Abstract
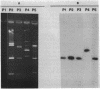
In 1990, over a 6-month period, an increase from 1 to 10% in the incidence of pristinamycin resistance among coagulase-negative staphylococci was observed in four intensive care units of a Parisian hospital. Twenty-three such isolates, as well as 25 pristinamycin-susceptible Staphylococcus epidermidis isolates, were collected and typed by analyzing various bacterial constituents. Two structurally related plasmids of 7.3 and 14.3 kb, carrying the gene vga encoding resistance to pristinamycin, were detected in the 23 pristinamycin-resistant coagulase-negative staphylococci which were identified as S. epidermidis. Although related by numerous common characteristics, 20 of these 23 isolates could be divided into two types, A (17 isolates) and B (three isolates). These types were characterized on the basis of their plasmid contents and hybridization patterns obtained when the EcoRI-digested DNA was probed with plasmid pIP1551 containing an internal fragment of the insertion sequence IS256. These findings suggest that the dissemination of type A epidemic strains was, in large part, responsible for the outbreak.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allignet J., Loncle V., Mazodier P., el Solh N. Nucleotide sequence of a staphylococcal plasmid gene, vgb, encoding a hydrolase inactivating the B components of virginiamycin-like antibiotics. Plasmid. 1988 Nov;20(3):271–275. doi: 10.1016/0147-619x(88)90034-0. [DOI] [PubMed] [Google Scholar]
- Allignet J., Loncle V., el Sohl N. Sequence of a staphylococcal plasmid gene, vga, encoding a putative ATP-binding protein involved in resistance to virginiamycin A-like antibiotics. Gene. 1992 Aug 1;117(1):45–51. doi: 10.1016/0378-1119(92)90488-b. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Fouace J. M., Bieth G. Physical studies of a Staphylococcus aureus plasmid mediating resistance to streptogramins, lincosamins and aminoglycosides. Ann Microbiol (Paris) 1977 Nov-Dec;128B(4):431–437. [PubMed] [Google Scholar]
- Byrne M. E., Rouch D. A., Skurray R. A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989 Sep 30;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- Chesneau O., Aubert S., Morvan A., Guesdon J. L., el Solh N. Usefulness of the ID32 staph system and a method based on rRNA gene restriction site polymorphism analysis for species and subspecies identification of staphylococcal clinical isolates. J Clin Microbiol. 1992 Sep;30(9):2346–2352. doi: 10.1128/jcm.30.9.2346-2352.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocito C. Antibiotics of the virginiamycin family, inhibitors which contain synergistic components. Microbiol Rev. 1979 Jun;43(2):145–192. doi: 10.1128/mr.43.2.145-192.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Buyser M. L., Morvan A., Aubert S., Dilasser F., el Solh N. Evaluation of a ribosomal RNA gene probe for the identification of species and subspecies within the genus Staphylococcus. J Gen Microbiol. 1992 May;138(5):889–899. doi: 10.1099/00221287-138-5-889. [DOI] [PubMed] [Google Scholar]
- De Buyser M. L., Morvan A., Grimont F., el Solh N. Characterization of Staphylococcus species by ribosomal RNA gene restriction patterns. J Gen Microbiol. 1989 Apr;135(4):989–999. doi: 10.1099/00221287-135-4-989. [DOI] [PubMed] [Google Scholar]
- De Meester C., Rondelet J. Microbial acetylation of M factor of virginiamycin. J Antibiot (Tokyo) 1976 Dec;29(12):1297–1305. doi: 10.7164/antibiotics.29.1297. [DOI] [PubMed] [Google Scholar]
- Dublanchet A., Soussy C. J., Squinazi F., Duval J. Résistance de Staphylococcus aureus aux streptogramines. Ann Microbiol (Paris) 1977 Apr;128A(3):277–287. [PubMed] [Google Scholar]
- Dyke K. G., Aubert S., el Solh N. Multiple copies of IS256 in staphylococci. Plasmid. 1992 Nov;28(3):235–246. doi: 10.1016/0147-619x(92)90055-f. [DOI] [PubMed] [Google Scholar]
- El Solh N., Bismuth R., Allignet J., Fouace J. M. Résistance à la pristinamycine (ou virginiamycine) des souches de Staphylococcus aureus. Pathol Biol (Paris) 1984 May;32(5):362–368. [PubMed] [Google Scholar]
- El Solh N., Fouace J. M., Pillet J., Chabbert Y. A. Plasmid DNA content of multiresistant Staphylococcus aureus strains. Ann Microbiol (Paris) 1981 Sep-Oct;132B(2):131–156. [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Goffic F. L., Capmau M. L., Bonnet D., Cerceau C., Soussy C., Dublanchet A., Duval J. Plasmid-mediated pristinamycin resistance. PAC IIA: a new enzyme which modifies pristinamycin IIA. J Antibiot (Tokyo) 1977 Aug;30(8):665–669. doi: 10.7164/antibiotics.30.665. [DOI] [PubMed] [Google Scholar]
- Iglesias A., Ceglowski P., Trautner T. A. Plasmid transformation in Bacillus subtilis. Effects of the insertion of Bacillus subtilis rRNA genes into plasmids. Mol Gen Genet. 1983;192(1-2):149–154. doi: 10.1007/BF00327660. [DOI] [PubMed] [Google Scholar]
- Le Goffic F., Capmau M. L., Abbe J., Cerceau C., Dublanchet A., Duval J. Plasmid mediated pristinamycin resistance: PH 1A, a pristinamycin 1A hydrolase. Ann Microbiol (Paris) 1977 Nov-Dec;128B(4):471–474. [PubMed] [Google Scholar]
- Ross J. I., Eady E. A., Cove J. H., Cunliffe W. J., Baumberg S., Wootton J. C. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol Microbiol. 1990 Jul;4(7):1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Walcher-Salesse S., Monzon-Moreno C., Aubert S., el Solh N. An epidemiological assessment of coagulase-negative staphylococci from an intensive care unit. J Med Microbiol. 1992 May;36(5):321–331. doi: 10.1099/00222615-36-5-321. [DOI] [PubMed] [Google Scholar]
- Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression--a review. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]
- el Solh N., Allignet J., Bismuth R., Buret B., Fouace J. M. Conjugative transfer of staphylococcal antibiotic resistance markers in the absence of detectable plasmid DNA. Antimicrob Agents Chemother. 1986 Jul;30(1):161–169. doi: 10.1128/aac.30.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Solh N., Fouace J. M., Shalita Z., Bouanchaud D. H., Novick R. P., Chabbert Y. A. Epidemiological and structural studies of Staphylococcus aureus R plasmids mediating resistance to tobramycin and streptogramin. Plasmid. 1980 Jul;4(1):117–120. doi: 10.1016/0147-619x(80)90087-6. [DOI] [PubMed] [Google Scholar]