Abstract
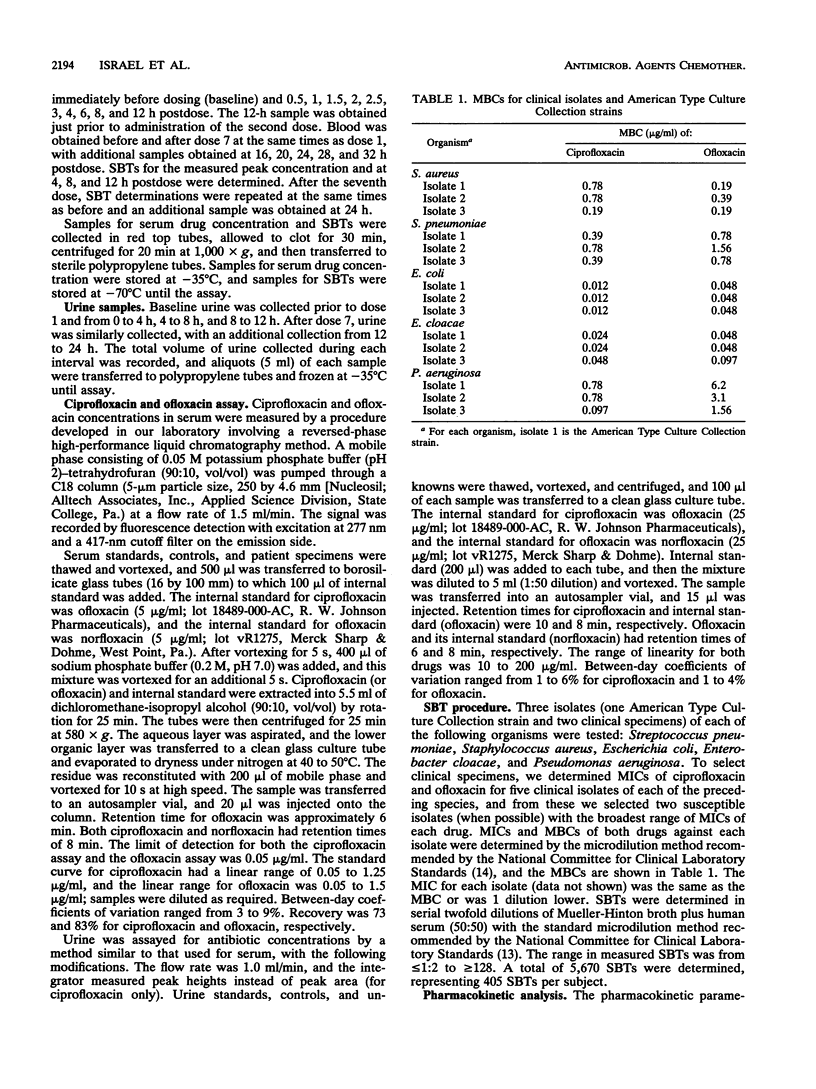
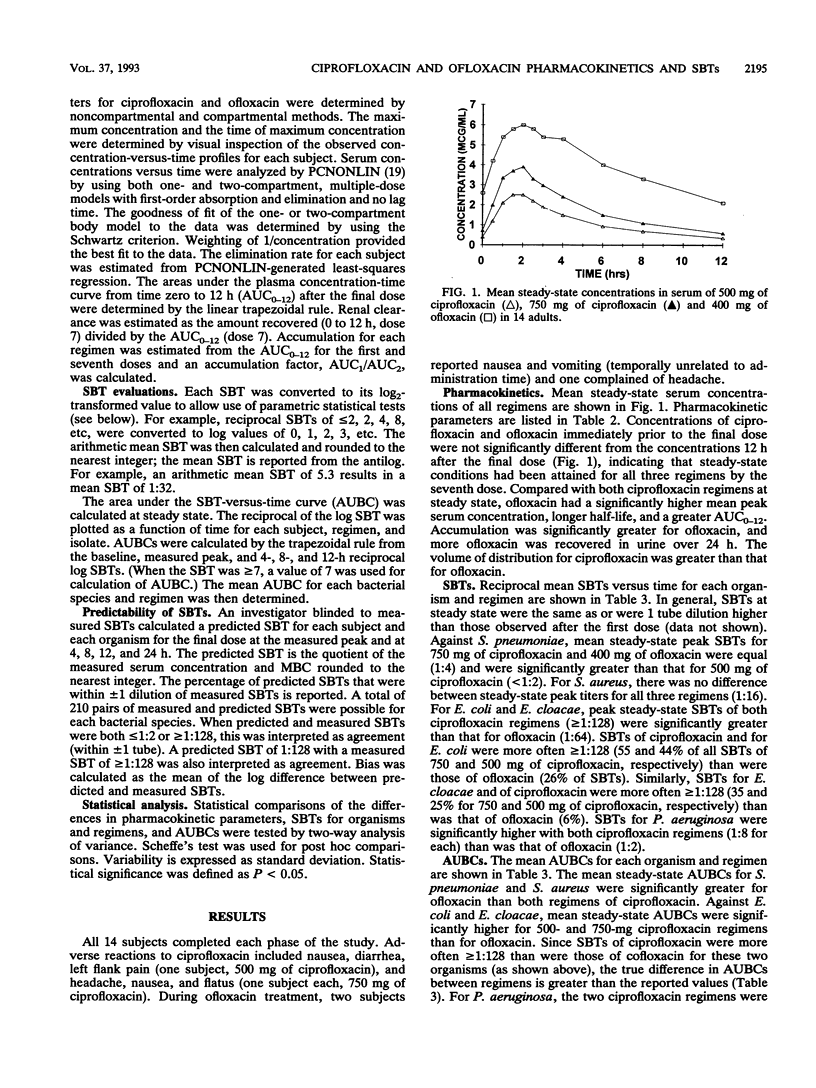
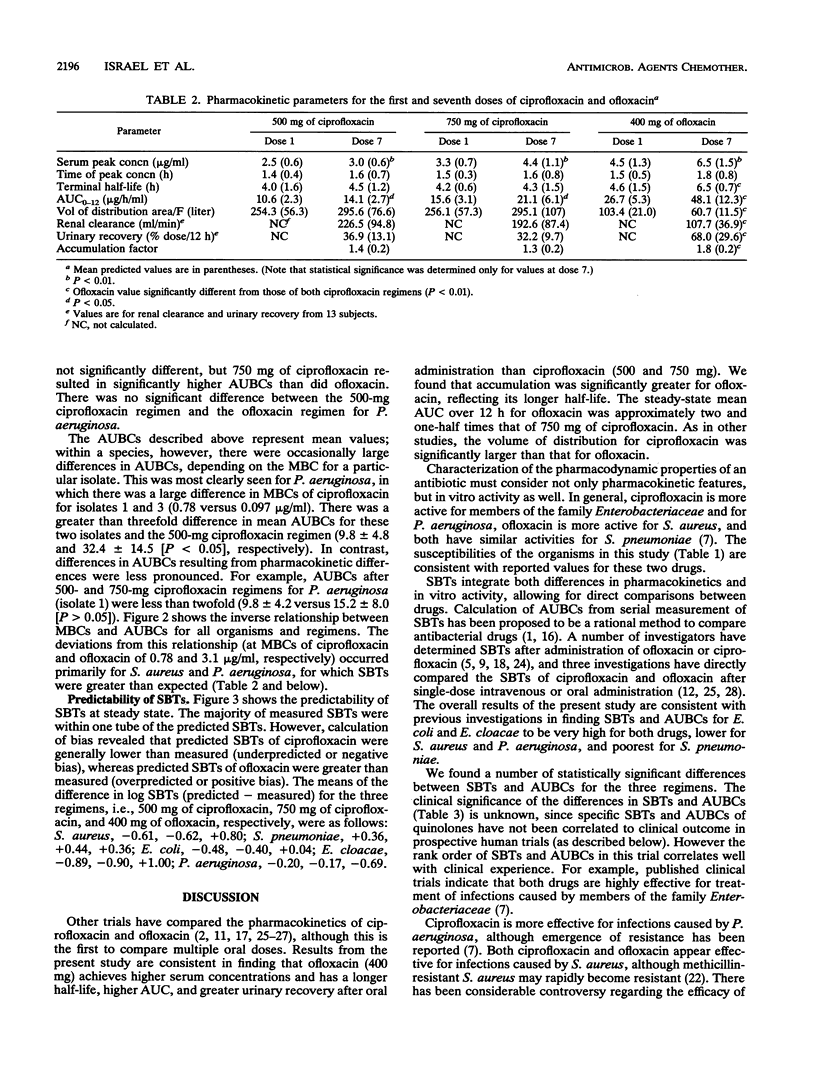
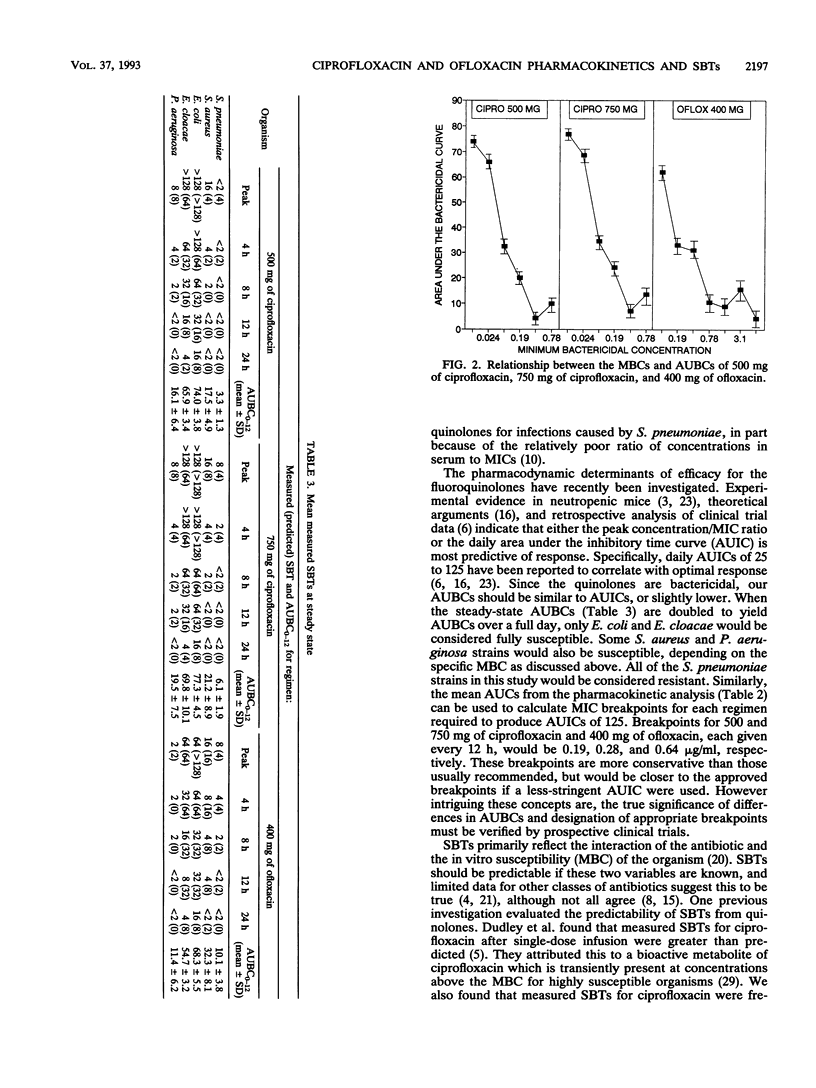
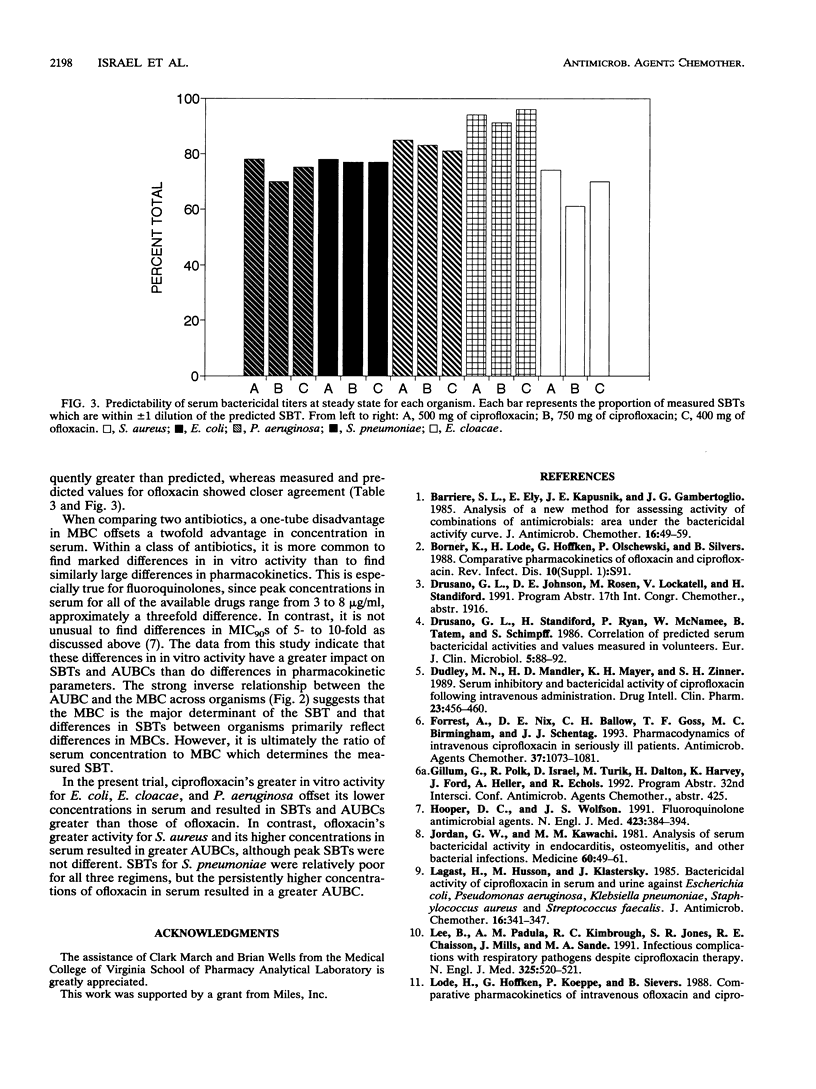
Fourteen adult males participated in a randomized three-way crossover study to compare the pharmacokinetics and serum bactericidal titers (SBTs) of 500 mg of ciprofloxacin (regimen A), 750 mg of ciprofloxacin (regimen B), and 400 mg of ofloxacin (regimen C) administered every 12 h for seven doses. Mean steady-state peak concentrations in serum for regimens A, B, and C were 3.0, 4.4, and 6.5 micrograms/ml, respectively (P < 0.01, all comparisons) and mean half-lives were 4.5, 4.3, and 6.5 h, respectively (P < 0.05, C versus A and B). Mean steady-state areas under the concentration-time curve were 14.1, 21.1, and 48.1 micrograms/h/ml for regimens A, B, and C, respectively (P < 0.05, all comparisons). SBTs were determined at different times postdose for three isolates each of Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli, Enterobacter cloacae, and Pseudomonas aeruginosa. Mean steady-state peak SBTs for regimens A, B, and C, respectively, were as follows: S. pneumoniae, < 1:2, 1:8, 1:8, S. aureus, 1:16, 1:16, 1:16; E. coli, 1: > or = 128, 1: > or = 128, 1:64; E. cloacae, 1: > or = 128, 1: > or = 128, 1:64; P. aeruginosa, 1:8, 1:8, 1:2. These differences in SBTs within each genus were statistically significant. The majority of predicted SBTs were within one dilution of measured SBTs. Areas under the serum bactericidal time curves for E. coli, E. cloacae, and P. aeruginosa were significantly higher for ciprofloxacin; areas under the serum bactericidal time curves for S. pneumoniae and S. aureus were significantly greater for ofloxacin. Ofloxacin achieved higher concentrations in serum than ciprofloxacin, but differences in in vitro activity were a more important determinant of SBTs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barriere S. L., Ely E., Kapusnik J. E., Gambertoglio J. G. Analysis of a new method for assessing activity of combinations of antimicrobials: area under the bactericidal activity curve. J Antimicrob Chemother. 1985 Jul;16(1):49–59. doi: 10.1093/jac/16.1.49. [DOI] [PubMed] [Google Scholar]
- Drusano G., Standiford H., Ryan P., McNamee W., Tatem B., Schimpff S. Correlation of predicted serum bactericidal activities and values measured in volunteers. Eur J Clin Microbiol. 1986 Feb;5(1):88–92. doi: 10.1007/BF02013475. [DOI] [PubMed] [Google Scholar]
- Dudley M. N., Mandler H. D., Mayer K. H., Zinner S. H. Serum inhibitory and bactericidal activity of ciprofloxacin following intravenous administration. DICP. 1989 Jun;23(6):456–460. doi: 10.1177/106002808902300603. [DOI] [PubMed] [Google Scholar]
- Forrest A., Nix D. E., Ballow C. H., Goss T. F., Birmingham M. C., Schentag J. J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993 May;37(5):1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991 Feb 7;324(6):384–394. doi: 10.1056/NEJM199102073240606. [DOI] [PubMed] [Google Scholar]
- Jordan G. W., Kawachi M. M. Analysis of serum bactericidal activity in endocarditis, osteomyelitis, and other bacterial infections. Medicine (Baltimore) 1981 Jan;60(1):49–61. doi: 10.1097/00005792-198101000-00005. [DOI] [PubMed] [Google Scholar]
- Lagast H., Husson M., Klastersky J. Bactericidal activity of ciprofloxacin in serum and urine against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus and Streptococcus faecalis. J Antimicrob Chemother. 1985 Sep;16(3):341–347. doi: 10.1093/jac/16.3.341. [DOI] [PubMed] [Google Scholar]
- Lee B. L., Padula A. M., Kimbrough R. C., Jones S. R., Chaisson R. E., Mills J., Sande M. A. Infectious complications with respiratory pathogens despite ciprofloxacin therapy. N Engl J Med. 1991 Aug 15;325(7):520–521. doi: 10.1056/nejm199108153250719. [DOI] [PubMed] [Google Scholar]
- Lode H., Höffken G., Olschewski P., Sievers B., Kirch A., Borner K., Koeppe P. Comparative pharmacokinetics of intravenous ofloxacin and ciprofloxacin. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):73–79. doi: 10.1093/jac/22.supplement_c.73. [DOI] [PubMed] [Google Scholar]
- Machka K., Milatovic D. Serum bactericidal activity of ciprofloxacin and ofloxacin in volunteers. Eur J Clin Microbiol. 1987 Feb;6(1):59–62. doi: 10.1007/BF02097195. [DOI] [PubMed] [Google Scholar]
- Peterson L. R., Shanholtzer C. J. Tests for bactericidal effects of antimicrobial agents: technical performance and clinical relevance. Clin Microbiol Rev. 1992 Oct;5(4):420–432. doi: 10.1128/cmr.5.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schentag J. J., Nix D. E., Adelman M. H. Mathematical examination of dual individualization principles (I): Relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP. 1991 Oct;25(10):1050–1057. doi: 10.1177/106002809102501003. [DOI] [PubMed] [Google Scholar]
- Standiford H. C., Drusano G. L., Forrest A., Tatem B., Plaisance K. Bactericidal activity of ciprofloxacin compared with that of cefotaxime in normal volunteers. Antimicrob Agents Chemother. 1987 Aug;31(8):1177–1182. doi: 10.1128/aac.31.8.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W. Serum bactericidal test. Clin Microbiol Rev. 1988 Jan;1(1):19–26. doi: 10.1128/cmr.1.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Weinstein M. P., Reller L. B. Correlation of serum bactericidal activity with antimicrobial agent level and minimal bactericidal concentration. J Infect Dis. 1982 Feb;145(2):160–168. doi: 10.1093/infdis/145.2.160. [DOI] [PubMed] [Google Scholar]
- Trucksis M., Hooper D. C., Wolfson J. S. Emerging resistance to fluoroquinolones in staphylococci: an alert. Ann Intern Med. 1991 Mar 1;114(5):424–426. doi: 10.7326/0003-4819-114-5-424. [DOI] [PubMed] [Google Scholar]
- Weinstein M. P., Deeter R. G., Swanson K. A., Gross J. S. Crossover assessment of serum bactericidal activity and pharmacokinetics of ciprofloxacin alone and in combination in healthy elderly volunteers. Antimicrob Agents Chemother. 1991 Nov;35(11):2352–2358. doi: 10.1128/aac.35.11.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Griggs D., Andrews J. M. Pharmacokinetics of the quinolones in volunteers: a proposed dosing schedule. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S83–S89. [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Comparative pharmacokinetics of ofloxacin and ciprofloxacin. Am J Med. 1989 Dec 29;87(6C):31S–36S. [PubMed] [Google Scholar]
- Zeiler H. J., Beermann D., Wingender W., Förster D., Schacht P. Bactericidal activity of ciprofloxacin, norfloxacin and ofloxacin in serum and urine after oral administration to healthy volunteers. Infection. 1988;16 (Suppl 1):S19–S23. doi: 10.1007/BF01650502. [DOI] [PubMed] [Google Scholar]
- Zeiler H. J., Petersen U., Gau W., Ploschke H. J. Antibacterial activity of the metabolites of ciprofloxacin and its significance in the bioassay. Arzneimittelforschung. 1987 Feb;37(2):131–134. [PubMed] [Google Scholar]



