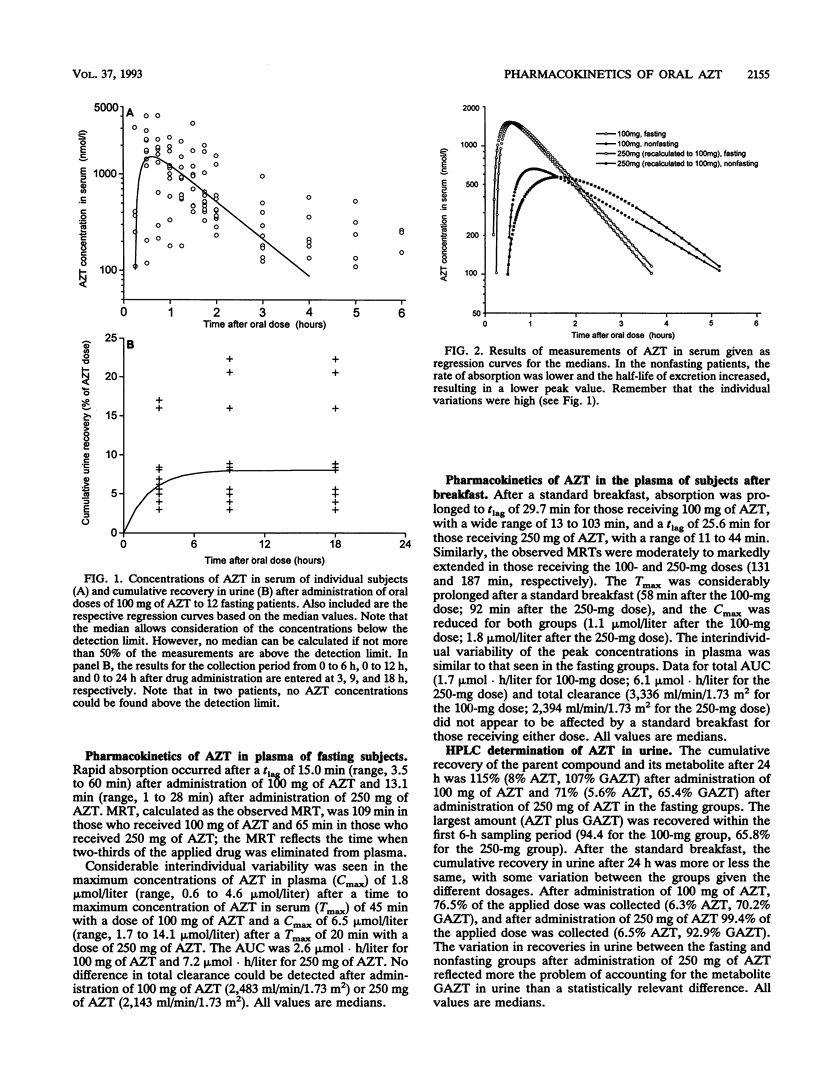
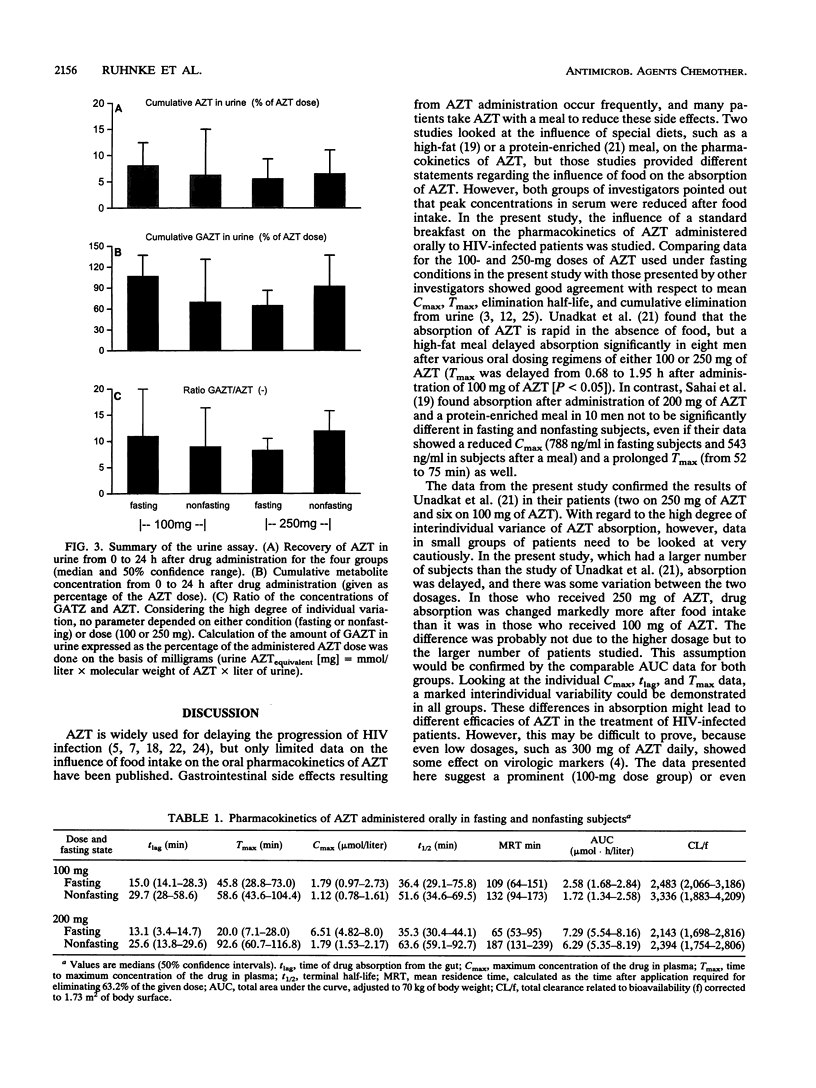
The influence of a standard breakfast on the single-dose pharmacokinetics of zidovudine (AZT) after oral administration of 100 and 250 mg of AZT was studied in 27 subjects with advanced human immunodeficiency virus infection (Centers for Disease Control stage IV). Concentrations of AZT and the 5'-glucuronide metabolite (GAZT) in serum and urine were measured by a high-pressure liquid chromatographic method. Pharmacokinetic analysis was done by an open one-compartment model as well as noncompartmentally. The results were summarized as medians with 50% confidence ranges because of the high degree of interindividual variability. Peak levels in plasma were moderately reduced after administration of 100 mg AZT in the nonfasting group (1.79 mumol/liter in the fasting group [F], 1.12 mumol/liter in the group that received breakfast [B]) and were markedly reduced after administration of 250 mg AZT (6.51 mumol/liter [F], 1.79 mumol/liter [B]). The terminal half-life in plasma was prolonged almost twofold after breakfast with 100 and 250 mg of AZT (100 mg, 36.4 min [F] and 51.6 min [B]; 250 mg, 35.3 min [F] and 63.6 min [B]). Recoveries (AZT and GAZT) in urine varied with both dosages, reflecting more a problem of accounting for the metabolite GAZT in urine than a relevant difference (100 mg, 115% [F] and 76.5% [B]; 250 mg, 71% [F] and 99.4% [B]). Our data suggest that absorption of AZT in human immunodeficiency virus-infected subjects is extremely variable, with a high degree of interindividual differences. Furthermore, breakfast had a marked influence on the absorption of AZT, suggesting that the drug should be taken in a fasting state.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E. Influences of diet and nutrition on clinical pharmacokinetics. Clin Pharmacokinet. 1988 Jun;14(6):325–346. doi: 10.2165/00003088-198814060-00002. [DOI] [PubMed] [Google Scholar]
- Balis F. M., Pizzo P. A., Eddy J., Wilfert C., McKinney R., Scott G., Murphy R. F., Jarosinski P. F., Falloon J., Poplack D. G. Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J Pediatr. 1989 May;114(5):880–884. doi: 10.1016/s0022-3476(89)80158-1. [DOI] [PubMed] [Google Scholar]
- Blum M. R., Liao S. H., Good S. S., de Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988 Aug 29;85(2A):189–194. [PubMed] [Google Scholar]
- Collier A. C., Bozzette S., Coombs R. W., Causey D. M., Schoenfeld D. A., Spector S. A., Pettinelli C. B., Davies G., Richman D. D., Leedom J. M. A pilot study of low-dose zidovudine in human immunodeficiency virus infection. N Engl J Med. 1990 Oct 11;323(15):1015–1021. doi: 10.1056/NEJM199010113231502. [DOI] [PubMed] [Google Scholar]
- Collins J. M., Unadkat J. D. Clinical pharmacokinetics of zidovudine. An overview of current data. Clin Pharmacokinet. 1989 Jul;17(1):1–9. doi: 10.2165/00003088-198917010-00001. [DOI] [PubMed] [Google Scholar]
- Drew R. H., Weller S., Gallis H. A., Walmer K. A., Bartlett J. A., Blum M. R. Bioequivalence assessment of zidovudine (Retrovir) syrup, solution, and capsule formulations in patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1989 Oct;33(10):1801–1803. doi: 10.1128/aac.33.10.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Good S. S., Reynolds D. J., de Miranda P. Simultaneous quantification of zidovudine and its glucuronide in serum by high-performance liquid chromatography. J Chromatogr. 1988 Sep 23;431(1):123–133. doi: 10.1016/s0378-4347(00)83075-3. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Klecker R. W., Jr, Collins J. M., Yarchoan R., Thomas R., Jenkins J. F., Broder S., Myers C. E. Plasma and cerebrospinal fluid pharmacokinetics of 3'-azido-3'-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clin Pharmacol Ther. 1987 Apr;41(4):407–412. doi: 10.1038/clpt.1987.49. [DOI] [PubMed] [Google Scholar]
- Koeppe P., Hamann C. A program for non-linear regression analysis to be used on desk-top computers. Comput Programs Biomed. 1980 Dec;12(2-3):121–128. doi: 10.1016/0010-468x(80)90058-6. [DOI] [PubMed] [Google Scholar]
- Langtry H. D., Campoli-Richards D. M. Zidovudine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1989 Apr;37(4):408–450. doi: 10.2165/00003495-198937040-00003. [DOI] [PubMed] [Google Scholar]
- Lotterer E., Ruhnke M., Trautmann M., Beyer R., Bauer F. E. Decreased and variable systemic availability of zidovudine in patients with AIDS if administered with a meal. Eur J Clin Pharmacol. 1991;40(3):305–308. doi: 10.1007/BF00315215. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spire B., Dormont D., Barré-Sinoussi F., Montagnier L., Chermann J. C. Inactivation of lymphadenopathy-associated virus by heat, gamma rays, and ultraviolet light. Lancet. 1985 Jan 26;1(8422):188–189. doi: 10.1016/s0140-6736(85)92026-4. [DOI] [PubMed] [Google Scholar]
- Unadkat J. D., Collier A. C., Crosby S. S., Cummings D., Opheim K. E., Corey L. Pharmacokinetics of oral zidovudine (azidothymidine) in patients with AIDS when administered with and without a high-fat meal. AIDS. 1990 Mar;4(3):229–232. doi: 10.1097/00002030-199003000-00008. [DOI] [PubMed] [Google Scholar]
- Welling P. G. Influence of food and diet on gastrointestinal drug absorption: a review. J Pharmacokinet Biopharm. 1977 Aug;5(4):291–334. doi: 10.1007/BF01061694. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Klecker R. W., Weinhold K. J., Markham P. D., Lyerly H. K., Durack D. T., Gelmann E., Lehrman S. N., Blum R. M., Barry D. W. Administration of 3'-azido-3'-deoxythymidine, an inhibitor of HTLV-III/LAV replication, to patients with AIDS or AIDS-related complex. Lancet. 1986 Mar 15;1(8481):575–580. doi: 10.1016/s0140-6736(86)92808-4. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Mitsuya H., Myers C. E., Broder S. Clinical pharmacology of 3'-azido-2',3'-dideoxythymidine (zidovudine) and related dideoxynucleosides. N Engl J Med. 1989 Sep 14;321(11):726–738. doi: 10.1056/NEJM198909143211106. [DOI] [PubMed] [Google Scholar]